Abstract
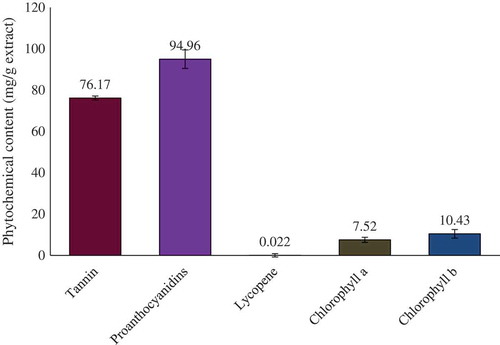
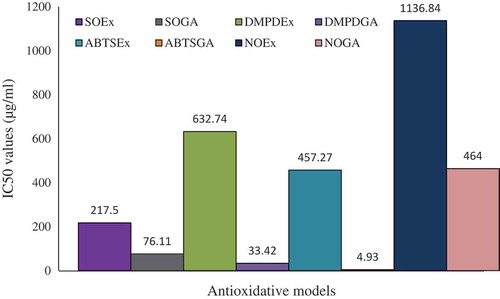
This study investigated the quantitative phytochemical contents, total phenolics, total flavonoids, total carotenoids, antioxidative capacity, tannin, proanthocyanidins, lycopene, chlorophyll a, and chlorophyll b of the Stephania japonica extract. Comprehensive antioxidative effects of the extract were also investigated. Quantitative assays were conducted through both spectrophotometric and high-performance liquid chromatographic methods. Antioxidative effects were measured through FeCl3 reducing power, metal chelating power, reducing power, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) scavenging activity, N, N-dimethyl-1,4-diaminobenzene free radical scavenging activity, superoxide radical scavenging activity, and nitric oxide scavenging effect. The contents of total phenolics, total flavonoids, total carotenoids, total antioxidative capacity, tannin, proanthocyanidins, lycopene, chlorophyll a, and chlorophyll b were found to be 47.32 ± 0.75 mg tannic acid equivalent, 61.41 ± 1.58 mg catechin equivalent, 63.29 ± 2.21 mg, 22.85 ± 0.70 mg ascorbic acid equivalent, 76.17 ± 0.97 mg tannic acid equivalent, 94.96 ± 4.49 mg catechin equivalent, 22.19 ± 0.79 µg, 22.19 ± 0.79 µg, 7.52 ± 1.24 mg, and 10.43 ± 2.11 mg, respectively, in 1 g of ethanol extract. A high concentration of epicatechin, p-coumaric acid, and rutin hydrate and moderate concentration of caffeic acid and quercetin was detected in the extract. The IC50 value for ferric reducing power assay, metal chelating assay, reducing power assay, ABTS scavenging assay, N, N-dimethyl-1,4-diaminobenzene scavenging assay, superoxide scavenging assay and nitric oxide (NO) assay were 465.06 ± 7.32 µmol ascorbic acid/g, 1656.52, 270.55, 457.27, 632.74, 217.5, and 464.00 µg/mL, respectively. No beta carotene was detected in the extract. The extract was demonstrated to be a very potential source of antioxidative metabolites.
INTRODUCTION
Physiological and biochemical processes in living cells may generate free radicals and other reactive oxygen species by-products. These free radicals cause oxidative damage to DNA, proteins and lipids, eventually leading to many chronic diseases, such as diabetes, atherosclerosis, coronary heart disease, cancer, aging, and other degenerative diseases, in humans.[Citation1] Plants are enriched with free radical scavenging molecules, such as alkaloids, amines, betalains, coumarins, vitamins, terpenoids, phenolic acids, lignins, stilbenes, tannins, flavonoids, quinones and other metabolites, which are endowed with antioxidant activity.[Citation2,Citation3] Researchers have shown that many of these antioxidant compounds possess antiatherosclerotic, antitumor, anticarcinogenic, inflammatory, antimutagenic, antibacterial, and antiviral activities.[Citation4,Citation5] Antioxidants act as a cooperative network employing a series of different redox reactions. The ingestion of natural antioxidants has been associated with reduced risks of cancer, cardiovascular disease, diabetes, and other diseases associated with ageing, and in recent years, there has been a worldwide trend toward the use of the natural phytochemical present in different fruits, leafy plants, vegetables, seeds, and crops.[Citation6–Citation10]
Stephania japonica belonging to Menisperaceae family consists of 70 genera and approximately 400 species with a great deal of pharmaceutically important compounds. Traditionally, this plant is used in facial paralysis, abortifacient, reproductive effects, cuts and wounds, and stomachache.[Citation11–Citation16] Various alkaloids, such as tertiary phenolic biscoclaurine type alkaloid stepholine, hasubanonine were isolated from roots of S. japonica.[Citation17,Citation18] The root extract has shown multidrug resistance modulator effect.[Citation19] Despite a noticeable study on this plant, comprehensive and quantitative analysis of phytochemical metabolites are not documented at all. We investigated the phytochemical contents, especially the total phenolics, flavonoids, proanthocyanidins (PCO), total carotenoids, lycopene, β-carotene, chlorophyll a, and chlorophyll b by spectrophotometric analysis and caffeic acid (CA), epicatechin, catechin, rutin hydrate (RH), and querecetine (QU) by high-performance liquid chromatography (HPLC) analysis in whole plant extract of S. japonica. Along with other known antioxidative metabolites chlorophyll a, chlorophyll b, and their derivatives are also considered as electron donors as evidenced by their ability to reduce free radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical.[Citation20] Diseases related the traditional uses of this plant are also evidenced to oxidative stress. A number of existing data reveals that in many of the infertility cases, the cause of sterility is the disabled sperm as a result of the oxidative stress.[Citation21] Oxidative stress is strongly involved in the pathogenesis of non-healing wounds.[Citation22] Oxidative stress-induced damage has also been hypothesized to play a role in spontaneous abortion, idiopathic recurrent pregnancy loss, defective embryogenesis, and drug-induced teratogenicity.[Citation23] In response to pathogens, the stomach induces oxidative stress, which is related to the development of gastric organic disorders such as gastritis, gastric ulcers, and gastric cancer, as well as functional dyspepsia.[Citation24] Therefore, the in vitro-antioxidative effects of the extract in ferric reducing power (FRAP) assay, metal chelating assay and reducing power (RP) assay, superoxide assay, N,N-dimethyl-1,4-diaminobenzene (DMPD) assay, ABTS assay and NO assay models were investigated.
MATERIALS AND METHODS
Chemicals and Reagents
Folin-Ciocalteau reagent, DPPH, ferric chloride, ascorbic acid, tannic acid, and catechin were purchased from Sigma Co. St. Louis, Missouri, USA. Methanol, hydrochloric acid, sodium hydroxide, aluminium chloride, sodium carbonate, sodium hydroxide, and potassium ferricyanide were purchased from Merck, Darmstadt, Germany. All the chemicals and reagents were of analytical grade. Gallic acid (GA), (+)-catechin hydrate (CH), vanillic acid (VA), CA, (−)-epicatechin (EC), p-coumaric acid (PCA), RH, ellagic acid (EA), and QU were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile (HPLC), methanol (HPLC), acetic acid (HPLC), and ethanol was obtained from Merck (Darmstadt, Germany).
Collection of Plant Materials
Whole Stephania japonica plants were collected from the plantation area of BCSIR laboratories Chittagong in August, 2011. The plant was taxonomically identified and authenticated by Industrial Botany Research Division of BCSIR laboratories Chittagong. A voucher specimen of the sample has been preserved in the herbarium of BCSIR Laboratory, Chittagong with the accession number Y-117.
Preparation of Plant Extract
The fresh plant S. japonica was washed with distilled water, chopped into small pieces, air dried under shade, ground into coarse powder, and stored in an airtight container. Plant powder (900 g) was soaked in ethanol (95%) for 7 days at room temperature (28 ± 1°C) with occasional stirring. Ethanol extract was filtered off through a cotton plug and then with Whatman No. 1 filter paper. The extract was concentrated under reduced pressure below 50°C through a rotary vacuum evaporator (EYELA Rikakikai Co., Ltd. Tokyo). The concentrated extracts were collected in a petri dish and air dried at room temperature. The whole process was repeated three times. The concentrated extract (60 g blackish-green) was stored at 4°C until use.
HPLC System
Chromatographic analyses were carried out on a Thermo Scientific Dionex UltiMate 3000 rapid separation liquid chromatography (RSLC) systems (Thermo Fisher Scientific Inc., MA, USA), coupled to a quaternary rapid separation pump (LPG-3400RS), Ultimate 3000RS autosamplier (WPS-3000) and rapid separation diode array detector (DAD-3000RS). Phenolic compounds were separated on an Acclaim® C18 (4.6 × 250 mm; 5 µm) column (Dionix, USA) which was controlled at 30°C using a temperature controlled column compartment (TCC-3000). Data acquisition, peak integration, and calibrations were performed with Dionix Chromeleon software (Version 6.80 RS 10).
Chromatographic Conditions
The phenolic composition of the ethanolic extract of S. japonica was determined by HPLC, as described by Sarunya and Sukon with some modifications.[Citation25] The mobile phase consisted of acetonitrile (solvent A), acetic acid solution pH 3.0 (solvent B), and methanol (solvent C). The system was run with the following gradient elution program: 0 min, 5%A/95%B; 10 min, 10%A/80%B/10%C; 20 min, 20%A/60%B/20%C; and 30 min, 100%A. There was a 5 min post-run at initial conditions for equilibration of the column. The flow rate was kept constant throughout the analysis at 1 mL/min and the injection volume was 20 µL. For ultraviolet (UV) detection, the wavelength program was optimized to monitor phenolic compounds at their respective maximum absorbance wavelengths as follows: λ 280 nm held for 18.0 min, changed to λ 320 nm and held for 6 min, and finally changed to λ 380 nm and held for the rest of the analysis and the DAD was set at an acquisition range from 200 nm to 700 nm. The detection and quantification of GA, CH, VA, CA, and EC was done at 280 nm, of PCA, RH, and EA at 320 nm, and of QU at 380 nm, respectively.
Preparation of Standard and Sample
A stock standard solution (100 µg/mL) of each phenolic compound was prepared in methanol by weighing out approximately 0.0050 g of the analyte into 50 mL volumetric flask. The mixed standard solution was prepared by dilution the mixed stock standard solutions in methanol to give a concentration of 20 µg/mL for each polyphenols except CA (8 µg/mL) and QU (6 µg/mL). All standard solutions were stored in the dark at 5°C and were stable for at least 3 months. The calibration curves of the standards were made by serial dilution of the stock standards (five set of standard dilutions) with methanol to yield 1.25–20 µg/mL for GA, CH, VA, EC, PCA, RH, EA; 0.5–8.0 µg/mL for CA, and 0.375–6.0 µg/mL for QU. The calibration curves were constructed from chromatograms as peak area versus concentration of standard. A solution of ethanolic extract of S. japonica at a concentration of 5 mg/mL was prepared in ethanol by vortex mixing (Branson, USA) for 30 min. The samples were stored in the dark at low temperature (5°C). Spiking the sample solution with phenolic standards was done for additional identification of individual polyphenols. Prior to HPLC analysis, all solutions (mixed standards, sample, and spiked solutions were filtered through 0.20 µm nylon syringe filter (Sartorius, Germany) and then degassed in an ultrasonic bath (Hwashin, Korea) for 15 min.
Determination of Total Phenolic Content (TPC)
The amount of TPC was determined by the Folin-Ciocalteu reagent method with some modifications.[Citation26] Briefly, a 0.5 mL of extract and 0.5 mL of Folin-Ciocalteu reagent (0.5 N) were mixed and incubated at room temperature for 5 min. Then 2.0 mL saturated sodium carbonate was added and further incubated for 30 min at room temperature and absorbance measured at 765 nm. Tannic acid was used as a positive control.[Citation27] The contents of total phenolics was determined by using the linear regression equation:
where x is absorbance and y is concentration of tannic acid.
Determination of Total Flavonoid Content
Total flavonoid content was determined by aluminium chloride method using catechin as a standard.[Citation28] Briefly, a 1.0 mL of test sample and 4.0 mL of water were added to a volumetric flask (10 mL volume). After 5 min of adding 0.3 mL of 5% sodium nitrite, 0.3 mL of 10% aluminium chloride was added. After 6 min incubation at room temperature, 2.0 mL of 1 M Sodium hydroxide was added to the reaction mixture. Immediately the final volume was made up to 10 mL with distilled water. The absorbance of the reaction mixture was measured at 510 nm against a blank spectrophtometrically (Shimadzu UV-1609, Japan).Total flavonoid content was calculated as catechin (mg/100 g) using the following equation based on the calibration curve:
where x was the absorbance and y was the catechin concentration.
Determination of Total Carotenoids
Total carotenoids and chlorophylls a and b were determined according to Rainha et al.[Citation29] Methanolic solutions of plant extracts of the appropriate concentration (1.0 to 4.0 mg/mL) were analyzed in a UV/VIS spectrophotometer at 470 nm. The concentrations of carotenoids were determined according to the equations reported by Lichtenthaler and Wellburn as follows:[Citation30]
Determination of Tannin
Quantitative estimation of tannin was carried out using the modified vanillin – HCl method.[Citation31] Vanillin reagent (0.5%, 5 mL) was added to the extract (1 mL) and the absorbance of the color developed after 20 min at 30°C was read at 500 nm. Standard curve was prepared expressing the results as catechin equivalents, i.e, the amount of catechin (mg/100 g) which gives a color intensity equivalent to that given by tannin after correcting for blank. Tannin content was calculated as catechin (mg/100 g) using the following equation based on the calibration curve:
where x was the absorbance and y was the catechin concentration.
Determination of PCO Content
Quantitative estimation of PCO was carried out using the modified vanillin–HCl method.[Citation31] Vanillin reagent (0.5%, 5 mL) was added to the extract (1 mL, concentration) and the absorbance of the color developed after 20 min at 30°C was read at 500 nm. A standard curve was prepared expressing the results as catechin equivalents, i.e., the amount of catechin (mg/100 g) which gives a color intensity equivalent to that given by PCO after correcting for blank. PCO content was calculated as catechin (mg/100 g) using the following equation based on the calibration curve:
where x was the absorbance and y was the catechin concentration.
Determination of Chlorophylls A and B
Chlorophylls a and b were determined according to Rainha et al.[Citation29] Methanolic solutions of plant extracts of the appropriate concentration (1.0 to 4.0 mg/mL) were analyzed in a UV/VIS spectrophotometer at 653 and 666 nm. The concentrations of chlorophylls α and b were determined according to the equations reported by Lichtenthaler and Wellburn as follows:[Citation30]
Estimation of β-Carotene and Lycopene
β-carotene and lycopene were determined from the dried ethanolic extract according to Kumari et al.[Citation32] A 100 mg of extract was mixed with 10 mL of acetone–hexane mixture (4:6) for 1 min and filtered. The absorbance was recorded at three different wavelengths (453, 505, and 663 nm). The β-carotene content was calculated by:
The results were presented as μg of carotenoid and lycopene/g of extract.
Assay of Antioxidative Effect
Determination of total antioxidant capacity
Total antioxidative activity was assayed by phosphomolybdenum method with some modifications.[Citation33] The basic principle of the assay is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of a green phosphate Mo (V) complex at acidic pH. S. japonica extract (0.3 mL) was combined with a mixture of 3.0 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were then capped and incubated at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the solution was measured at 695 nm against blank. Methanol (0.3 mL) in the place of extract was used as the blank. The antioxidative effect was expressed as the mg of equivalents of ascorbic acid equivalents in mg.
Determination of FRAP
FRAP assay was carried out according to the established methods with some modifications.[Citation34] FRAP reagent was prepared from acetate buffer (1.6 g sodium acetate and 8 mL acetic acid made up to 500 mL; pH 3.6), 10 mM 2,4,6-tris (1-pyridyl)-5-triazine (TPTZ) solution in 40 mM HCL and 20 mM iron (III) chloride solution in proportion of 10:1:1 (v/v), respectively. The FRAP reagent was prepared fresh daily and was warmed to 37°C in an oven prior to use. A total of 200 μL of sample extract was added to 4 mL of the FRAP reagent and mixed well. The absorbance was measured at 593 nm using UV-visible spectrophotometer (UV-Vis 1210, Shimadzu Corporation, and Japan). Samples were measured in three replicates. Standard curve of ascorbic acid (125, 250, 500, 750, and 1000 µmol) was prepared using the similar procedure. The results were expressed as μM ascorbic acid/g extract sample.
Determination metal chelating effect
The chelation of ferrous ions is estimated using the method of Chanda et al. with some modifications.[Citation26] A 4.0 mL of the different concentrations of extract was added to a solution of 0.2 mL ferrous chloride (2 mM). The reaction was initiated by the addition of 0.2 mL of ferrozine (5 mM) and incubated at room temperature for 10 min and then the absorbance was measured at 562 nm. Ethylenediaminetetraacetic acid (EDTA) was used as a positive control.
Determination of RP
The RP was determined by the method described by Jayanthi et al.[Citation35] Various concentrations of the extract in corresponding solvents were mixed with phosphate buffer (2.5 mL) and potassium ferricyanide (2.5 mL). This mixture was kept at 50°C in water bath for 20 min. After cooling, 2.5 mL of 10% trichloro acetic acid was added and centrifuged at 3000 rpm for 10 min whenever necessary. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and a freshly prepared ferric chloride solution (0.5 mL). The absorbance was measured at 700 nm. Control was prepared in similar manner excluding sample. Ascorbic acid at various concentrations was used as standard. Increased absorbance of the reaction mixture indicated increase in RP. IC50 was concentration of extract or standard to require absorbance 0.50.
ABTS free radical scavenging assay
ABTS assay was carried out according to the method of Re et al. with some modification.[Citation36] The ABTS radical method is one of the most used assays for the determination of the concentration of free radicals.[Citation37] It is based on the neutralization of a radical-cation arising from the one-electron oxidation of the synthetic chromophore 2,2‘-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; ABTS•): ABTS• – e- ABTS•+ . This reaction is monitored spectrophotometrically by the change of the absorption spectrum.
Seven mmol·L-1 ABTS• and 4.95 mmol·L-1 potassium peroxodisulphate are mixed and dissolved in distilled water. The solution is then diluted with distilled water in a 1:9 v/v ratio (10 mL is quantitatively transferred into 100 mL calibrated flask and diluted). The solution is incubated for 12 h in the dark and the reagent is usable for 7 days if stored in the dark at 4°C. Briefly, the reaction mixture (3.5.0 mL) consist of 3.0 mL of ABTS and 0.50 mL of various concentrations of methanol extract and it is incubated for 10 min in dark, then the absorbance is measured at 734 nm against distilled water as a blank and control is prepared by ABTS reagents and distilled water in place of sample extract. In this assay, the positive control is GA. The percentage of inhibition can be calculated using the formula:
where A0 is the absorbance of control and A1 is the absorbance of test.
DMPD free radical scavenging assay
DMPD assay was carried out according Gülçin method with some modification.[Citation38] The compound DMPD is converted in solution to a relatively stable and colored radical form by the action of ferric salt. After addition of a sample containing free radicals, these are scavenged and as a result of this scavenging, the colored solution is decolorized.[Citation38]
Sodium acetate buffer (1) in distilled water (0.2 mol·L-1, pH 5.25 adjusted with concentrated acetic acid) and 0.74 mmol·L-1 ferric chloride (2) in distilled water were prepared. DMPD, 36.7 mmol·L-1 (3), is dissolved in distilled water and solution was prepared. DMPD solution must be prepared at the time of use due to its low stability. These three solutions (solutions no. 1, 2, and 3) are mixed in a 20:1:1 (v/v/v) ratio. The reaction mixture (4.0 mL) consist of 3.0 mL of DMPD and 1.0 mL of various concentrations of methanol extract and it is incubated for 6 min, then the absorbance is measured at 505 nm against distilled water as a blank and control is prepared by DMPD reagents and sodium acetate buffer in place of sample extract. In this assay, the positive control is GA and ascorbic acid. The percentage of inhibition can be calculated using the formula:
where A0 is the absorbance of control and A1 is the absorbance of test.
Scavenging of Superoxide Radical by Alkaline Dimethyl Sulfoxide (DMSO) Method
Superoxide scavenger inhibits the formation of a red dye formazan. In alkaline DMSO method, superoxide radical was generated by the addition of sodium hydroxide to air saturated DMSO.[Citation39] To the reaction mixture containing 0.1 mL of NBT (1 mg/mL solution in DMSO) and 3 mL of the various concentration of the extract, 1 mL of alkaline DMSO was added to give a final volume of 4.1 mL and the absorbance was measured at 560 nm. The same procedure was repeated for the standard GA. The percentage inhibition was calculated using following equation:
where A0 is the absorbance of control and A1 is the absorbance of test.
Nitric Oxide Scavenging Activity
Various concentrations of the extract were mixed with 2.5 mL of sodium nitroprusside and made up to 3.0 mL with phosphate-buffered saline (PBS). Then the mixture was incubated for 15 min at 25°C. After incubation, 0.5 mL of the reaction mixture was removed and 0.5 mL of the Griess reagent was added. Then the absorbance was measured at 546 nm.[Citation40] The percentage inhibition was calculated by comparing the results of the test with those of controls not treated with the extract, as per the following formula:
Statistical Analysis
All the experiments were carried out in triplicate, and the results were expressed as mean ± SD. Data were analyzed by one-way analysis of variance (ANOVA) using the statistical software statistical package for social science (SPSS, version 22.0, IBM corporation, NY). Values were considered significant with the p < 0.05.
RESULTS
Phytochemical Quantification
Quantitative phytochemical analyses showed the presence of tannin, PCO, lycopene, chlorophylls a and b, phenolics, flavonoids, and carotenoids in the crude extract of whole S. japonica. Total phenolic, total flavonoid, total carotenoids, and total antioxidant capacity were calculated from the regression analysis and their values are summarized in . The quantitative comparisons of tannin, PCO, lycopene, chlorophyll a, and chlorophyll b in grams of methanol extract are graphically presented in .
Table 1 Spectrophotmetric determination of the phytochemicals of Stephania japonica leaf extract
HPLC Analysis of Phytochemicals
Identification and quantification of individual phenolic compounds in the ethanol extract of S. japonica was analysed by HPLC. An especially high concentration of epicatechin (100.58 mg), p-coumaric acid (PCA) (232.76 mg), and RH (383.81 mg) and moderate concentration of CA (48.63 mg) and querecetin (51.46 mg) were found in 100 g of dry extract. The chromatogram for the separations of polyphenols in ethanol extract is shown in . The content of each phenolic compound was calculated from the corresponding calibration curve and presented as the mean of five determinations as shown in .
FIGURE 2 HPLC chromatogram of Stephania japonica extract. Peaks were compared with reference metabolites and identified as: 1: caffeic acid; 2: (–)-epicatechin; 3: p-coumaric acid; 4: rutin hydrate; and 5: querecetine.
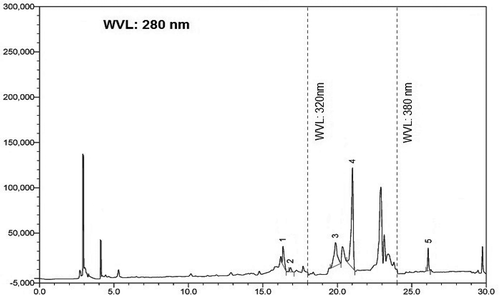
Table 2 Contents of polyphenolic compounds in the ethanol extract of Stephania japonica leaf (n = 5).
Determination of Total Antioxidant Capacity
The total antioxidant capacity in the ethanolic extract of S. japonica was determined using the linear regression equation (y = 52.128x + 1.3407; RCitation2 = 0.9964; where x is absorbance and y is ascorbic acid concentration in microgram) of the calibration curve and was expressed as ascorbic acid equivalent. The total antioxidant capacity of the extract is 22.85 ± 0.70 (n = 3) mg ascorbic acid equivalent/g extract ().
Determination of FRAP
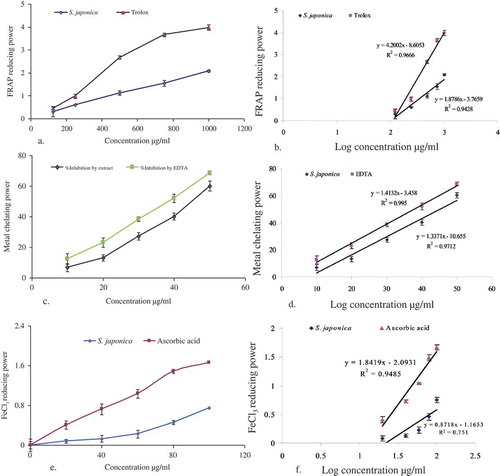
The antioxidant capacity of the extract of S. japonica is determined by the ability of the antioxidants in the extract to reduce ferric iron to ferrous in FRAP reagent, which consists of TPTZ prepared in sodium acetate buffer, pH 3.6. The reduction of ferric iron in FRAP reagent will result in the formation of a blue product (ferrous – TPTZ complex) whose absorbance can be read at 593 nm. The absorbance decrease is proportional to the antioxidant content.[Citation41] The ferric ions reducing activities of the extract at different concentrations are calculated through the regression analysis (). The antioxidant activity compared to the standard (3a) and the FRAP was shown, respectively, in and .
Table 3 Inhibition concentration of Stephania japonica extract in different antioxidative models
FIGURE 3 Antioxidative effects (a, c, e) and comparative IC50 values (b, d, f) of S. japonica extract. Data are presented as mean ± SD (values are small to be visualized in the lines) for triplicate. Data were analyzed by statistical package for social science (SPSS) for windows followed by Tukey’s post-hoc test for multiple comparisons, version 18.0, (p < 0.05) from each other.
Metal Chelating Activity
The iron chelating effect of S. japonica extract and standard antioxidant ascorbic acid is shown in . Inhibition concentration (IC50) was calculated from regression analysis showed in . The IC50 value of S. japonica extract and reference chelating agent EDTA were found to be 1656.52 and 37.83 µg/mL, respectively ().
RP
The RP of the extract was found to be correlated with increasing absorbance (at 700 nm) as compared with ascorbic acid, a known antioxidant. S. japonica extract and ascorbic acid showed a dose dependent reducing activity in FeCl3 assay (). Regression analysis for reducing activity versus log concentration showed the IC50 value for S. japonica was 270.55 µg/mL which was close to that of ascorbic acid (31.12 µg/mL; ).
ABTS Radical Scavenging Activity
In this assay, the activity of extract was found to be increased in a dose-dependent manner at different concentrations. The S. japonica extract exhibited the highest inhibition 32.11%, whereas the standard GA showed 96.87%. Regression analysis for extract and GA are shown in and . The extract exhibited an IC50 value of 457.27 µg/mL ().
FIGURE 4 Regression analysis to determine the IC50 values of S. japonica extract comparing with gallic acid (reference molecule) through various in vitro models. Scavenging assay for A: superoxide radical and B: gallic acid; C: DMPD; D: Gallic acid; E: ABTS; F: Gallic acid; G: Nitric oxide; H: Gallic acid.
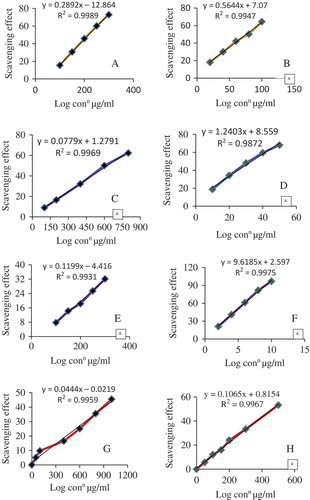
DMPD Scavenging Activity
The S. japonica extract was analyzed for the ability to scavenge the DMPD radicals. Dark color of DMPD•+ radical cation solution becomes lighter and absorbance of solution becomes lower, in the presence of an antioxidant compound. The DMPD•+ radical cation solution shows a maximum absorbance at 505 nm. The DMPD•+ radical quenching was found to increase with an increase in concentration of the extract. Regression analysis for the scavenging assay and inhibition concentration is shown in and . The IC50 value of the extract was found to be 632.74 µg/mL which was statistically significant (p < 0.05) compared to that of standard GA is 33.42 µg/mL ().
Superoxide Scavenging Activity
Superoxide radical scavenging activity of S. japonica extract was assessed by alkaline DMSO method. The plant extract strongly inhibited the superoxide radical generation. In the hydrogen peroxide radical scavenging assay, the extract was found to be equipotent with rutin but less potent when compared to ascorbic acid. Superoxide scavenging assay was measured in context of inhibition concentration. Regression analysis for both extract and GA is plotted in and . The IC50 value of S. japonica extract is 217.5 µg/mL extract which was compared with that (76.11 µg/mL) of standard GA, the reference molecule. The values were tabulated in .
Nitric Oxide Scavenging Activity
Stephania japonica extract also caused a moderate dose-dependent inhibition of nitric oxide ( and ). GA was used as a reference compound and 90.82 ± 4.75 μg/mL GA was needed for 50% inhibition. The IC50 value of the extract was less than that of the standard. At 1.0 mg/mL, the percentage inhibition of the plant extract was 45.52%, whereas that of GA was 53.2%. IC50 of S. japonica extract is 464.00 µg/mL extract ().
DISCUSSION
Plant phenolics, such as polyphenols, have greatly drawn the attention of food and medical scientists due to their strong in vitro and in vivo antioxidant activities. They are the potential agents to scavenge free radicals, break radical chain reaction, and chelate metals.[Citation42] Structural variations of plant phenolic classes strongly differentiate their effects as free radical scavengers and potentials as food antioxidants.[Citation43] This hypothesis is rigorously reflected in our phytochemical group tests. Quantitative determination of plant metabolites showed the phytochemical prevalence as; PCO > total carotenoids > flavonoids > total phenolics > chlorophyll a > chlorophyll b > lycopene in the extract.
PCO have strong antioxidant properties in vitro. It is also established that foods rich in PCO have high oxygen radical absorbance capacity. Scientists continue to research the relevance of antioxidant properties in vitro and potential effects of PCOs on cancer or cardiovascular disease as determined in laboratory studies.[Citation44].PCOs, among various roles, are considered to exert exciting antiatherosclerotic effects which are also promoted by antioxidative agent.[Citation45] High concentrations of PCO in S. japonica, therefore, indicates its potential to be useful in cancerous cases.
In this study, TPCs of the extract was expressed in tannic acid equivalent since tannic acid is one of the major polyphenolic compounds that occur in plants. Plant extracts containing high levels of tannic acid or any compound equivalent to that may be able to scavenge excessive free radicals such as superoxide anion radicals and peroxy radicals in the human body and protect human cells or tissues against oxidative stress.[Citation46] A blue-colored solution was produced when the extract reacted with Folin-Ciocalteau reagent and sodium carbonate. The blue color of phospho-molybdic-phosphotungstic-phenol complex became more concentrated as the TPCs of the extract increased. The present results suggest that plant extract contained the remarkable TPC, since its extract produced the most concentrated blue solution. These results are very much consistent with the presence of higher amount of phenolics and lycopene in other plants used in cancerous incidence.[Citation47]
Determination of the ferric reducing antioxidant power is a simple and direct test of antioxidant capacity. The assay of reducing activity is based on the reduction of FeCitation3+ to the FeCitation2+ form in the presence of antioxidants in the tested samples. The FeCitation2+ was then monitored by measuring the formation of Perl’s Prussian blue at 700 nm. The RP of a compound may serve as a significant indicator of its potential antioxidant activity Zhao et al.[Citation48] In this study, the FRAP of S. japonica was compared with a reference agent Trolox demonstrating that the extract has very good status of FRAP.
Metal ion chelating capacity is significant because they reduce the concentration of catalyzing transition metal in lipid peroxidation. Chelating properties may be attributed to endogenous chelating agents, mainly phenolics. Some phenolic compounds consist of very properly oriented functional groups that can chelate metal ions.[Citation49] It was established that chelating agents, which form σ-bonds with a metal, are effective as secondary antioxidants since they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion.[Citation50] Metal ion chelating activity of the extract was tested against ferrous ion (FeCitation2+) to evaluate the potential of plant extract as secondary antioxidants. The metal ion chelating ability of an extract measures how effectively the compounds in it can compete with ferrozine for ferrous ion. Ferrozine can quantitatively form complexes with FeCitation2+. The presence of antioxidant compounds in S. japonica extract can disrupt the formation of ferrozine-FeCitation2+ complex resulting in decolorization of red or purple color of the complex.[Citation51] In this study, the purple color of the reaction solutions was changed in the presence of extract although the metal ion chelating effect of the extract was lower than EDTA, a standard chelating agent. The results suggest that compounds in extract can be compared positively moderate metal ion chelating effects.
The reducing properties of the plant extracts are generally associated with the presence of reductants, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom.[Citation52] Reductants are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. The data obtained in the present study suggest that it is likely to contribute significantly toward the observed antioxidant effects. Like the antioxidant activity, the RP of the extract increases with increasing concentration. The effect was clarified using the inhibition concentration (IC50 of plant extract) which was 270.55 µg/mL for extract and 27.38 µg/mL for standard ascorbic acid. This value indicates that the extract has strong RP.
ABTS assay is an excellent tool for determining the antioxidant activity of hydrogen-donating antioxidants and of chain-breaking antioxidants.[Citation53] ABTS assay is often used in evaluating total antioxidant power of single compounds and complex mixtures of various plants.[Citation37] In this assay, ABTS radical mono cation was generated directly in stable form from potassium peroxodisulfate. The noticeable IC50 value as well as the radical scavenging activity of S. japonica extract indicates its ability to scavenge free radicals, thereby preventing lipid oxidation via a chain-breaking reaction. Here, the extract’s radical scavenging activity showed a direct role of its phenolic compounds in free radical scavenging.
Superoxide radical is known to be a very harmful species to cellular components as a precursor of more reactive species.[Citation54] The superoxide radical is known to be produced in vivo and can result in the formation of hydrogen peroxide via dismutation reaction. Robak and Glyglewski[Citation55] reported that flavonoids are effective antioxidants mainly because they scavenge superoxide anions.[Citation55] Our results suggest that the plant extract is a more potent scavenger of superoxide radical than the standard GA. The extract is found to be an efficient scavenger of superoxide radical generated in alkaline DMSO system. The result also clearly indicates that the plant extract has a noticeable effect as scavenging superoxide radical.
Nitric oxide formed during their reduction with oxygen or with superoxide, such as NO2, N2O4, N3O4, is very reactive. These radicals are responsible for altering the structure and functional behavior of many cellular components. The S. japonica extract showed better activity in competing with oxygen to react with nitric oxide and thus inhibited the generation of anions and the activity is comparable to the standards used. The plant secondary metabolites may have the property to counteract the effect of nitric oxide formation and in turn may be considerably interested in preventing the ill effects of excessive nitric oxide generation in the human body. Further the scavenging activity may also help to arrest the chains or reactions initiated by excess generation of nitric oxide that are dangerous to the human health. Nitric oxide is also implicated for inflammation, cancer and other pathological conditions.[Citation56]
CONCLUSIONS
The phytochemical contents, TPCs, total antioxidant capacities, and the potential antioxidant activities of S. japonica leaf extract were assessed for the first time. The antioxidant capacity and TPC suggest that the antioxidant activities of the medicinal plant can be mainly ascribed to the phenol compounds. Besides their strong antioxidant activities, make the plant as a promising sources of natural antioxidants and other bioactive compounds in the food and pharmaceutical industries.
ACKNOWLEDGMENT
The authors wish to thank the Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka for their support in progress of this research.
REFERENCES
- Harman, D. Aging: Phenomena and Theories; Annals of the New York Academy of Sciences: New York, NY, 1998.
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. Journal of Agriculture and Food Chemistry 2001, 49, 5165–5170.
- Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Activity of Betalains from Plants of the Amaranthaceae. Journal of Agricultural and Food Chemistry 2003, 51, 2288–2294.
- Sala, A.; Recio, M.D.; Giner, R.M.; Manez, S.; Tournier, H.; Schinella, G.; Rios, J.L. Antiinflammatory and Antioxidant Properties of Helichrysum Italicum. The Journal of Pharmacy and Pharmacology 2002, 54, 365–371.
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Activities of Plant-Derived Polyphenolic Flavonoid. Free Radical Research 1995, 22, 375–383.
- Ashokkumar, D.; Mazumder, U.K.; Gupta, M.; Senthilkumar, G.P.; Selvan, V.T. Evaluation of Antioxidant and Free Radical Scavenging Activities of Oxystelma Esculentum in Various in Vitro Models. Journal of Complementary and Integrated Medicine 2008, 5, 1–17.
- Veerapur, V.P.; Prabhakar, K.R.; Parihar, V.P.; Kandadi, M.R.; Ramakrishana, S.; Mishra, B.; Rao, S.B.S.; Srinivasan, K.K.; Priyadarsini, K.I.; Unnikrishnan, M.K. Ficus Racemosa Stem Bark Extract: A Potent Antioxidant and a Probable Natural Radioprotector. Evidence Based Complementary and Alternative Medicine 2009, 6, 317–324.
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Hu, C. Antioxidant Properties of a North American Gingseng Extract. Molecular and Cellular Biochemistry 2000, 203, 1–10.
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Želmièka, M.; Rivas-Gonzalo, J.C. Measurement of Antioxidant Activity of Wine Catechins, Procyanidins, Antocyanins and Piranoantocyanins. International Journal of Molecular Science 2007, 8, 797–809.
- Wang, S.Y.; Jiao, H. Correlation of Antioxidant Capacities to Oxygen Radical Scavenging Enzyme Activities in Blackberry. Journal of Agricultural and Food Chemistry 2000, 48, 5672–5676.
- Yusuf, M.; Wahab, M.A.; Yousuf, M.; Chowdhury, J.U. Some Tribal Medicinal Plants of Chittagong Hill Tracts, Bangladesh. Bangladesh Journal Plant Taxonomy 2007, 14, 117–128.
- Murty, P.P.; Venkaiah, M. Some Abortifacient Plants Used by the Tribal People of Andhra Pradesh, India. Journal of Phytology 2010, 2, 7–12.
- Mitra, S.; Mukherjee, S.K. Some Abortifacient Plants Used by the Tribal People of West Bengal. Natural Product Radiance 2009, 8, 167–171.
- Mukherjee, S.; Banerjee, R.; Upadhyay, S.N.; Hazra, J.; Poddar, K.N.; Mukherjee, A.; Saha, A. Reproductive Effects of Ethnomedicinal Formulation of Tape-Vine Leaves in Female Rats. Biological and Pharmaceutical Bulletin 2006, 29, 1916–1922.
- Gupta, A.; Nagariya, A.K.; Mishra, A.K.; Bansal, P.; Kumar, S.; Gupta, V.; Singh A.K. Ethno-Potential of Medicinal Herbs in Skin Diseases: An Overview. Journal of Pharmacy Research 2010, 3, 435–441.
- Tomas, S.C.Li. Chinese and Related North American Herbs: Phytopharmacology and Therapeutic Values; CRC Press: Washington, DC, 2002; 146 pp.
- Tomita, M.; Ibuka, T. Studies on the Alkaloids of Menispermaceous Plants. Cciv. Alkaloids of Formosan Stephania Japonica Miers. (2). The Structure of a New Tertiary Phenolic Biscoclaurine Type Alkaloid “Stepholine.” Yakugaku Zasshi 1963, 83, 940–944.
- Watanabe, Y., Matsumura, H. Studies on the Alkaloids of Menispermaceous Plants. CCII. Alkaloids of Stephania Japonica Miers. (Supplement 8). Structure of Hasubanonine. (1). Hasubanol. Yakugaku Zasshi 1963, 83, 991–996.
- Hall, A.M.; Chang, C.J. Multidrug-Resistance Modulators from Stephania Japonica. Journal of Natural Products 1997, 60, 1193–1195.
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant Effects of Chlorophyll and Pheophytin on the Autoxidation of Oils in the Dark. II. The Mechanism of Antioxidative Action of Chlorophyll. Journal of American Oil Chemical Society 1985, 62, 1387–1390.
- Nenkova, G.; Alexandrova, A. A Review: Oxidative Stress and Its Role in Reproduction. Advances in Bioscience and Biotechnology 2013, 4, 37–43.
- Schäfer, M.; Werner, S. Oxidative Stress in Normal and Impaired Wound Repair. Pharmacological Research 2008, 58, 165–171.
- Gupta, S.; Ashok Agarwal, A.; Banerjee, J.; Alvarez, L.G. The Role of Oxidative Stress in Spontaneous Abortion and Recurrent Pregnancy Loss: A Systematic Review. CME Review Article 2007, 62, 335–347.
- Suzuki, H.; Nishizawa, T.; Tsugawa, H.; Mogami, S.; Hibi, T. Roles of Oxidative Stress in Stomach Disorders. Journal of Clinical Biochemistry and Nutrition 2011, 50, 35–39.
- Sarunya, C.; Sukon, P. Method Development and Determination of Phenolic Compounds in Broccoli Seeds Samples. Chiang Mai Journal of Science 2006, 33, 103–107.
- Chanda, S.; Dave, R. In Vitro Models for Antioxidant Activity Evaluation and Some Medicinal Plants Possessing Antioxidant Properties: An Overview. African Journal of Microbiology Research 2009, 3, 981–996.
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. Journal of Agriculture and Food Chemistry 2003, 51, 609–614.
- Shwetha, C.; Latha, K.P.; Pushpa, B.; Sruthi, A.; Vaidya, V.P. Phytochemical Screening and Evaluation of in-vitro Antioxidant Activity, Total Phenolics, and Total Flavonoids of Holarrena antidysentrica Leaf Extracts. International Journal of Research in Pharmacy and Chemistry 2011, 1, 546–550.
- Rainha, N.; Lima, E.; Baptista, J.; Rodrigues, C. Antioxidant Properties, Total Phenolic, Total Carotenoid, and Chlorophyll Content of Anatomical Parts of Hypericum foliosum. Journal of Medicinal Plants Research 2011, 5, 1930–1940.
- Lichtenthaler, H.; Wellburn, A. Determination of Total Carotenoids and Chlorophylls A and B of Leaf in Different Solvents. Biochemical Society Transactions 1985, 11, 591–592.
- Abdelseed, B.H.; Abdalla, A.H.; Yagoub, A.E.G.A.; Ahmed, I.A.M.; Babiker, E. Some Nutritional Attributes of Selected Newly Developed Lines of Sorghum (Sorghum bicolor) after Fermentation. Journal of Agricultural Science and Technology 2011, 13, 399–409.
- Kumari, D.; Reddy, S.M.; Upadhyay, R.C. Antioxidant Activity of Three Species of Wild Mushroom Genus Cantharellus Collected from North-Western Himalaya, India. International Journal of Agricultural Biology 2011, 13, 415–418
- Sadhu, S.K.; Okuyama, E.; Fujimoto, H.; Ishibashi, M. Separation of Leucas aspera, a Medicinal Plant of Bangladesh, Guided by Prostaglandin Inhibitory and Antioxidant Activities. Chemical and Pharmaceutical Bulletin 2003, 51, 595–598.
- Maizura, M.; Aminah, A.; Wan Aida, W.M. Total Phenolic Content and Antioxidant Activity of Kesum (Polygonum minus), Ginger (Zingiber officinale), and Turmeric (Curcuma longa) Extract. International Food Research Journal 2011, 18, 529–534.
- Jayanthi, P.; Lalitha, P. Reducing Power of the Solvent Extracts of Eichhornia crassipes (Mart.) Solms. International Journal of Pharmacy and Pharmaceutical Sciences 2011, 3, 126–128.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying An Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 Medicinal Plant Extracts for Antioxidant Capacity and Total Phenols. Food Chemistry 2006, 94, 550–557.
- Gülçin, I. Antioxidant Properties of Resveratrol: A Structure-Activity Insight. Innovative Food Science and Emerging 2010, 11, 210–218.
- Laloo, D.; Sahu, A.N. Antioxidant Activities of Three Indian Commercially Available Nagakesar: An in Vitro Study. Journal of Chemical and Pharmaceutical Research 2011, 3, 277–283.
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite and [15N]Nitrate in Biological Fluids. Analytical Biochemistry 1982, 126, 131–138.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) As a Measure of “Antioxidant Power” the FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Liu, Q.; Yao, H. Antioxidant Properties of Barley Seed Extracts. Food Chemistry 2006, 102, 732–737.
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant Potential of Synthetic and Natural Antioxidants and Its Effect on Warmedover-Flavour in Different Species of Meat. Food Chemistry 2007, 105, 908–916.
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in Health Care: Current and New Trends. Current Medicinal Chemistry 2004, 11, 1345–1359.
- Yamakoshi, J.; Kataoka, S.; Koga, T.; Ariga, T. Proanthocyanidin-Rich Extract from Grape Seeds Attenuates the Development of Aortic Atherosclerosis in Cholesterol-Fed Rabbits. Atherosclerosis 1999, 142, 139–149.
- Rangka-dilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of Free Radical Scavenging and Antityrosinase Activities of Standardized Longan Fruit Extract. Food and Chemical Toxicology 2006, 45, 328–336.
- Abreu, W.C.D.; Barcelos, M.D.F.P.; Boas, E.V.D.D.V.; Silva, E.P.D. Total Antioxidant Activity of Dried Tomatoes Marketed in Brazil. International Journal of Food Properties 2014, 17, 639–649.
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of Antioxidant Activities and Total Phenolic Contents of Typical Malting Barley Varieties. Food Chemistry 2007, 107, 296–304.
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemistry and Antioxidant Properties of Essential Oil and Oleoresins Extracted from the Seeds of Tomer (Zanthoxylum armatum DC). International Journal of Food Properties 2013, 16, 288–300.
- Oktay, M.; Gulcin, I.; Kufrevioglu, I. Determination of in Vitro Antioxidant Activity of Fennel (Foeniculum vulgare). Lebens-Wiss Technology 2003, 36, 263–271.
- Ravisankar, N.; Sivaraj, C.; Seeni, S.; Joseph, J.; Raaman, N. Antioxidant Activities and Phytochemical Analysis of Methanol Extract of Leaves of Hypericum hookerianum. International Journal of Pharmacy and Pharmaceutical Sciences 2014, 6, 456–460.
- Kumar, S.R.; Rajkapoor, B.; Perumal, P. Antioxidant Activities of Indigofera cassioides Rottl. Ex. DC. Using Various in Vitro Assay Models. Asian Pacific Journal of Tropical Biomedicine 2012, 2, 256–261.
- Leong, L.P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chemistry 2002, 76, 69–75.
- Arunachalam, K.; Murugan, R.; Parimelazhagan, T. Evaluation of Antioxidant Activity and Nutritional and Chemical Composition of Ficus Amplissima Smith Fruit. International Journal of Food Properties 2014, 17, 454–468.
- Robak, J.; Gryglewski, I.R. Flavonoids are Scavengers of Superoxide Anions. Biochemical Pharmacology 1988, 37, 837–841.
- Hofseth, L.J. Nitric Oxide As a Target of Complementary and Alternative Medicines to Prevent and Treat Inflammation and Cancer. Cancer Letter 2008, 268, 10–30.