ABSTRACT
This study aimed to determine the phytochemical profile and nutraceutical properties of nopal cladodes (Opuntia ficus-indica) at different stages of maturity. Medium-age cladodes showed the highest total saponins, phytosterols, and indigestible fiber, as well as the highest in vitro antioxidant capacity and digestive enzymes inhibitory activity. Furthermore, these cladodes presented the highest content of p-hydroxybenzoic acid, p-coumaric acid, rutin, narcissin, nicotiflorin, β-sitosterol, and sitosteryl-3-β-glucopyranoside, as well as several amino acids, organic acids, and fatty acids. Whereas young cladodes contained the highest concentration of condensed and hydrolyzable tannins. These results demonstrated that maturity affects the nutritional and nutraceutical properties of nopal cladodes.
Introduction
Nopal (Opuntia ficus-indica) is a drought-tolerant plant cultivated in arid and semi-arid regions. This plant produces edible stems known as cladodes, which are widely consumed in Mexico. Interestingly, its production and consumption is extending to other countries due to its high nutritional and nutraceutical value, since nopal cladodes are rich in pectin, mucilage, vitamins, polyphenols, and minerals.[1]
Mexican traditional medicine widely recommends the consumption of nopal cladodes for the treatment of several diseases, one being diabetes. Several studies have reported the antidiabetic effect of Opuntia, for instance, the administration of steamed (Opuntia ficus indica) cladodes to diabetic patients reduced postprandial blood glucose and serum insulin peaks.[2] Similar results were obtained with Opuntia streptacantha, which decreased the hyperglycemic peak in healthy rabbits.[3]
These beneficial effects have been associated to several phytochemical compounds.[4] For instance, a petroleum ether extract of nopal cladodes exerted hypoglycemic effects in streptozotocin (STZ)-induced diabetic mice, which was related to their high content of phytosterols, polyunsaturated fatty acids, and phytol.[4] Furthermore, polysaccharides of O. monacantha cladodes improved blood glucose and serum lipid levels in STZ-induced diabetic rats.[5] Similarly, isorhamnetin glycosides-rich extract of nopal cladodes stimulated insulin secretion and improved blood lipid profile in obese mice, preventing the development of metabolic abnormalities associated to diet-induced obesity.[6]
The profile of phytochemicals and nutrients in plants is not only variety-dependent, it also depends on others factors such as cultivation conditions and physiological stage.[7] For instance, older cladodes showed a higher content of insoluble fiber, whereas soluble fiber decreases with increasing age. Additionally, it has been reported that protein and starch decreases in mature cladodes, whereas simple carbohydrates increase.[8,9]
We have previously evaluated the acute hypoglycemic effect of nopal cladodes at different maturity stages in healthy and diabetic rats, and small and medium cladodes (40–70 g) exerted the greatest beneficial effect on postprandial blood glucose as compare to large cladodes (293 g). This effect was associated to the higher fiber content in cladodes of early maturity stage, which increases the ability to form suspensions with higher viscosity, promoting glucose entrapment.[10] Nevertheless, a nutrimental and phytochemical profile is necessary to understand the differential hypoglycemic effect of nopal cladodes at different maturity stages. Therefore, the aim of this study is to determine the indigestible fraction content, as well as the phytochemical and low molecular weight metabolite profile of nopal cladodes at different maturity stages, and to associate their bioactive composition with their antioxidant and digestive enzymes inhibitory capacities.
Materials and Methods
Plant Material
Opuntia ficus-indica var. Milpa Alta was used in this study, which is the cactus specie of major economic relevance in the world.[1] Fresh cactus cladodes were collected at September 2012 from a commercial orchard located at El Refugio, Guanajuato, Mexico, and were identified by C. Mondragon Jacobo from the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Cladodes were classified according to their age (days) and size (weight and length) as young (12 days, 40 ± 10 g), medium (20 days, 74 ± 20 g), and old (30 days, 293 ± 70 g). All cladodes were in a maturity stage adequate for human consumption. Cladodes were washed with distilled water and disinfected using commercial 10% sodium hypochlorite solution, and thorns were manually removed with a stainless steel knife. Afterward, cladodes were diced (about 2 × 2 cm) and dehydrated in a tray drier at 40°C for 24 h. The dried material was ground using a mill with a mesh size of 80 (particle size <0.18 mm). Nopal flours were stored in sealed containers at room temperature and protected from light and oxygen to prevent oxidation.
Determination of Total Polyphenols and Flavonoids
Polyphenols extraction was performed according to Hassan.[11] Briefly, 0.5 g of dry sample were mixed with 20 mL of methanol/water acidified with HCl (50:50 v/v; pH 2), the mixture was shaken for 1 h at room temperature, centrifuged at 4000 × g for 10 min, and supernatants were recovered. Afterward, 20 mL of acetone/water (70:30 v/v) were added to the residue, and the mixture was shaken and centrifuged as previously described. Methanol and acetone extracts were mixed and used for the quantification of total polyphenols[12] and total flavonoids.[13] Results were expressed as milligrams of gallic acid equivalent/g of dry sample (mg GAE/g) and mg of catechin equivalent/g of dry sample (mg CE/g), respectively.
Determination of Condensed Tannins
Condensed tannins content was determined as described by Zurita[14] using the residue obtained in the methanol/acetone extraction. Samples (100 mg) were mixed with 6 mL of 95:5 n-buthanol/HCl and 0.2 mL of iron reagent (2% w/v ferric ammonium sulphate in 2 mol/L HCl). Mixtures were incubated in a boiling water bath for 50 min. Then, the mixture was adjusted to 25 mL with n-butanol/HCl, and absorbances were measured at 450 and 555 nm. Results were expressed as mg of proanthocyanidins equivalent/g of dry sample (mg PAE/g).
Determination of Hydrolyzable Tannins
The residue obtained in the methanol/acetone extraction (20 mg) was hydrolyzed with 2 mL of methanol and 200 μL of sulphuric acid (20 h, 85°C). Then, the methyl gallate released from the reaction was oxidized with potassium iodate (pH 5.5, 30°C), and the chromophore was measured at 525 nm. Results were expressed as mg of methyl gallate equivalent/g of dry sample (mg MGE/g).[15]
Determination of Total Phytosterols
Samples (1 g) were saponified with 15 M KOH in methanol (100 mL). This solution was heated at 80°C for 60 min. Then, 20 mL of water and 40 mL of hexane were added. Samples were centrifuged at 5000 × g during 5 min, and supernatants were recovered. Solvents were removed at 45°C with N2, and then 80 mL of isopropanol were added. An aliquot (300 µL) was mixed with 3 mL of free cholesterol kit reagent, containing cholesterol oxidase and peroxidase (Spinreact, Spain). Samples were incubated for 5 min at 37ºC, and absorbances were measured at 505 nm. Results were expressed as mg of β-sitosterol equivalents/g of dry sample (mg SE/g).
Determination of Total Saponins
Dry samples (5 g) were defatted with 50 mL of petroleum ether, then the solvent was evaporated to complete dryness, and samples were dissolved in 150 mL of 75% ethanol. Samples were subjected to reflux at 70°C for 4 h, and then the extract was filtered and evaporated at 40°C. The dried residue was extracted three times with 40 mL of n-butanol, the solvent was evaporated to dryness, and samples were dissolved in 25 mL of methanol. Finally, an aliquot (50 µL) were mixed with 0.2 mL of 5% vanillin (8% w/v), and 0.8 mL of sulfuric acid (72% v/v). The mixture was incubated at 70°C for 15 min, and then cooled on ice. Then, 5 mL of glacial acetic acid were added; and absorbances were measured at 550 nm. Results were expressed as mg sitosteryl 3β-D-glucopyranoside equivalents/g of dried sample (mg SGE/g).
Identification of Phytochemical Compounds
The phytochemical profile of nopal cladodes was assessed using an Agilent 1200 high performance liquid chromatography (HPLC)-diode array detector (DAD) system connected to a SL quadrupole mass spectrometer Agilent 1100 equipped with an electrospray interface, using a Phenomenex C18 column (250 mm × 4.6 mm, 5 µm). Mass spectrometer was operated in negative ion mode, using the following conditions: capillary voltage, 4000 V; nebulizer pressure, 40 psi; drying gas flow rate, 10 L/min; gas temperature, 300°C; skimmer voltage, 50 V; octapole, 150 V; and fragmentor voltage, 130 V. LC-MS accurate mass spectra were recorded across the range of m/z 50−1200. Two different systems were used for the separation of phytochemicals compounds.[16]
System I (Phenolic Acids and Flavonoids)
Samples were extracted with acidified methanol/water as previously described. Then, solvents were evaporated to dryness, and samples were dissolved in 200 μL of methanol. The mobile phase consisted of (A) acetic acid-water 2:98 and (B) acetic acid-water-acetonitrile 2:48:50 under gradient conditions. Absorbances were monitored at 280 nm (hydroxybenzoic acids and flavonols) and 320 nm (hydroxycinnamic acids and stilbenes).
System II (Phytosterols and Saponins)
Dried samples (0.5 g) were extracted with 4 mL of hexane during 6 h, and then the mixture was centrifuged at 19,000 × g for 5 min. The hexanic phase was recovered, evaporated to dryness, and dissolved in 200 μL of acetonitrile. The mobile phase consisted of (A) acetonitrile-acetic acid-methanol-water 48:2:25:25 and B) acetonitrile-acetic acid 98:2 under gradient conditions. Absorbances were measured at 203 nm.
Identification of Low Molecular Weight Metabolites
Samples (10 mg) were mixed with 1 mL of methanol, sonicated for 15 min, and centrifuged at 12,000 × g at 4°C for 10 min. Supernatants were recovered, filtered with 0.45 μm pore membranes, and concentrated with a nitrogen gas stream. Then, samples were derivatized with 50 µL of BSTFA (N,O-bis[Trimethylsilyl]trifluoroacetamide + 1% TMCS [trimethylchlorosilane]).
Samples (1 µL) were injected into an Agilent GC Series 7890A (Wilmington, DE, USA) coupled to an Agilent single quadrupole MS detector (Agilent 5975C), with electron energy set at 70 eV, recording across a mass range of 50–800 m/z. A HP‐5MS capillary column (30 m × 0.25 mm, 0.25 μm) was used. The injector temperature was set at 250°C in split-less mode. Initial oven temperature was 100°C, held for 1 min and raised at 6°C/min to 220°C, which was held for 1.23 min, then raised at 10°C/min to 290°C, after raised at 40°C/min to 310°C and held for 7.5 min. The flow rate of the carrier gas (helium) was maintained at 1 mL/min.[16] Preliminary compound identification was assessed with the National Institute of Standards and Technology (NIST) and Wiley electron impact mass spectral library search using the ChemStation (Agilent Technologies) software. Peaks with similarity index ≥70% were assigned compound names.
Determination of Non-Digestible Fraction
Soluble and insoluble indigestible fractions were determined following the procedure of Saura-Calixto.[17] Briefly, 300 mg of each sample were digested with 0.2 mL of pepsin (300 mg/mL solution in HCl-KCl 0.2 M buffer, pH 1.5, 1 h, 37°C) and 1 mL of α-amylase (120 mg/mL solution in tris-maleate buffer 0.1 M, pH 6.9, 16 h, 37°C). Samples were centrifuged (3000 × g for 15 min) and residues were dried and quantified gravimetrically. Supernatants were dialyzed against water for 48 h at 25°C. Then, dialysates were lyophilized and weighed to determine the content of soluble indigestible fraction. Results were expressed as g of fraction/kg of dry sample.
Free Radical Scavenging Assays
DPPH• and ABTS•+ free radical scavenging activities were measured in the polyphenolic extract as described by Brand-Williams[18] and Re,[19] respectively. Results were expressed as half maximum inhibitory concentration (IC50, μg/mL).
Digestive Enzyme Inhibition Assays
α-Amylase, α-glucosidase, and pancreatic lipase inhibitory activities were evaluated in the polyphenolic extract according to the methods described by Kandra,[20] Apostolidis,[21] and McDougall,[22] respectively.
Statistical Analysis
All results were expressed as mean values ± standard error (SE). Data were analyzed by one-way analysis of variance (ANOVA) and differences among treatments were determined by comparison of means using Tukey’s test. The level of statistical significance was considered at p < 0.05. Pearson correlation was used to evaluate associations between phytochemicals, antioxidant capacity, and digestive enzymes inhibitory activity. All statistical analyses were carried out with JMP software (v11.0, SAS Institute).
Results and Discussion
Phytochemical Profile
Phytochemicals exert anti-hyperglycemic properties through several mechanisms, such as protection against oxidative damage and inhibition of carbohydrate and lipid digestion.[23] Nevertheless, their content in plants depend of several factors such as variety, maturity, and cultivation conditions.[7] Therefore, we evaluated the phytochemical profile of O. ficus-indica cladodes harvested at different maturity stages.
The highest content of polyphenols and flavonoids were found in medium age cladodes, whereas old cladodes showed the lowest content of total polyphenols. Interestingly, medium-age cladodes presented 13 and 40% more polyphenols, and 33 and 41% more flavonoids than young and old cladodes, respectively (). The variation of polyphenols in fruit and vegetables during maturity may be attributed to changes in primary and secondary metabolism. Two different trends have been observed in polyphenols; a steady decrease or increase during maturation. However, these effects vary from plant to plant or even in different organs of the same plant.[24]
Table 1. Total phytochemicals content of O. ficus-indica cladodes at different stages of maturity.
The phytochemical profile of nopal cladodes at different maturity stages was assessed by HPLC-DAD-mass spectrometer detector (MSD). Regarding polyphenols, 15 phenolic acids and 13 flavonoids were identified (, ), from which only 11 have been identified previously in nopal cladodes.[25] Interestingly, different polyphenol profiles were observed in nopal cladodes harvested at different maturity stages. For instance, young and medium cladodes presented a higher content of several phenolic acids and flavonoids as compared to old cladodes, such as p-hydroxybenzoic acid (42%), p-coumaric acid (50%), ferulic acid (17%), rutin (33%), narcissin (31%), and nicotiflorin (37%). Moreover, several polyphenols were only identified in young and medium age cladodes, such as chlorogenic acid, protocatechuic acid, sinapic acid, rosmarinic acid, ellagic acid, procyanidins B1 and B2, gallocatechin gallate, and epicatechin gallate.
Table 2. Phytochemical profile of O. ficus-indica cladodes at different stages of maturity assessed by HPLC-DAD-MSD.
Figure 1. Representative chromatogram of A: Low molecular weight metabolites assessed by GC-MSD; B: phenolic compounds; C: saponins and phytosterols evaluated by HPLC-DAD-MSD of O. ficus-indica cladodes.
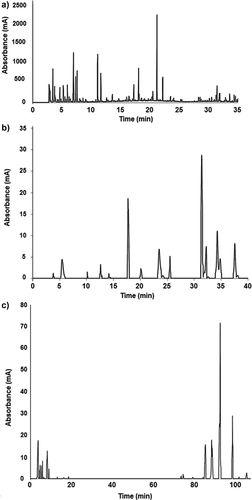
Interestingly, several compounds identified in cladodes of early maturation have been associated to beneficial health effects. For instance, chlorogenic acid has been reported to exert antidiabetic effects by delaying intestinal glucose absorption and inhibiting hepatic gluconeogenesis,[26] whereas gallocatechin gallate and epicatechin gallate-rich green tea extracts decrease triglyceride and insulin resistance in type 2 diabetic patients.[27]
On the other hand, tannins are polyphenols of high molecular weight with several health beneficial properties. These compounds are classified into two groups according to their monomer composition: hydrolysable tannins formed by phenolic acids, and condensed tannins or proanthocyanidins formed by flavonoids.[28] Interestingly, nopal cladodes of early stage maturation showed a higher content of condensed and hydrolysable tannins as compared to old cladodes (25–63 and 17–31%, respectively; ). Similar results were reported by Qudsieh,[29] who studied the effect of maturity stages on yellow cane, and found that tannins decreased rapidly during maturity, reaching a reduction of about 90%.
In addition to polyphenols, the health benefits of nopal cladodes can be attributed to other phytochemicals such as phytosterols and saponins.[30] Phytosterols are compounds that present similar structures to cholesterol, which vary only in carbon side chains and/or the presence or absence of a double bond.[31] On the other hand, saponins are compounds of high molecular weight formed by triterpene or steroid aglycones with one or more sugar chains.[32] Studies have shown that phytosterols and saponins exert health beneficial effects by decreasing blood lipids, improving blood glucose response, among others mechanisms.[31,32]
The highest concentration of phytosterols and saponins was found in medium age cladodes, whereas old cladodes presented the lowest content. Interestingly, medium age cladodes presented 7 and 30% more saponins, and 27 and 44% more phytosterols than young and old cladodes, respectively (, ). Similar results were reported by Lima,[33] who reported that steroidal saponins in Brachiaria spp. decreased with maturation, whereas Le Fur[34] found that young grape berries presented a higher content of phytosterols than older fruits. Decreased phytosterols content in old cladodes may be related to their conversion into steroidal hormones and vitamins, which regulate growth and development of immature tissues in plants.[35]
Six phytosterols were identified in nopal cladodes, such as β-sitosterol, Δ7-stigmasterol, and stigmastanol (). Interestingly, this is the first study that identify campestanol in nopal cladodes. Regarding the maturity stage, young and medium cladodes presented the highest amount of all individual phytosterols. Furthermore, medium cladodes showed a higher content of β-sitosterol as compared to young and old cladodes (32 and 66%, respectively). Interestingly, β-campesterol was identified only in nopal cladodes harvested at early maturity stages.
Similarly, Luo[4] tentatively identified sitosterol and stigmastanol in a petroleum ether extract of O. ficus-indica var. Milpa Alta; however, the effect of maturity on the phytosterol profile of nopal cladodes has not been evaluated previously. The greater antidiabetic effect of nopal cladodes harvested at early maturity as compared to old cladodes[10] may be related to their higher content of phytosterols, since Luo[4] reported that a phytosterol-rich extract of Opuntia cladodes decreased blood glucose levels in STZ-induced diabetic mice.
Additionally, four steroid saponins were identified in nopal cladodes, campesteryl-, stigmasteryl-, and sitosteryl-3-β-glucopyranoside, and isorhamnetin-3-O-rutinoside, which were found in greater amount in young and medium age cladodes (). Interestingly, the presence of saponins has been reported in a methanolic extract of O. monocantha cladodes;[36] nevertheless, the profile of these bioactive compounds has not been reported. Several studies have suggested that saponins exert several health benefits such as anti-carcinogenic, anti-hyperglycemic, and hypolypidemic effects.[31]
Low Molecular Weight Metabolites Profile
In addition to phytochemicals, nutritional compounds are affected during plants maturation.[37] Therefore, the profile of low molecular weight metabolites, such as amino acids, carbohydrates, organic acids, and fatty acids of nopal cladodes was assessed by GC-MSD (, ). Several studies have reported that young nopal cladodes present high levels of several amino acids, such as glutamine, serine, and valine.[38] However, the amino acid profile of older cladodes has not been reported previously.
Table 3. Low molecular weight metabolites of O. ficus-indica cladodes at different stages of maturity assessed by GC-MSD.
In this study, 12 amino acids were identified in nopal cladodes (). Interestingly, young and medium cladodes showed several amino acids that were not detected in old cladodes, such as alanine, isoleucine, and asparagine, whereas threonine was detected only in old cladodes. Furthermore, cladodes harvested at early maturation stages presented a higher content of most amino acids than older cladodes. Similarly, it has been reported that young nopal cladodes present a higher amount of protein than older cladodes,[10] which may be related to a higher metabolic activity in early maturity stages. In addition, nitrogen transport occurs from mature to young tissues.[39]
Regarding carbohydrates profile, two compounds were identified in nopal cladodes, glucose and arabinose (). Accordingly, Nobel[40] reported arabinose as the main sugar of O. ficus indica mucilage. Interestingly, arabinose was detected in higher amounts in old cladodes, whereas glucose was found in greater amount in young cladodes (). The rate of accumulation of these carbohydrates in plants relays on photosynthesis and respiration, which are influenced by several factors among them, growth and development.[41]
On the other hand, nine organic acids were identified in nopal cladodes (). Feitosa[42] reported malic acid as the majoritarian organic acid of nopal cladodes. Young cladodes presented several organic acids that were not identified in medium and old cladodes, such as malonic acid, lauric acid, and tartaric acid. Furthermore, young and medium cladodes presented a greater amount of glycolic acid, threonic acid, and citric acid as compared to old cladodes (25–44, 41–52, and 65–82%, respectively). Similar results were reported by Stintzing[38] who found a lower content of malonic, citric, and tartaric acid in old cladodes. Interestingly, medium and old cladodes showed a higher amount of succinic acid and malic acid as compared to young cladodes (up to 19 and 22%, respectively).
It has been reported that organic acids are decreased in plants during advanced maturation stages due to an increased membrane permeability, allowing acids to be stored in respiring cells. Furthermore, altered membrane permeability reduces organic acids translocation from leaves, as well as their synthesis.[43] Organic acids are primary metabolites that have been reported to exert beneficial effects against several oxidative diseases, such as diabetes, due to their antioxidant properties.[44]
Additionally, six fatty acids were identified in nopal cladodes, mainly 16 and 18 carbon-length compounds (). Young and medium cladodes presented a higher amount of palmitic acid as compared to old cladodes (22–26%). Interestingly, linoleic and α-linolenic acids were only identified in young and medium cladodes. Conversely, medium and old cladodes presented a higher amount of myristic and oleic acid as compared to young cladodes (3.7- and 1.8-fold, respectively). Accordingly, it has been reported that fat content decreases with increasing maturity.[9]
Indigestible Fraction Content
Nopal cladodes represent an important source of dietary fiber, mainly insoluble, which are considered partly responsible of nopal beneficial effects.[9] Recently, the quantification of dietary indigestible fraction (DIF) has been proposed as substitute for dietary fiber, since DIF includes dietary fiber and other components that reach colon, such as resistant starch, resistant proteins, tannins, and other associated compounds. Altogether, these compounds show resistance to the action of digestive enzymes and contribute to the health beneficial effects reported to dietary fiber.[17]
All nopal cladodes presented a higher content of insoluble DIF as compared to soluble DIF (). Interestingly, young and medium cladodes presented a higher content of soluble and insoluble DIF as compared to old cladodes (11–20 and 12–13%, respectively). It has been reported that the insoluble moiety reduces postprandial glycemia by several mechanisms, such as reduction of digestion rate and carbohydrates absorption. Furthermore, when DIF reaches colon, it serves as a substrate for fermentative microflora, producing short-chain fatty acids. These compounds have been reported to improve insulin sensitivity and to decrease hepatic glucose production.[45] On the other hand, DIF decreases cholesterol intestinal absorption by linking with biliary acids.[46]
Table 4. Indigestible fraction, antioxidant capacity, and inhibitory activity of digestive enzymes of O. ficus-indica cladodes at different stages of maturity.
Antioxidant Capacity
Antioxidant capacity is mainly related to compounds with the ability to counteract free radicals formation. ABTS and DPPH are assays widely used to determine foods antioxidant capacity, and these assays evaluate their ability to donate electrons and hydrogen atoms, respectively.[47] It has been proposed that foods and raw plant extracts with IC50 values <50 µg/mL exert a high antioxidant capacity.[48] Therefore, nopal cladodes could be considered with high antioxidant capacity (). Interestingly, nopal cladodes exhibited a higher capacity to donate electrons than hydrogen atoms, since lower IC50 values were observed in ABTS assay.
The antioxidant capacity of nopal cladodes may be related to their phytochemical composition; therefore, correlations between bioactive compounds and antioxidant capacity were assessed (). Total polyphenols presented a significant correlation with 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay, whereas total flavonoids were correlated with 2,2- diphenyl-1-picrylhydrazyl (DPPH) and ABTS assays. Interestingly, no significant correlation was found between the antioxidant capacity and tannins. Conversely, it has been reported that tannins show a high radical scavenging activity due to their high number of hydroxyl groups.[49]
Table 5. Correlations between phytochemicals, antioxidant capacity, and digestive enzymes inhibitory activity O. ficus-indica cladodes at different stages of maturity.
Furthermore, nopal antioxidant capacity was correlated with several individual phenolic acids (chlorogenic, caftaric, coutaric, p-coumaric, sinapic, ferulic, and rosmarinic acids) and flavonoids (procyanidins B1, epicatechin gallate, rutin, epigallocatechin gallate, quercetin-3-O-glucoside, coumestrol, and narcissin). Additionally, several phytosterols, such as β-sitosterol and stigmasterol, and one saponin, sitosteryl 3β-glucopyranoside, were correlated with ABTS scavenging activity. Interestingly, several phytochemicals that showed a significant correlation with antioxidant capacity were found in greater concentration in young and medium cladodes.
Digestive Enzymes Inhibitory Activity
Several phytochemicals identified in nopal cladodes, such as polyphenols and saponins, have been reported to inhibit digestive enzymes involved in the breakdown of lipids and carbohydrates, leading to positive effects on obesity and blood glucose control.[50,51] Therefore, the capacity of nopal cladodes to inhibit carbohydrate digestive enzymes, such as α-amylase and α-glucosidase, was evaluated. Pancreatic α-amylase is involved in starch hydrolysis to oligosaccharides, which are further hydrolyzed to glucose and other monosaccharides by intestinal α-glucosidases. Therefore, the inhibition of these enzymes may result in the delay of glucose intestinal digestion and absorption.[52]
All nopal cladodes showed inhibitory activity against both carbohydrate digestive enzymes, presenting a maximum inhibition of 55–65% against α-amylase and ~25% against α-glucosidase (data not shown), presenting IC50 values of 13.5–28.7 µg/mL (). Interestingly, medium and old cladodes showed a greater inhibitory activity against α-amylase and α-glucosidase, as observed in lower IC50 values than young cladodes (26–37 and 30–35%, respectively; ). Acarbose, a drug widely used to inhibit these digestive enzymes, showed a maximum inhibition of 81 and 89%, respectively, presenting IC50 values of 5.4 and 3.9 µg/mL, respectively (data not shown). Therefore, nopal cladodes exhibit a low inhibitory activity against these enzymes as compared to acarbose.
Becerra-Jiménez[53] reported that water, water–ethanol, and acidified ethanol extracts of O. streptacantha cladodes showed no inhibitory activity against α-glucosidase. These discrepancies may be related to the use of different plant species, growth conditions, and maturity stage during harvest, since these parameters affect the content and composition of bioactive metabolites.[7,54]
Nopal cladodes inhibitory activity against pancreatic lipase was also evaluated. This enzyme hydrolyzes dietary triglycerides into fatty acids, which are further absorbed. Therefore, its inhibition may lead to decreased serum triglyceride levels.[22] All nopal cladodes showed an inhibitory activity against pancreatic lipase, with IC50 values of 16–25 µg/mL (), presenting a maximum inhibition of 38–45% (data not shown). This is the first study that reports the ability of nopal cladodes to inhibit this digestive enzyme. Interestingly, young and medium cladodes exhibited a higher capacity to inhibit this enzyme as compared to old cladodes (38 and 54%, respectively). Orlistat is a drug widely used to inhibit lipid absorption through lipase activity inhibition, and showed a maximum inhibition of 86% and IC50 values of 9.06 µg/mL (data not shown). Therefore, nopal cladodes exert a low inhibitory activity against this enzyme as compared to orlistat.
The inhibitory activity of nopal cladodes against α-amylase and α-glucosidase showed a significant correlation with condensed tannins content, as well as several individual phenolic acids and flavonoids, such as coutaric acid, syringic acid, epicatechin, and quercetin-3-O-glucoside (). Regarding pancreatic lipase inhibition, significant correlations were observed with hydrolizable tannins, and most phenolic acids and flavonoids identified in this study. Furthermore, several phytosterols, such as β-campesterol, campestanol, Δ5-avenasterol, and stigmastanol, as well as all saponins identified in this work, showed a significant correlation with pancreatic lipase inhibition assay ().
Conclusion
The maturity stage of nopal (O. ficus-indica) cladodes at harvest plays an important role in their nutrimental and phytochemical profile, which may be reflected in their health beneficial properties. Medium age nopal cladodes (20-days-old) showed a high inhibitory activity against α-amylase, α-glucosidase, and pancreatic lipase, which was related to their content of several polyphenols, phytosterols and saponins. On the other hand, young cladodes showed the highest concentration of tannins, as well as amino acids, organic acids, and fatty acids, whereas old cladodes showed the lowest content of bioactive compounds and exerted a low antioxidant capacity and in vitro digestive enzymes inhibitory activity. Therefore, this study could be useful to determine the optimal maturity stage to harvest nopal cladodes to increase their nutraceutical potential.
References
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and Medicinal Use of Cactus Pear (Opuntia spp.) Cladodes and Fruits. Frontiers in Bioscience 2006, 1, 2574–2589.
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torres, N. The Effect of Nopal (Opuntia Ficus Indica) on Postprandial Blood Glucose, Incretins, and Antioxidant Activity in Mexican Patients with Type 2 Diabetes after Consumption of Two Different Composition Breakfasts. Journal of the Academy of Nutrition and Dietetics 2014, 114, 1811–1818.
- Roman-Ramos, R.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Anti-Hyperglycemic Effect of Some Edible Plants. Journal of Ethnopharmacology 1995, 48, 25–32.
- Luo, C.; Zhang, W.; Shenga, C.; Zheng, C.; Yao, J.; Miaoa, Z. Chemical Composition and Antidiabetic Activity of Opuntia Milpa Alta Extracts. Chemistry & Biodiversity 2010, 7, 2869–2879.
- Yang, N.; Zhao, M.; Zhu, B.; Yang, B.; Chenb, C.; Cui, C.; Jiang, C. Anti-Diabetic Effects of Polysaccharides from Opuntia Monacantha Cladode in Normal and Streptozotocin-Induced Diabetic Rats. Innovative Food Science and Emerging Technologies 2008, 9, 570–574.
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Díaz, A.M.; Antunes-Ricard, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A.; Serna-Saldivar, S.O.; Tovar, A.R. The Effect of Isorhamnetin Glycosides Extracted from Opuntia Ficus-Indica in a Mouse Model of Diet Induced Obesity. Food & Function 2015, 6, 805–815.
- Cohen, S.; Kennedy, J. Plant Metabolism and the Environment: Implications for Managing Phenolics. Food Science & Nutrition 2010, 50, 620–643.
- Hernández-Urbiola, M.I.; Contreras-Padilla, M. Study of Nutritional Composition of Nopal (Opuntia Ficus Indica cv. Redonda) at Different Maturity Stages. The Open Nutrition Journal 2010, 4, 1–6.
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia Ficus Indica) at Varied Ages in an Organic Harvest. International Journal of Environmental Research and Public Health 2011, 8, 1287–1295.
- Nuñez-López, M.A.; Paredes-López, O.; Reynoso-Camacho, R. Functional and Hypoglycemic Properties of Nopal Cladodes (O. Ficusi Indica) at Different Maturity Stages Using in Vitro and in Vivo Tests. Journal of Agricultural and Food Chemistry 2013, 61, 10981–10986.
- Hassan, F.; Ismail, A.; Abdulhamid, A.; Azlan, A. Identification and Quantification of Phenolic Compounds in Bambangan (Mangifera Pajang Kort.) Peels and Their Free Radical Scavenging Activity. Journal of Agricultural and Food Chemistry 2011, 59, 9102–9111.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic and Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–153.
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical Activity and Polyphenol Composition of Local Brassicaceae Edible Varieties. Food Chemistry 2006, 99, 464–469.
- Zurita, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Improved Procedure to Determine Non-Extractable Polymeric Proanthocyanidins in Plant Foods. International Journal of Food Sciences and Nutrition 2012, 63, 936–939.
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of Hydrolyzable Tannins (Gallotannins and Ellagitannins) after Reaction with Potassium Iodate. Journal of Agricultural and Food Chemistry 2002, 50, 1785–1790.
- Lomas-Soria, C.; Pérez-Ramírez, I.F.; Caballero-Pérez, J.; Guevara-Gonzalez, R.G.; Guevara-Olvera, L.; Loarca-Piña, G.; Guzman-Maldonado, H.S.; Reynoso-Camacho, R.; Cooked Common Beans (Phaseolus Vulgaris L.) Modulate Renal Genes in Streptozotocin-Induced Diabetic Rats. The Journal of Nutritional Biochemistry 2015, 26, 761–768.
- Saura-Calixto, F.; García-Alonso, A.; Goñi, I.; Bravo, L. In Vitro Determination of the Indigestible Fraction in Foods: An Alternative to Dietary Fiber Analysis. Journal of Agricultural and Food Chemistry 2000, 48, 3342–3347.
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Science and Technology 1995, 28, 25–30.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology & Medicine 1999, 26, 1231–1237.
- Kandra, L.; Zajacz, A.; Remenyik, J.; Gyemant, G. Kinetic Investigation of a New Inhibitor for Human Salivary Alpha-Amylase. Biochemical and Biophysical Research Communications 2005, 34, 824–828.
- Apostolidis, E.; Kwon, Y.; Shetty, K. Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese Against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innovative Food Science and Emerging Technologies 2007, 8, 46–54.
- McDougall, G.; Kulkarni, N.; Stewart, D. Berry Polyphenols Inhibit Pancreatic Lipase Activity in Vitro. Food Chemistry 2009, 115, 193–199.
- Ali, A. Anti-Diabetic Potential of Phenolic Compounds: A Review. International Journal of Food Properties 2013, 16, 91–103.
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of Maturity on Phenolics (Phenolic Acids and Flavonoids) Profile of Strawberry Cultivars and Mulberry Species from Pakistan. International Journal of Molecular Sciences 2012, 13, 4591–4607.
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.; De León, Á.; Fomsgaard, I.S.; De la Rosa, A.B. Proximate Composition, Phenolic Acids, and Flavonoids Characterization of and Wild Nopal (Opuntia spp.). Journal of Food Composition and Analysis 2010, 23, 525–532.
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic Acid Stimulates Glucose Transport in Skeletal Muscle Via Ampk Activation: A Contributor to the Beneficial Effects of Coffee on Diabetes. PLOS One 2012, 7, e32718.
- Chia-Yu, L.; Chien-Jung, H.; Lin-Huang, H.; I-Ju, C.; Jung-Peng, C.; Chung-Hua, H. Effects of Green Tea Extract on Insulin Resistance and Glucagon-Like Peptide 1 in Patients with Type 2 Diabetes and Lipid Abnormalities: A Randomized, Double-Blinded, and Placebo-Controlled Trial. PLOS One 2014, 9, e91163.
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Critical Reviews in Food Science and Nutrition 1998, 38, 421–464.
- Qudsieh, H.Y.; Yusof, S.; Osman, A.; Rahman, R.A. Effect of Maturity on Chlorophyll, Tannin, Color, and Polyphenol Oxidase (PPO) Activity Of Sugarcane Juice (Saccharum Officinarum Var. Yellow Cane). Journal of Agricultural and Food Chemistry 2002, 50, 1615–1618.
- El-Mostafa, K.; El-Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El-Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia Ficus-Indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901.
- Jones, P.J.; AbuMweis, S.S.; Phytosterols as Functional Food Ingredients: Linkages to Cardiovascular Disease and Cancer. Current Opinion in Clinical Nutrition & Metabolic Care 2009, 12, 147–151.
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S.; Phytochemical and Pharmacological Properties of Gymnema Sylvestre: An Important Medicinal Plant. BioMed Research International 2014, ID 830285.
- Lima, F.G.; Haraguchi, M.; Pfister, J.A.; Guimaraes, V.Y.; Andrade, D.F.; Ribeiro, C.S.; Costa, G.L.; Araujo, A.L.; Fioravanti, M.S. Weather and Plant Age Affect the Levels of Steroidal Saponin and Pithomyces Chartarum Spores in Brachiaria Grass. International Journal of Pharmacognosy and Phytochemical Research 2013, 2, 45–53.
- Le Fur, Y.; Hory, C.; Bard, M.; Olsson, A. Evolution of Phytosterols in Chardonnay Grape Berry Skins During Last Stages of Ripening. Vitis 1994, 33, 127–131.
- Jang, M.S.; Han, K.S.; Kim, S.K. Identification of Brassinos-Teroids and Their Biosynthetic Precursors from Seeds of Pumpkin. Bulletin of the Korean Chemical Society 2000, 21, 161–164.
- Nadeem, M.B.; Zubair, M.; Rizwan, K.; Rasool, N.; Hussain, L.B.; Akram, S.; Hussain, T.B.; Shahid, M.; Hameed, M.; Uddin, V.A. Biological Activities of Opuntia Monacantha Cladodes. Journal of the Chemical Society of Pakistan 2012, 34, 990–995.
- Khawas, P.; Deka, S.C. Comparative Nutritional, Functional, Morphological and Diffractogram Study on Culinary Banana (Musa ABB) Peel at Various Stages of Development. International Journal of Food Properties 2016, 19, 289–299.
- Stintzing, F.C.Carle;, R. Cactus Stems (Opuntia spp.): A Review on Their Chemistry, Technology, and Uses. Molecular Nutrition & Food Research 2005, 49, 175–194.
- Nobel, P.S. Nutrient levels in Cacti-Relation to Nocturnal Acid Accumulation and Growth. American Journal of Botany 1983, 70, 1244–1253.
- Nobel, P.S.; Cavelier, J.; Andrade, J.L. Mucilage in Cacti: Its Apoplastic Capacitance Associated Solutes, and Influence on Tissue Water Relations. Journal of Experimental Botany 1992, 43, 641–648.
- Fulkerson, W.J.; Donaghy, D.J. Plant-Soluble Carbohydrate Reserves and Senescence—Key Criteria for Developing an Effective Grazing Management System for Ryegrass-Based Pastures: A Review. Australian Journal of Experimental Agriculture 2001, 41, 261–275.
- Feitosa, F.F.; Warren, J.S.; Brown, W.H.; Whiting, F.M. Amino and Organic Acids of the Prickly Pear Cactus (Opuntia Ficus Indica L.). Journal of the Science of Food and Agriculture 1984, 35, 421–425.
- Glewa, R.H.; Ayazb, F.A.; Sanzc, C.; VanderJagta, D.J.; Huangd, H.S.; Chuangd, L.T.; Strnade, M. Changes in Sugars, Organic Acids and Amino Acids in Medlar (Mespilus Germanica L.) During Fruit Development and Maturation. Food Chemistry 2003, 83, 363–369.
- Oliveira, A.; Pereira, J.; Andradec, P.; Valentãoc, P.; Seabrac, R.; Silva, B. Organic Acids Composition of Cydoniaoblonga Miller Leaf. Food Chemistry 2008, 111, 393–399.
- Higgins, J.A. Whole Grains, Legumes, and the Subsequent Meal Effect: Implications for Blood Glucose Control and the Role of Fermentation. Journal of Nutrition and Metabolism 2012, 829238. doi: 10.1155/2012/829238
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53, 4290–4302.
- Muñoz, U.; Atha, D.E.; Ma, J.; Nee, M.H.; Kennelly, E. Antioxidant Capacities of Ten Edible North American Plants. Phytotherapy Research 2002, 16, 63–65.
- Riedl, K.M.; Carando, S.; Alessio, H.M.; McCarthy, M.; Hagerman, A.E. Antioxidant Activity of Tannins and Tannin-Protein Complexes: Assessment in Vitro and in Vivo. Free Radicals in Food. In Chemistry, Nutrition, and Health Effects; Morello, M.J.; Shahidi, F.; Ho, C.h.; Ed.; 2009, 14, 188–200.
- Hu, X.; Wang, S.; Xu, J.; Wang, D.; Chen, Y.; Yang, G. Triterpenoid Saponins from Stauntonia Chinensis Ameliorate Insulin Resistance Via the Amp-Activated Protein Kinase and IR/IRS-1/PI3 K/AKT Pathways in Insulin-Resistant HepG2 Cells. International Journal of Molecular Sciences 2014, 15, 10446–10458.
- Rideout, T.C.; Ramprasath, V.; Griffin, J.D.; Browne, R.W.; Harding, S.V.; Jones, P.J. Phytosterols Protect Against Diet-Induced Hypertriglyceridemia in Syrian Golden Hamsters. Lipids in Health and Disease 2014, 13, 5.
- Po-Hsien, L.; Yu-Wen, L.; Wen-Chien, L.; Jyh-Ming, H.; Da-Wei, H. In Vitro Hypoglycemic Activity of the Phenolic Compounds in Longan Fruit (Dimocarpus Longan Var. Fen Ke) Shell Against α-Glucosidase and β-Galactosidase. International Journal of Food Properties 2016, 19, 1786–1797.
- Becerra-Jiménez, J.; Andrade-Cettob, A. Effect of Opuntia Streptacantha Lem. on α-Glucosidase Activity. Journal of Ethnopharmacology 2012, 139, 493–496.
- Herrera-Hernández, M.G.; Guevara-Lara, F.; Reynoso-Camacho, R.; Guzmán-Maldonado, H. Effects of Maturity Stage and Storage on Cactus Berry (Myrtillocactus Geometrizans) Phenolics, Vitamin C, Betalains and Their Antioxidant Properties. Food Chemistry 2011, 129, 1744–1750.
