Abstract
The main objective of this research was to conduct a theoretical investigation into the antioxidant properties of chlorogenic acid. Calculations based on the density functional theory were performed using the B3LYP exchange-correlation functional and the 6-311G standard basis set to determine the theoretical parameters, such as bond dissociation enthalpy and spin density. H atom abstraction from −OH groups was the most representative reaction pathway chosen for this study. From these calculations, it was estimated that bond dissociation enthalpies of hydroxyl groups were in the order of 3’-OH > 4’-OH > 2-OH > 1-OH. Spin densities of O-atoms of free radicals formed (after the removal of H atoms) from the hydroxyl groups of aromatic rings were lower than that of hydroxyl groups of alicyclic rings. On the basis of the calculated results, H atom abstraction from −OH groups of aromatic ring should be the main mechanism for the antioxidant potential of chlorogenic acid. Moreover, a comparison of bond dissociation enthalpy values for different phenolic acid antioxidants showed that the chlorogenic acid is a potent antioxidant. This theoretical investigation might be helpful for the future applications of chlorogenic acid in the field of the pharmacy and food industries.
INTRODUCTION
Phenolic acids constitute a family of natural compounds that can be found in a wide variety of foods and in the plant kingdom. Antioxidant and radical scavenging activities, along with many useful characteristics, have been attributed to phenolic acids.[Citation1] These are extensively studied due to their action as “free radical scavengers” and their broad range of applications.[Citation2,Citation3] Free radicals are highly reactive species, as they can attack important biological molecules such as carbohydrates, proteins, lipids, DNA, and RNA, resulting in several kinds of diseases, such as cancer, arthritis, aging, and heart disease. Therefore, antioxidants are needed to prevent from these destructive chain reactions by scavenging the free radicals through electron transfer mechanism.[Citation4,Citation5]
Moreover, most of the commercial antioxidants have side effects and also their efficiency decreases with time due to the instability of formed radicals. A single radical cannot provide better protection, because nutrient antioxidants generally have a very narrow range of activity against a single type of free radical.[Citation6] Therefore, plant-based antioxidants were used along with nutrient antioxidants to enhance their activity. Some of the most useful plant-based antioxidants are flavonoids and plant pigments. Recent studies show that flavonoids are more potent and effective against a broader range of free radicals than nutrient antioxidants. Plant-based bioflavonoids can effectively protect the human body from oxidative stress; a conclusion based on the substantial epidemiological evidences.
Chlorogenic acid is widely recognized to have antioxidant activities and a broad range of biological properties, such as anticarcinogenic, antiviral, antimutagenic, and enhancing cellular defense, which is attributed to scavenging species of oxygen and nitrogen.[Citation7] Chlorogenic acid can be found in many plants,[Citation8] especially in Phyllostachys edulis,[Citation9] which is commonly known as bamboo. It is also one of the major phenolic compounds identified in peach,[Citation10] prunes,[Citation11] and coffee. Chlorogenic acid can also be found in the shoots of Calluna vulgaris.[Citation12] Structurally, chlorogenic acid is an ester formed between caffeic acid and L-quinic acid. [Citation13]
During the past decade, the density functional theory (DFT) has been widely applied for exploring the antioxidant potential of the phenolic compounds.[Citation14–Citation16] As the theoretical investigations provide the same information in less time, easily, economically, and with reasonable accuracy. Phenolic compounds generally exert their protective activities by three different mechanisms: hydrogen atom transfer (HAT), electron transfer-proton transfer (ETPT), and sequential proton loss-electron transfer (SPLET). All the mechanisms are believed to play important roles in determining radical scavenging activities of antioxidants in various environmental conditions.[Citation17] It has been shown that radical scavenging activities of phenolic antioxidants are related to the phenolic O-H bond dissociation enthalpy (BDE), and spin density.[Citation18,Citation19]
This study was performed to determine the antioxidant potential of chlorogenic acid, a plant-based antioxidant. Moreover, BDE and spin density were computed in order to evaluate the antioxidant potential of chlorogenic acid. These are the most critical theoretical parameters to explore the antioxidant potential of phenolic acids. To the best of the author’s knowledge, the antioxidant potential of chlorogenic acid by DFT has not yet been documented elsewhere.
MATERIALS AND METHODS
DFT was used in all the computational calculations. Computational calculations were performed with the Gaussian 09 W suite of programs.[Citation20] The lowest energy structures of the species were determined by conformational analysis. All chemical systems were optimized in their ground electronic state. In all DFT calculations, B3LYP functional[Citation21] and 6-311G basis set was used for the optimization of all parent molecule and their radicals. Optimized geometry of the neutral molecule, after H-atom removed from OH groups was used for geometry optimization of radicals. For the geometry optimization of parent molecule, the restricted approach was applied, while for the free radicals the unrestricted approach was used. Total enthalpies of species X, H(X), are calculated using the following formula:
where E0 is total electronic energy, ZPE stands for zero-point energy; ∆Htrans, ∆Hrot, and ∆Hvib are the translational, rotational, and vibrational contributions to the enthalpy. Finally, RT represents PV-work term. The homolytic BDE O–H is determined using formula:
where H(ArO) is the enthalpy of the radical produced by the removal of H, H(H·) is the enthalpy of hydrogen atom [–0.4979 Hartree] and H(ArOH) is the enthalpy of neutral molecule.
RESULTS AND DISCUSSION
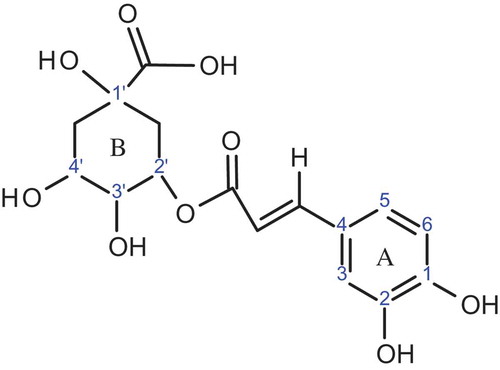
In this study, computational evaluation of antioxidant potential of chlorogenic acid was carried out. As shown in , the structure of chlorogenic acid is consists of two rings A and B. Ring A is aromatic while ring B is alicyclic. There are five hydroxyl groups present in the structure of chlorogenic acid, two are present in aromatic ring while three in the alicyclic ring. We have focused on only four hydroxyl groups. We did not study the 1’-OH hydroxyl group because during the optimization of radical formed after the abstraction of H atom from 1’-OH, ring B was broken down due to the steric hindrance of carboxylic group.
BDE
The hydrogen donating capacity of phenolic compounds and their free radicals can be explained by an important parameter i.e., BDE. The BDE is related to the breaking of O–H bond, resulted in the abstraction of H atom. With the help of this tool, the stability of hydroxyl bonds can also be estimated.[Citation22] Lower BDE value indicates the high antioxidant potential. shows the BDE values computed under gas phase.
TABLE 1 BDEs of different hydroxyl groups of chlorogenic acid
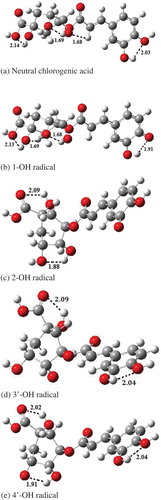
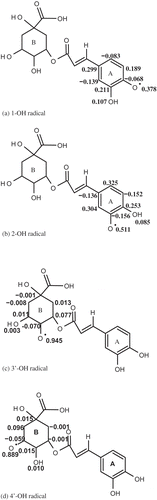
On the basis of the calculated results, BDEs are in following order 3’-OH > 4’-OH > 2-OH > 1-OH. This trend can be explained on the basis of intramolecular hydrogen bonding. shows the intramolecular hydrogen bonding in neutral chlorogenic acid and its radicals. BDE value for 1-OH is lower than all other radicals. Lower BDE values are observed due to the presence of stronger intramolecular hydrogen bonding, as it can stabilize the free radical and consequently decrease the BDE values. BDE values for 2-OH are higher than 1-OH. Because 2-OH has two intramolecular hydrogen bonds, while 1-OH has four intramolecular hydrogen bonds. Therefore, intramolecular hydrogen bonds are relatively stronger in 1-OH than that in 2-OH.
BDE values for hydroxyl groups of ring A are lower than that of hydroxyl groups of ring B. This is due to the better delocalization of unpaired electrons in ring A. The number of intramolecular hydrogen bonds in 3’-OH and 4’-OH are less than that in 1-OH and 2-OH. So BDEs of 3’-OH and 4’-OH are higher due to the absence of delocalization and less number of intramolecular hydrogen bonds. In case of the hydroxyl groups in ring B, BDE of 3’-OH is greater than that of 4’-OH. That can be explained on the basis of number of intramolecular hydrogen bonds, as there are two intramolecular hydrogen bonds in 3’-OH radical, while three in 4’-OH radical.
Spin Density
The importance of antioxidants is due to their ability to stop the chain reaction, which can cause many diseases. During the free radical scavenging mechanism, the antioxidant itself becomes a free radical; but it does not start chain reactions due to its stability. Stability of the formed free radical plays a key in antioxidant activity of a compound. It is necessary to calculate the spin densities of free radicals formed from antioxidants after the abstraction of H atom. Spin densities can also provide the information about the speed of free radical scavenging reaction. More delocalized the spin density results in the easier radical formation and faster the speed of scavenging reaction. Spin density is directly related with BDE, as lower spin density results in the decrease of the BDE value.[Citation23] Spin densities can also provide the information about reactivity of various active sites in the molecule.
As shown in , spin densities of O-atom (formed after the abstraction of H atom) of various free radicals of chlorogenic acid are in the following trend: 3’-OH > 4’-OH > 2-OH > 1-OH. This trend indicates the strong relation between BDE and spin density. The fact is that, the more delocalized spin density; stabilize the free radical and results in the decrease of BDE value. The O-atom of 1-OH radical from which H atom is abstracted has lowest spin density due to the aromatic ring and stronger intramolecular hydrogen bonds. These factors decrease the electron density from oxygen atom and increase the stability of radical.
This extraordinary stability is responsible for lower BDE of 1-OH. The antioxidant activity of chlorogenic acid is mainly due to this 1-OH group. For the compounds, those have more than one hydroxyl groups, antioxidant activity is determined from one O-H bond, which has lowest BDE value. As shown in , we have compared the antioxidant potential of chlorogenic acid with some good phenolic acid antioxidants. This comparison was based on BDE values of phenolic acids. Moreover, the comparison shows that the chlorogenic acid has strong antioxidant potential.
FIGURE 4 Comparison of BDEs of some phenolic acids with chlorogenic acid.[Citation16,Citation18,Citation19]
![FIGURE 4 Comparison of BDEs of some phenolic acids with chlorogenic acid.[Citation16,Citation18,Citation19]](/cms/asset/422c3757-643b-4a51-85fb-76bf06a75597/ljfp_a_1042588_f0004_oc.jpg)
CONCLUSIONS
In conclusion, this work showed that the chlorogenic acid exhibited excellent antioxidant activities, mainly due to the hydroxyl groups of aromatic ring those possesses lower BDE values and greater stability of free radicals. On the basis of comparison, the BDE values of previously reported phenolic acids are in good agreement with our results. It is hoped that present study will be helpful for better utilization of chlorogenic acid in the food and pharmacy industries.
REFERENCES
- Garzon, A.; Bravo, I.; Barbero, A.J.; Albaladejo, J. Mechanistic and Kinetic Study on the Reactions of Coumaric Acids with Reactive Oxygen Species: A DFT Approach. Journal of Agricultural and Food Chemistry 2014, 62(40), 9705–9710.
- Cadenas, E.; Packer, L. Handbook of Antioxidants; Marcel Dekker: New York, 1996.
- Rice-Evans, C.A.; Diplock, A.T. Current Status of Antioxidant Therapy. Free Radical Biology and Medicine 1993, 15(1), 77–96.
- Kabanda, M.M.; Mammino, L.; Murulana, L.C.; Mwangi, H.M.; Mabusela, W.T. Antioxidant Radical Scavenging Properties of Phenolic Pent-4-En-1-Yne Derivatives Isolated From Hypoxis Rooperi. A DFT Study in Vacuo and in Solution. International Journal of Food Properties 2014, 18(1), 149–164.
- Esmaeili, A.; Mohabi, N. Experimental and Theoretical Determination of the Antioxidant Properties of Aromatic Monoterpenes of Thymol and 2,5,6-Trifluorothymol. International Journal of Food Properties 2014, 17(5), 1162–1168.
- Rice-Evans, C.; Packer, L. Flavonoids in Health and Disease; Marcel Dekker: New York, 1998.
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. Journal of Agricultural and Food Chemistry 2012, 60(46), 11625–11630.
- Clifford, M.N. The Analysis and Characterization of Chlorogenic Acids and Other Cinnamates. In Methods in Polyphenol Analysis; Santos-Buelga, C., Williamson, G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2003; 314–337.
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys Edulis). Journal of Agricultural and Food Chemistry 2001, 49(10), 4646–4655.
- Crisosto, G.W.C.a.C.H. Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue. Journal of the American Society for Horticultural Science 1995, 120(5), 135–138.
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical Composition and Potential Health Effects of Prunes: A Functional Food. Critical Reviews in Food Science and Nutrition 2001, 41(4), 251–286.
- Jalal, M.A.F.; Read, D.J.; Haslam, E. Phenolic Composition and Its Seasonal Variation in Calluna Vulgaris. Phytochemistry 1982, 21(6), 1397–1401.
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence, and Dietary Burden. Journal of the Science of Food and Agriculture 1999, 79(3), 362–372.
- Mahmood, A.; Saqib, M.; Ali, M.; Abdullah, M.I.; Khalid, B. Theoretical Investigation for the Designing of Novel Antioxidants. Canadian Journal of Chemistry 2013, 91(2), 126–130.
- Saqib, M.; Iqbal, S.; Naeem, S.; Mahmood, A. DFT for Exploring the Antioxidant Potential of Homogentisic and Orsellinic Acids. Pakistan Journal of Pharmaceutical Sciences 2013, 26(6), 1209–1214.
- Mohajeri, A.; Asemani, S.S. Theoretical Investigation on Antioxidant Activity of Vitamins and Phenolic Acids for Designing a Novel Antioxidant. Journal of Molecular Structure 2009, 930(1–3), 15–20.
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS One 2015, 10(3), e0121276.
- Cao, H.; Cheng, W.-X.; Li, C.; Pan, X.-L.; Xie, X.-G.; Li, T.-H. DFT Study on the Antioxidant Activity of Rosmarinic Acid. Journal of Molecular Structure: THEOCHEM 2005, 719(1–3), 177–183.
- Zhang, J.; Xiong, Y.; Peng, B.; Gao, H.; Zhou, Z. Density Functional Study on the Bioactivity of Ellagic Acid, Its Derivatives and Metabolite. Computational and Theoretical Chemistry 2011, 963(1), 148–153.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M. A.; Cheeseman, J.; … Fox, D.J. Gaussian 09; Gaussian Inc.: Wallingford, CT, 2009.
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Physical Review B 1988, 37(2), 785–789.
- Zhang, H.-Y.; Sun, Y.-M.; Wang, X.-L. Substituent Effects on O-H Bond Dissociation Enthalpies and Ionization Potentials of Catechols: A DFT Study and Its Implications in the Rational Design of Phenolic Antioxidants and Elucidation of Structure–Activity Relationships for Flavonoid Antioxidants. Chemistry—A European Journal 2003, 9(2), 502–508.
- Parkinson, J.C.; Mayer, M.P.; Radom, L. An Assessment of Theoretical Procedures for the Calculation of Reliable Radical Stabilization Energies [Dagger][Double Dagger]. Journal of the Chemical Society, Perkin Transactions 2 1999, 11, 2305–2313.