Abstract
Conventionally, organically, and locally grown varieties of carrot, tomato, rhubarb, raspberry, strawberry, and bell peppers were analyzed by differential pulse voltammetry to determine the impact of cultivation conditions on produce antioxidant content. The potential (indicating antioxidant activity) and area (indicating antioxidant capacity) of the primary peaks were evaluated. Overall, no consistent trends were observed across cultivation conditions, suggesting that cultivation conditions have minimal impact on antioxidant content. This study revealed differential pulse voltammetry to be a sensitive and rapid method for antioxidant determination, with a detection and quantitation limit of 8.38 × 10−5 and 2.79 × 10−4 mol/L, respectively.
INTRODUCTION
Oxygen is vital to aerobic life. However, despite its necessity, oxygen is also deleterious to mammalian bodies, including humans. This is because, during the biological synthesis of adenosine triphosphate (ATP), free radicals termed reactive oxygen species (ROS) are produced as by-products.[Citation1–Citation3] In the cell, ROS induce the radical oxidation of proteins, lipids, and nucleic acids, causing cellular damage and degeneration, which in turn, leads to a condition known as oxidative stress.[Citation2,Citation4–Citation7] Oxidative stress has been linked to several diseases and disorders, including Alzheimer’s disease, cirrhosis, sickle cell disease, rheumatoid arthritis, Parkinson’s disease, diabetes mellitus, cardiovascular disease, arteriosclerosis, and even cancer.[Citation2,Citation5,Citation8–Citation16] Oxidative stress is also thought to be a principal contributor to the degenerative processes of aging.[Citation10,Citation11]
Antioxidants, such as vitamin C (ascorbic acid), vitamin E, flavonoids, and polyphenols, are compounds which either prevent or mitigate oxidative stress by donating electrons to ROS, thereby neutralizing the ability of ROS to act as free radicals. Consequently, increased consumption of foods high in antioxidants has been associated with many health benefits.[Citation17–Citation20] One of the principal results of this association had been increased concern among the general public with diet antioxidant content, and many food manufacturers have begun to address this concern by producing foods purported to be higher in antioxidants than traditional varieties.[Citation21–Citation23] A key example of this is the rapid growth of the organic foods industry.[Citation21,Citation23]
Organically grown foods (foods grown without the use of pesticides, herbicides, genetic modification, or sewage fertilization) are purported to be healthier than conventionally grown foods due not only to the lack of chemical treatment, but also because organically grown foods are said to possess a higher content of nutrients, including antioxidants.[Citation21,Citation25] However, the reality of the health benefits afforded by organically grown foods remains a matter of constant debate, with many studies presenting contradictory information.[Citation23,Citation24] For instance, in comparing the total phenolic content of strawberries, Asami et al.[Citation26] found organically grown strawberries to consistently possess higher phenolic levels than the conventionally grown variety, while Hakkinen and Torronen[Citation27] found organic cultivation conditions to have minimal impact on the phenolic content of strawberries. The evaluation of organically grown produce (OGP) is further complicated by the fact that most studies restrict their analyses to small sample sizes of similar produce types (e.g., berries).[Citation22]
An additional factor complicating the comparative evaluation of cultivation conditions is the variability of traditional antioxidant assays. The majority of antioxidant evaluative techniques rely upon spectrophotometric chemical assays that indirectly measure antioxidant content based on an observed color change. These assays, however, are generally non-specific, time-consuming and costly, and can be influenced by the color of the sample being analyzed.[Citation28] Furthermore, the results from different assays are often not comparable.[Citation28,Citation29] In contrast, electrochemical techniques are insensitive to differences in color and have been documented to be simple, rapid, and cost-effective alternatives to traditional chemical assays.[Citation30–Citation32] Therefore, this study aimed to determine the impact of cultivation conditions on the antioxidant capacity (thermodynamic efficiency of an antioxidant) and activity (reaction kinetics of an antioxidant) of fruits and vegetables using an electrochemical technique known as differential pulse voltammetry (DPV).
In this study, eight fruits and vegetables were analyzed in their conventionally, organically, and locally grown varieties. For the purposes of this article, conventionally grown produce (CGP) refers to produce typically purchased from mainstream groceries; OGP refers to produce certified by the United States Department of Agriculture (USDA) as having been grown without the use of “irradiation, sewage sludge, synthetic fertilizers, prohibited pesticides, [or] genetically modified organisms;”[Citation25] and locally grown produce (LGP) refers to produce grown by non-commercial farms/greenhouses/gardens local to the region of central Alberta, Canada. Furthermore, as opposed to past studies which have focused on one particular category of produce, produce samples were chosen to represent a diverse range of botanical classifications, including vegetable fruit (tomato), root (carrot), and petioles (rhubarb), as well as aggregate (raspberry), accessory (strawberry), and berry (red, green, and yellow bell pepper) fruits.
MATERIALS AND METHODS
Materials
Monosodium phosphate, disodium phosphate, anhydrous ethanol, o-phosphoric acid, and gallic acid (GA) were obtained from Fisher Scientific, and potassium chloride from J.T. Baker. All chemicals were of reagent grade purity. Produce types analyzed included: carrot, tomato, rhubarb, raspberry, strawberry, red bell pepper, yellow bell pepper, and green bell pepper. For all produce types, three varieties were analyzed: CGP, OGP, and LGP. To prevent overlap of these definitions, LGP was obtained from non-commercial sources which were not USDA certified (i.e., the LGP samples were not certified as organic). Furthermore, since cultivar type can affect antioxidant content,[Citation33–Citation35] where possible the CGP and OGP were of the same brand. For example, CGP and OGP raspberries were both produced by Driscolls. CGP and OGP samples were purchased from mainstream groceries, while LGP was obtained directly from farms, gardens, and greenhouses located in central Alberta, Canada. For rhubarb, only the OGP and LGP varieties were analyzed, since CGP could not be obtained.
Extraction
Prior to extraction, surface contaminants were removed by rinsing produce samples well with tap water. For all samples, only the traditionally consumed portion was analyzed. All samples were extracted under organic conditions. For extraction, 25 g of finely chopped sample was weighed into a 250-mL Erlenmeyer flask and diluted with 60 mL methanol and 10 mL 0.3 mol/L HCl. The flask was then sparged with nitrogen gas for 2 min and sealed. Samples were stirred for 24 h in the absence of light, before being suction filtered through Whatman No. 4. filter paper. Methanol in the filtrate was evaporated under reduced pressure, and the resultant residue diluted with 50 mL of 0.1 mol/L pH 2.0 phosphate buffer. Phosphate buffer was prepared from 0.04 mol/L disodium phosphate and 0.06 mol/L monosodium phosphate, with 0.1 mol/L KCl as supporting electrolyte and 6 mol/L o-phosphoric acid used to bring the solution to pH 2.0.
Instrumentation
DPV was performed using a Pine Instrument Company bipotentiostat model AFCBP1 and AfterMathTM software. Analyses were carried out in a three-electrode cell using a glassy-carbon working electrode, a Pt counter electrode and an Ag|AgCl reference electrode. Prior to each run, the working electrode was polished on a Buehler Microcloth® pad with successive aqueous slurries of 1.0, 0.3, and 0.05 micron Buehler Micropolish II® alumina powder. Following each polish, the electrode was sonicated in distilled water for 5 min. After the 0.05 micron polish, the electrode was sonicated again in distilled water for 5 min, followed by sonication in anhydrous ethanol for 5 min, after which the electrode was rinsed with distilled water and immediately used for analysis. Prior to each run, the Pt counter electrode was also polished on a wetted Buehler Microcloth® pad and rinsed with distilled water. DPV parameters: initial potential: 200 mV; final potential: 900 mV; height: 50 mV; width: 100 ms; period: 200 ms; increment: 1 mV. For each sample analyzed, the potential and area (from 200–900 mV) of the primary peak was measured.
Sampling Plan
Three samples of each produce type (e.g., three boxes of CGP strawberries, three LGP red bell peppers) were randomly chosen and subjected to organic extraction (). Extracts were sparged with nitrogen gas for 2 min, before being immediately analyzed by DPV. Each extract was analyzed twice, with the average of the two runs serving as the data point for that sample.
GA Calibration Curve
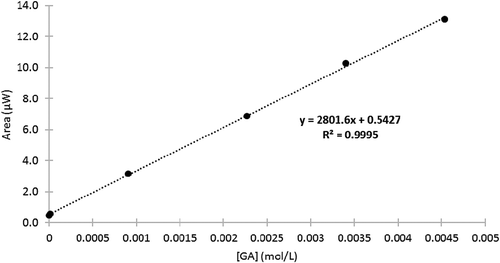
To enable the produce data to be expressed in GA equivalents, a calibration curve of peak area as a function of GA concentration was prepared ([GA] = 4.5 × 10−3 to 1.1 × 10−6 mol/L). Standards were prepared with the same solvent as was used to prepare the fruit and vegetable extracts (0.1 mol/L, pH 2.0 phosphate buffer with 0.1 mol/L KCl as supporting electrolyte). In addition, the curve was used for validation of the utilized method, including the determination of the method accuracy and precision, and the calculation of the limit of detection (LOD) and limit of quantitation (LOQ):
where s is the standard deviation of the intercept (n = 3) and m is the slope.[36]
Data Analysis
All results are given as the mean ± standard deviation. Data analysis was performed using After MathTM software and Microsoft® Excel 2013. Statistical analysis across cultivation method (i.e., OGP versus CGP, OGP versus LGP, CGP versus LGP) was carried out using two-tailed unequal variance Student’s t-tests, with results deemed significant at p < 0.05. Results were expressed in GA equivalents using the prepared calibration curve ():
FIGURE 2 Calibration curve expressing voltammogram peak area in microwatts (µW) as a function of gallic acid (GA) concentration; n = 6, (GA) = 4.5 × 10−3 to 1.1 × 10−6 mol/L.
where y is the area of the primary peak (in microwatts, µW) and x is the concentration of GA (mol/L).
RESULTS AND DISCUSSION
Overview
Representative voltammograms of analyzed produce samples can be seen in . All samples demonstrated an intense peak in the 310 to 510 mV region (). At pH 2.0, many antioxidant compounds exhibit an oxidation peak within this region, including but not limited to: protocatechuic acid (590 mV; +)-catechin (530 mV), anthocyanins (440–590 mV), quercetin (440 mV), rutin (510 mV), and GA (428 mV).Footnote‡[Citation9,Citation13,Citation37–Citation41] The potential of the principal peak of each sample is determined by the sample’s relative content of low and high oxidation potential antioxidants.[Citation16]
FIGURE 3 Representative differential pulse voltammograms for conventionally (solid line), locally (dotted line), and organically (dashed line) grown produce samples, showing current (µA, y-axis) as a function of applied voltage (mV, x-axis).
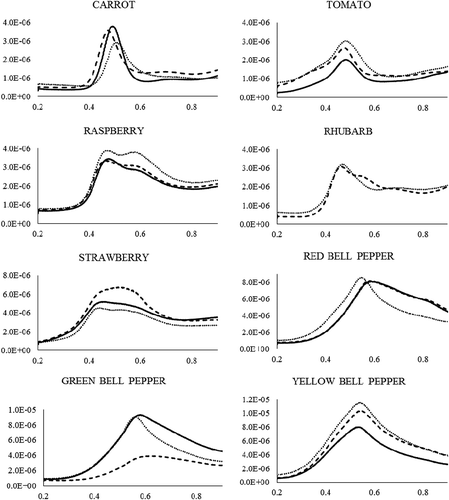
TABLE 1 Potential (Ep) and area (A, measured in nanowatts) of principal peak in differential pulse voltammograms of analyzed fruits and vegetables. Values are shown as the mean ± standard deviation (n = 3)
While it should be noted that secondary peaks were observed above 510 mV for some samples, these peaks were much less intense than the primary peak. These secondary peaks may indicate the presence of small amounts of higher oxidation potential antioxidants, such as vanillic acid (900 mV) or coumaric acid (850–950 mV).[Citation16,Citation39]
Antioxidant Activity
Antioxidant activity is a kinetic quantification measuring the reaction rate of an antioxidant with its target oxidant.[Citation8,Citation28,Citation31,Citation32] In DPV, the antioxidant activity of a particular sample is represented by the potential of the sample’s primary peak, with a lower potential corresponding to a higher activity.[Citation12] Differences in the relative amounts of low and high potential antioxidants in the produce samples determine the location of the primary peak. For instance, a sample containing a large amount of high activity antioxidants will demonstrate a low peak potential. Analysis of peak potential thus enables the meaningful comparison of the antioxidant activity of produce samples across cultivation conditions.
Among the samples analyzed, statistically significant differences were observed for only two produce types: carrot and raspberry. For carrots, the OGP variety demonstrated a significantly higher antioxidant activity than either CGP (p = 0.003) or LGP (p = 0.001), while CGP demonstrated a significantly higher activity than LGP (p = 0.017). For raspberry, OGP demonstrated a significantly higher activity than LGP (p = 0.024); however, no significant differences were observed between LGP and CGP, or between CGP and OGP. This lack of a steady trend among samples is consistent with several studies in the literature that have shown no consistent significant differences in nutritional value for OGP compared to CGP.[Citation27,Citation42–Citation44] However, while past studies restricted analysis solely to CGP and OGP, this work extended the analysis to a third set of cultivation conditions (LGP). Despite this, no consistent differences were observed, further suggesting that cultivation conditions have little impact on the antioxidant activity of produce. As well, since the specific genotype of produce can affect its antioxidant properties, it should be noted that the significant differences observed between the CGP and LGP varieties may be the result of variances in cultivar types as well as in cultivation conditions,[Citation33,Citation34] since it cannot be guaranteed that the LGP was of the exact same cultivar as the GCP or OGP.
Antioxidant Capacity
Antioxidant capacity, a thermodynamic measurement of an antioxidant’s efficiency, is indicative of antioxidant concentration.[Citation8,Citation28] Electrochemically, antioxidant capacity can be determined from the area of a voltammogram, with a greater area signifying a greater capacity.[Citation41,Citation45] Significant differences across cultivation conditions were observed for yellow bell pepper, strawberry, and rhubarb only. For yellow bell peppers, LGP proved to possess a greater capacity than either CPG (p = 0.019) or OGP (p = 0.041), with OGP surpassing CGP (p = 0.045). For strawberries, OGP possessed a significantly higher capacity than CGP (p = 0.002) or LGP (p = 0.039), while for raspberries, LGP had a higher capacity than CGP (p = 0.043). As with the determination of antioxidant activity, this lack of a consistent trend indicates that cultivation conditions have minimal impact on the antioxidant content of produce, an observation consistent with other previous comparisons between organic and CGP.[Citation27,Citation44,Citation46] Again, it should be noted that the significant differences observed between varieties may also have resulted from differences in cultivar type, particularly those differences observed between the LGP and the OGP or CGP varieties. In addition, OGP and CGP were often purchased from the same producer and thus, were likely to be of the same cultivar, but the specific genotype of LGP cannot be guaranteed.
Quantitative Determination of Antioxidant Content
Though traditional chemical and spectrophotometric assays commonly rely upon standards such as Trolox, in electrochemical studies the linear relationship between peak area and antioxidant concentration enables the use of stable antioxidants, such as GA, to be used as standards.[Citation45,Citation47–Citation49] Thus, using the prepared GA calibration curve (), the peak areas of the analyzed samples could be used to express the total antioxidant concentration as mg GA per 100 g of sample ().
TABLE 2 Antioxidant content of samples in gallic acid equivalents, based on gallic acid calibration curve. Values are shown as the mean ± standard deviation (n = 3)
For all three cultivation methods, the general ranking for antioxidant content was as follows: carrot < tomato < rhubarb < raspberry < strawberry < bell peppers. This is consistent with the values found in the literature (carrot < tomato < rhubarb < strawberry ≈ raspberry), though reference values could not be found for North American bell peppers.[Citation50] Overall, red and yellow bell peppers demonstrated the highest antioxidant content (33.6–52.9 mg/100 g), and tomatoes and carrots the lowest (0.9–5.6 mg/100 g). The highest antioxidant content was observed in LGP yellow bell peppers (52.9 mg/100 g), while the lowest was seen in CGP carrots (0.9 mg/100 g). For all of the samples analyzed, the highest antioxidant content was consistently observed in either the OGP or LGP variety; CGP did not ever demonstrate the highest antioxidant content among any of the produce types analyzed.
Method Validation
The GA calibration curve was also used to validate the utilized method, using a procedure adapted from Yilmaz et al. ().[36] A linear correlation between concentration and the area of the primary peak was observed over the entire concentration range (4.5 × 10−3 mol/L to 1.1 × 10−6 mol/L). The method demonstrated acceptable sensitivity, as indicated by the percent relative standard deviation (% RSD) of the slope (1.11%), and repeatability, as indicated by the % RSD of the potential (0.43%), height (1.64%), and area (1.73%) of the primary peak (determined from three independent analyses of a 2.27 × 10−3 mol/L GA standard). The LOD and LOQ were also within an acceptable range, found to be 8.38 × 10−5 mol/L and 2.79 × 10−4 mol/L, respectively. These values are comparable to those found in the literature for similar studies.[Citation51]
TABLE 3 Validation parameters of DPV method. Parameters derived from calibration curve of gallic acid in 0.1 mol/L phosphate buffer at pH 2.0 at a glassy carbon electrode. Repeatability of peak potential, height, and area are based on three independent scans of a 2.27 × 10−3 mol/L solution
CONCLUSIONS
Purported to be higher in nutrient content than conventional produce, organically grown foods have experienced a dramatic increase in popularity in recent years. However, the extent of the effects of cultivation practices on nutrients remains a matter of significant debate. This study used DPV to determine the effect of cultivation conditions on the activity and capacity of antioxidants in a variety of fruits and vegetables. No consistent trends across cultivation conditions were observed in terms of either antioxidant activity or capacity, and cultivation conditions were thus found to have little effect on antioxidant content of fruit and vegetable samples. However, it should be noted that organic farming is still a more environmentally friendly practice compared to conventional farming because no pesticides, fertilizers, and other pollution-causing chemicals are used. In summary, the results obtained from this study showed that DPV is a highly sensitive and precise technique for antioxidant determination in fruit samples.
ACKNOWLEDGMENTS
The authors would like to thank Mr. David King for his technical assistance and Mrs. Linda Ervin for her editorial aid. As well, we thank Paula Marentette and Alice Tymchatyn for their donation of LGP.
FUNDING
This work was carried out with financial support from the Summer Student Research Assistantship administered by the Augustana Campus of the University of Alberta.
Additional information
Funding
Notes
‡ When comparing experimental oxidation peaks to that of the literature, it should be noted that a one unit increase in pH leads to a negative shift of approximately 35 mV, while a one unit decrease in pH leads to a positive shift of 35 mV;[Citation41] the listed potential values were adjusted to the relevant pH using this rule.
REFERENCES
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress, and the Biology of Ageing. Nature 2000, 408 ( November), 239–247.
- Prior, R.L.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Americal Journal of Biomedical Sciences 2013, 5 (2), 126–139.
- Bast, A.; Haenen, G.R.M.M. Ten Misconceptions About Antioxidants. Trends in Pharmacological Sciences 2013, 34 (8), 430–436.
- Villanueva, C.; Kross, R.D. Antioxidant-Induced Stress. International Journal of Molecular Sciences 2012, 13 (2), 2091–2109.
- Pohanka, M.; Hynek, D.; Kracmarova, A.; Kruseova, J.; Ruttkay-Nedecky, B.; Sochor, J.; Adam, V.; Hubalek, J.; Masarik, M.; Eckschlager, T.; Kizek, R. Voltammetry Assay for Assessment of Oxidative Stress Linked Pathologies in Brain Tumor Suffered Childhood Patients. International Journal of Electrochemical Science 2012, 7, 11978–11992.
- Pekal, A.; Drozdz, P.; Pyrzynska, K. Comparison of the Antioxidant Properties of Commonly Consumed Commercial Teas. International Journal of Food Properties 2012, 15, 1101–1109.
- Peksel, A.; Imamoglu, S.; Altas Kiymaz, N.; Orhan, N. Antioxidant and Radical Scavenging Activities of Asphodelus Aestivus Brot. Extracts. International Journal of Food Properties 2013, 16 (February), 1339–1350.
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the Determination of Biological Antioxidant Capacity in Vitro: A Review. Journal of the Science of Food and Agriculture 2006, 86 (August), 2046–2056.
- Barros, L.; Falcão, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant Activity of Agaricus sp. Mushrooms by Chemical, Biochemical, and Electrochemical Assays. Food Chemistry 2008, 111 (1), 61–66.
- Ghiaba, Z.; Yousfi, M.; Hadjadj, M.; Saidi, M.; Dakmouche, M. Study of Antioxidant Properties of Five Algerian Date (Phoenix Dactylifera L.) Cultivars by Cyclic Voltammetric Technique. International Journal of Eectrochemical Science 2014, 9, 909–920.
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochemistry and Analytical Biochemistry 2012, 1 (1), 1–10.
- Blasco, A.J.; González, M.C.; Escarpa, A. Electrochemical Approach for Discriminating and Measuring Predominant Flavonoids and Phenolic Acids Using Differential Pulse Voltammetry: Towards An Electrochemical Index of Natural Antioxidants. Analytica Chimica Acta 2004, 511 (1), 71–81.
- Esch, J.R.; Friend, J.R.; Kariuki, J.K. Determination of the Vitamin C Content of Conventionally and Organically Grown Fruits by Cyclic Voltammetry. International Journal of Electrochemical Science 2010, 5, 1464–1474.
- Remanan, M.K.; Wu, J. Antioxidant Activity in Cooked and Simulated Digested Eggs. Food and Function 2014, 5 (7), 1464–1474.
- Dey, A.; Lakshmanan, J. The Role of Antioxidants and Other Agents in Alleviating Hyperglycemia Mediated Oxidative Stress and Injury in Liver. Food and Function 2013, 4 (8), 1148–1184.
- Ervin, E.M.; Kariuki, J.K. Effect of Extraction Method on Antioxidant Determination in Produce by Differential Pulse Voltammetry. International Journal of Electrochemical Science 2014, 9, 6235–6245.
- Bui, H.-T.; Makhlouf, J.; Ratti, C. Postharvest Ripening Characterization of Greenhouse Tomatoes. International Journal of Food Properties 2010, 13, 830–846.
- Gujral, H.S.; Angurala, M.; Sharma, P.; Singh, J. Phenolic Content and Antioxidant Activity of Germinated and Cooked Pulses. International Journal of Food Properties 2011, 14, 1366–1374.
- Sant’ana, L.D.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. International Journal of Food Properties 2014, 17 (1), 65–76.
- Taylor, P.; Snoussi, A. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (beta vulgaris L. Conditiva) Roots and Stems Extracts. International Journal of Food Properties 2014, 17 (9), 37–41.
- Aarset, B.; Beckmann, S.; Bigne, E.; Beveridge, M.; Bjorndal, T.; Bunting, J.; McDonagh, P.; Mariojouls, C.; Muir, J.; Prothero, A.; Reisch, L.; Smith, A.; Tveteras, R.; Young, J. The European Consumers’ Understanding and Perceptions of the “Organic” Food Regime: The Case of Aquaculture. British Food Journal 2004, 106 (2), 93–105.
- Woese, K.; Lange, D.; Boess, C.; Bo, K.W. A Comparison of Organically and Conventionally Grown Foods—Results of a Review of the Relevant Literature. Journal of the Science of Food and Agriculture 1997, 281 (3), 281–293.
- Bourn, D.; Prescott, J. A Comparison of the Nutritional Value, Sensory Qualities, and Food Safety of Organically and Conventionally Produced Foods. Critical Reviews in Food Science and Nutrition 2002, 42 (1), 1–34.
- Brandt, K.; Molgaard, J.P. Organic Agriculture: Does It Enhance or Reduce the Nutritional Value of Plant Foods? Journal of the Science of Food and Agriculture 2001, 81 (9), 924–931.
- United States Department of Agriculture National Organic Program. http://www.ams.usda.gov/AMSv1.0/NOPOrganicStandard (accessed January 14, 2015).
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the Total Phenolic and Ascorbic Acid Content of Freeze-Dried and Air-Dried Marionberry, Strawberry, and Corn Grown Using Conventional, Organic, and Sustainable Agricultural Practices. Journal of Agricultural and Food Chemistry 2003, 51 (5), 1237–1241.
- Hakkinen, S.H.; Torronen, A.R. Content of Flavonols and Selected Phenolic Acids in Strawberries and Vaccinium Species: Influence of Cultivar, Cultivation Site, and Technique. Food Research International 2000, 33, 517–524.
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of Measurement and Evaluation of Natural Antioxidant Capacity/Activity (IUPAC Technical Report). Pure and Applied Chemistry 2013, 85 (5), 957–998.
- Mitić, S.S.; Paunović, D.Đ.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.B.; Mitić, M.N. Phenolic Profiles and Total Antioxidant Capacity of Marketed Beers in Serbia. International Journal of Food Properties 2014, 17 (4), 908–922.
- Escarpa, A. Food Electroanalysis: Sense and Simplicity. Chemical Record (New York, N.Y.) 2012, 12 (1), 72–91.
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; Kizek, R. Electrochemistry As a Tool for Studying Antioxidant Properties. International Journal of Electrochemical Science 2013, 8, 8464–8489.
- Plaza, M.; Kariuki, J.; Turner, C. Quantification of Individual Phenolic Compounds’ Contribution to Antioxidant Capacity in Apple: A Novel Analytical Tool Based on Liquid Chromatography with Diode Array, Electrochemical, and Charged Aerosol Detection. Journal of Agricultural and Food Chemistry 2014, 62, 409–418.
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21 (2), 207–213.
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic Compounds, Lycopene, and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum esculentum). Journal of the Science of Food and Agriculture 2002, 82 (3), 323–330.
- Li, L.; Wang, S.; Chen, J.; Xie, J.; Wu, H.; Zhan, R.; Li, W. Major Antioxidants and in Vitro Antioxidant Capacity of Eleven Mango (Mangifera Indica L.) Cultivars. International Journal of Food Properties 2014, 17 (8), 1872–1887.
- Yilmaz, S.; Sadikoglu, M.; Saglikoglu, G.; Yagmur, S.; Askin, G. Determination of Ascorbic Acid in Tablet Dosage Forms and Some Fruit Juices by DPV. International Journal of Electrochemical Science 2008, 3, 1534–1542.
- Brett, A.M.O.; Ghica, M.E. Electrochemical Oxidation of Quercetin. Electroanalysis 2003, 15 (22), 1745–1750.
- Janeiro, P.; Brett, A.M.O. Redox Behavior of Anthocyanins Present in Vitis vinifera L. Electroanalysis 2007, 19 (17), 1779–1786.
- Simić, A.; Manojlović, D.; Segan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules (Basel, Switzerland) 2007, 12 (10), 2327–2340.
- Medvidovi, M.; Šeruga, M.; Jakobek, L.; Novak, I. Electrochemical and Antioxidant Properties of (+)-Catechin, Quercetin and Rutin. Croatica Chemica Acta 2010, 83 (2), 197–207.
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical Determination of Antioxidant Capacity of Fruit Tea Infusions. Food Chemistry 2010, 121 (3), 820–825.
- Durazzo, A.; Azzini, E.; Lazzé, M.C.; Raguzzini, A.; Pizzala, R.; Maiani, G.; Palomba, L.; Maiani, G. Antioxidants in Italian Head Lettuce (Lactuca sativa var. capitata L.) Grown in Organic and Conventional Systems Under Greenhouse Conditions. Journal of Food Biochemistry 2014, 38 (1), 56–61.
- Ismail, A.; Fun, C.S. Green Vegetables Organically and Conventionally Grown. Malaysian Journal of Nutrition 2003, 9 (1), 31–39.
- Lima, G.P.P.; Teixeira da Silva, J.A.; Bernhard, A.B.; Pirozzi, D.C.Z.; Fleuri, L.F.; Vianello, F. Organic and Conventional Fertilisation Procedures on the Nitrate, Antioxidants and Pesticide Content in Parts of Vegetables. Food Additives and Contaminants, Part B 2012, 5 (3), 188–193.
- Chevion, S.; Roberts, M.A.; Chevion, M. The Use of Cyclic Voltammetry for the Evaluation of Anioxidant Capacity. Free Radical Biology and Medicine 2000, 28 (6), 860–870.
- Stracke, B.A.; Rüfer, C.E.; Weibel, F.P.; Bub, A.; Watzl, B. Three-Year Comparison of the Polyphenol Contents and Antioxidant Capacities in Organically and Conventionally Produced Apples (Malus Domestica Bork. Cultivar “Golden Delicious”). Journal of Agricultural and Food Chemistry 2009, 57 (11), 4598–4605.
- Rebelo, M.J.; Rego, R.; Ferreira, M.; Oliveira, M.C. Comparative Study of the Antioxidant Capacity and Polyphenol Content of Douro Wines by Chemical and Electrochemical Methods. Food Chemistry 2013, 141 (1), 566–573.
- Ziyatdinova, G.K.; Salikhova, I.R.; Budnikov, H.C. Evaluation of the Antioxidant Capacity of Cognacs and Brandies by Differential Pulse Voltammetry. Journal of Analytical Chemistry 2014, 69 (12), 1165–1170.
- Ziyatdinova, G.K.; Budnikov, H.C. Evaluation of the Antioxidant Properties of Spices by Cyclic Voltammetry. Journal of Analytical Chemistry 2014, 69 (10), 990–997.
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; Barikmo, I.; Berhe, N.; Willett, W. C.; Phillips, K.M.; Jacobs, D.R.; Blomhoff, R. The Total Antioxidant Content of More Than 3100 Foods, Beverages, Spices, Herbs, and Supplements Used Worldwide. Nutrition Journal 2010, 9, 3.
- Pisoschi, A.M.; Pop, A.; Negulescu, G.P.; Pisoschi, A. Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes. Molecules 2011, 16 (2), 1349–1365.