ABSTRACT
In this study, graphene oxide was derivative with 5-aminoisophtalic acid by amidization reaction. The nanomaterial in suspension was denoted as graphene oxide-isophtalic acid. The graphene oxide-isophtalic acid suspension was covered on the glassy carbon electrode surface under the infrared lamb. The graphene oxide was characterized with transmission electron microscopy and x-ray diffraction. Surface characterization of the glassy carbon/graphene oxide-isophtalic acid was performed with x-ray photoelectron spectroscopy, cyclic voltammetry, and electrochemical impedance spectroscopy. The ultrasensitive nanoplatform for the simultaneous electrochemical square-wave anodic stripping voltammetry assay of Bi3+ and Cd2+ in aqueous solution has been developed on the glassy carbon/graphene oxide-isophtalic acid. The linearity range of Bi3+ and Cd2+ were 1.0×10−8 – 1.0×10−12 M (S/N = 3). The responses of species were practically unaltered with the increase of various species concentration. The detection limits of Cd2+ and Bi3+ were determined as 8.1×10−13 M and 1.06×10−13 M, respectively. The electrode performance was checked with tap water and commercially milk samples.
Introduction
Cadmium is an extremely toxic metal, it is an anti-corrosion metal and is used extensively in electroplating. Cadmium is also used in industrial paints and may present a hazard when sprayed.[Citation1] The carcinogenic effect of cadmium has been proven by scientists.[Citation2] According to researchers, cadmium and cadmium salts are very dangerous, that often stem from interference with some metabolic processes.[Citation3] Despite the high toxicity of the element, cadmium is widely used in various industry such as electroplating, paint and polymer, glass, printing inks, alloys, Ni-Cd batteries, solar cells, automotive, and control rods in nuclear reactors. The source of the cadmium pollution are generally phosphate fertilizers, sewage sludge, and various industrial products such as Ni-Cd batteries, plating, pigments, and plastics.[Citation4] Smoking cigarettes is the one of the largest sources of cadmium exposure. If the Cd2+ concentration is up to 15 µg/L in urine and 0.15 µg/L in human plasma, serious toxicity problems will be shown. Chronic disorders, gastrointestinal scintigraphy problems, and damage in liver are observed after long-time interaction with cadmium. A typical example that everybody knows that itai-itai disease is shown in Japan, where rice that was grown in cadmium contaminated irrigation water is consumed.[Citation5–Citation7]
In the case of bismuth, is well known as a natural diamagnetic and has the lowest thermal conductivity properties. This metal is generally very useful in various alloys, electrode and catalyst materials, ceramics, cosmetic and pharmaceutical raw materials, magnets, the paint industry, electronics, and x-ray diagnostic media.[Citation8,Citation9] The one of the important bismuth toxicity sources is an increasing use of bismuth alloys in recent years. Researchers claimed that bismuth shows a low toxicity according to other heavy metals. Bismuth destroys or denatures these groups of enzymes. The low toxicity of bismuth is connecting to insolubility of bismuth salts in the water. Despite this, the low toxicity of bismuth, also known as an environmental element of bismuth, chronic poisoning can be dangerous when interacting with bismuth.[Citation10]
Up until now, there been articles reporting for the electrochemical detection of Cd2+ and Bi3+. Ouyang et al. prepared the carbon nanotube modified with Hg–Bi included electrode nanomaterial on glassy carbon (GC) electrode for simultaneous analysis of Zn2+, Cd2+, and Pb2+.[Citation11] They calculated that the limits of detection for Zn2+, Cd2+ were lower than 2 ppb. Luo and co-workers studied an electrochemical method for the determination of Cd (II) using a bismuth derivative multi-walled carbon nanotubes doped carbon paste electrode with a detection limit of 0.3 ppb.[Citation12] Wei et al. prepared highly sensitive nanosensor for Pb2+ and Cd2+ by using SWV on porous magnesium oxide/nafion-based nanostructure modified GC. Detection limits of the developed methods were calculated as 2.1 pM for Pb2+ and 81 pM for Cd2+ with the linear calibration curves range from 3.3 to 22 nm and 40 to 140 nM for Pb2+ and Cd2+, respectively[Citation13] Mandil and co-workers fabricated the screen-printed electrode by modifying the carbon ink surface with multi-walled carbon nanotube (MWCNT) and bismuth film. They reported the limit of detection as 1.5 nM for Cd2+ with a 120 s deposition time.[Citation14] Afkhami et al. investigated a new highly sensitive simultaneous nanosensor for Pb2+ and Cd2+ by using square wave voltammetry (SWV) with MWCNT and a schiff base modified carbon paste electrode. They found the limit of detection as 0.74 ppb for Cd2+.[Citation15] Xu et al. prepared ultrasensitive nanosensor for Pb2+ and Cd2+ on MWCNT/Nafion/Bismuth composite GC electrode. They reported the limit of detection as 40 ng/L for Cd2+.[Citation16] Ashrafi and Vytřas developed to determine trace Bi3+ by using SWV on the Sb covered carbon paste electrode which was a detection limit of 1.55 ppb.[Citation17] Khaloo et al. analyzed the Bi3+ and Cu2+ on hanging mercury drop electrode (HMDE) by using differential pulse voltammetry (DPV). The detection limits were determined as 0.05 ppb for Bi3+.[Citation18] Lexa and Stulík prepared a tri-n-octylphosphinic oxide in a poly(vinyl chloride) modified mercury film electrode for Bi3+ and Cu2+. They determined from 0.002 to 0.5% of bismuth.[Citation19]
Graphene has been known as a novel carbon allotrope since 2004.[Citation20] Its structure is a flat monolayer of sp2-carbon atoms attached and arranged in a honeycomb lattice.[Citation21] Graphene oxide (GO) is a state of graphene-containing functional groups such as –OH, C=O, -COOH, etc. The single atomic layer carbon material has got high surface area, good thermal conductivity, outstanding electrical conductivity, and perfect optical properties.[Citation22] The graphene-based nanomaterials have found their way into various technological applications such as fuel cells,[Citation23] batteries/capacitors,[Citation24,Citation25] sensor studies,[Citation26–Citation34] nanocatalyzer,[Citation35] electronic devices,[Citation36] food,[Citation37] and medicine studies.[Citation38]
Recently, electrochemically[Citation39–Citation41] or self-ordered[Citation42] modified electrodes[Citation43–Citation46] are very popular because of its remarkable properties such as high sensitivity,[Citation47–Citation51] simplicity,[Citation52] low cost, and ease.[Citation53] In this study, we developed a different method to determine both Cd (II) and Bi (III) at the same time. The GC electrode was modified with GO, and then isophtalic acid terminated on GO modified GC electrode (GC/GO) for determination Cd (II) and Bi (III) nanosensor. The effects of some parameters were investigated to determination of Cd (II) and Bi (III) as pH, deposition time, and suspension solution volume. The nanomaterials were characterized by using transmission electron microscopy (TEM), x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). The GC/GO-IPA electrode was utilized for the determination of Cd2+ and Bi3+ ions by square-wave anodic stripping voltammetry (SWASV). The electrode response performance was checked via tap water and commercial milk samples.
Materials and methods
All highest purity chemicals were gathered from Merck, Sigma-Aldrich, Fluka, or Riedel chemical companies. The water was used as ultrapure water (UPW) with a resistance of 18.3 MΩ (Human Power 1+ purification system, S. Korea) for solutions and electrode cleaning. In all electrochemical experiments were studied under the purified Argon gas (99.999%) atmosphere. Electrochemical experiments were performed at room temperature (25 ± 1ºC) under a traditional three-electrode cell system for all electrochemical experiments. Ag/Ag+(10 mM AgNO3) in 0.1 M tetrabutylammonium tetrafluoroborate (NBu4BF4) in acetonitrile (CH3CN) reference electrode was used in non-aqueous media. Ag/AgCl/KCl(sat) reference electrode was used in aqueous media. A platinum wire was employed as a counter electrode. GC electrode (Bioanalytical Systems, MF-2012, USA) were used in electrochemical characterization such as CV and EIS. Electrochemical application of nanomaterial was performed on the same GC electrode. The GC electrode was cleaned and polished with 100 and 50 nm Al2O3 suspension (Baikowski Int. Corp., USA) on polishing cloth (Buehler, Lake Bluff, IL, USA) for approximately 5 min and then washed with UPW. Polished GC electrodes were sonicated (Ultrasonic Cleaner, SK1200H, China) in UPW for approximately 5 min in the following sonication GC electrodes were dipped in to mixture of 1:1 (v/v) isopropyl alcohol/CH3CN media.
Preparation of GO
GO nanoparticles were synthesized by using electrochemical exfoliation of graphite. As anode pristine graphite, as cathode a platinum foil and as the electrolyte solution a mixture of 2.4 g H2SO4 and 11 mL 30% KOH in 100 mL deionized water were used. The electrochemical oxidation was performed under the constant DC (Yıldırım Electronics, Turkey) potential of +20 V. The GO was sonicated with a Hermle (Z36HK, Germany) ultracentrifuge system under 10,000 rpm for 10 min and then washed with UPW. It was dried at 100°C for 10 min.
Nanoconstruction of GO-IPA and preparation of GC/GO-IPA
Two hundred milligrams of GO was added in 10 mM 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide/N-hydroxysulfosuccinimide (EDC/NHS) solution in 25 mL phosphate buffer solution (PBS; pH = 7) and stirred via magnetic stirring for 8 h. The GO suspension was participated with the centrifuge and then washed with UPW. The activated GO was added in 1 mM 5-aminoisophthalic acid included PBS (pH = 7) solution and then stirred for 8 h. The GO-IPA suspension was sonicated and washed three times. It was dried at 80°C for 1 day under vacuum. GO-IPA was diluted with 10 mL CH3CN as suspension solution. Bare GC electrodes were polished with Al2O3 slurry on a Buehler cloth. They were rinsed in an ultrasonic bath with isopropyl alcohol-CH3CN mixture (1:1, v/v) and UPW for 5 min, respectively. The bare GC electrode was modified with 10 μL of sonicated GO-IPA solution by micro-syringe, and then dried under an infrared (IR) lamp for 10 min. The modified electrode was denoted as GC/GO-IPA.
Characterization of the nanomaterials
The GO was characterized by using a JEOL 2100 HRTEM instrument (JEOL Ltd., Tokyo, Japan) and v. a Rikagu Miniflex XRD, using mono-chromatic Cu Kα radiation and operating at a voltage of 30 kV and a current of 15 mA. The XPS data was acquired a PHI 5000 Versa Probe (Φ ULVAC-PHI. Inc., Japan/USA) model XPS with monochromatized Al Kα radiation source (1486.6 eV) as an x-ray anode operated with 50 W at 10−Citation7 Pa. GC/GO-IPA nanofilm were characterized by using the XPS. Electrochemical measurements were performed using an Ivium compactstat potentiostat (Ivium Technologies, The Netherlands) equipped with C3 cell stand. The CV and EIS characterization of the modified electrode was carried out in the presence of 2.0 mM of Fe(CN)63-/4- in 100 mM KCl as redox probe couple.
Determination of Cd2+ and Bi3+
Immersing of the GC/GO-IPA into the measuring solution containing Cd2+ and Bi3+ ions led to the accumulations of the ions on the modified electrode surface via complexation between the cations and the modified surface in a 0.1 M acetate buffer solution (pH 4.5). After accumulation of the ions, the modified electrode was taken off and washed with UPW. Before application the SWASV, metal ions on the modified electrode were reduced to metallic forms at –1.0 V under the constant potential for 10 s in a measuring 0.1 M acetate buffer blank solution (pH 4.5). The GC/GO-IPA electrode was used for the determination of Cd2+ and Bi3+ ions in tap water and milk samples. The samples were used as received the pH for all samples were adjusted using 0.1 M acetate buffer solution. Additionally milk samples were 1:1 (v/v) diluted with UPW. The measurements of the samples were repeated five times (n = 5).
Results and discussion
To determine the optimal condition for maximum signal (current) of Cd (II) and Bi (III), some analytical parameters including pH, deposition time, and suspension solution volume were examined.
Characterization of the GO and GC/GO-IPA
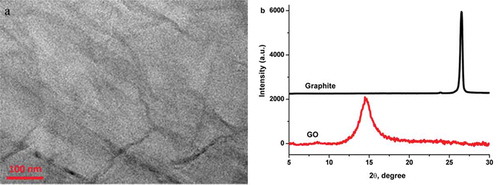
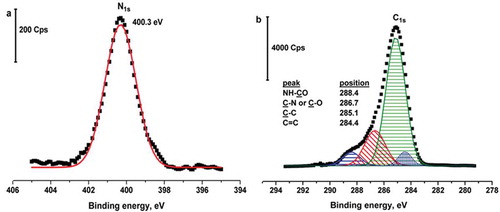
TEM imaging of GO is shown in . The exfoliated nanosheets are shown in the figure. The graphite and graphene oxide (GO) were characterized by XRD, as shown in . The (002) peak at 26.9º for pristine graphite is shown in . The (002) peak of GO is shifted to 14.6º in the figure.[Citation54] High-resolution XPS-core spectra of N1s and C1s of the GC/GO-IPA are shown in and , respectively. N1s binding energies of amide’s nitrogen (NH-C=O) included GC/GO-IPA was fitted at 406.3 eV.[Citation39] The C1s binding spectra for the GC/GO-IPA were deconvoluted into four peaks, which were NH-C=O at 288.4 eV, C=O or C-N, 286.7 eV, C-C at 285.1 eV and C=C at 284.4 eV in .[Citation55]
Bare GC and GC/GO-IPA surface were characterized using 2 mM Fe(CN)63-/4- redox couple solution in 100 mM KCl aqueous media and the resulting voltammograms were shown in . Cyclic voltammograms of the redox couple gave strong evidence that GO-IPA nanomaterial was formed on the GC surfaces, which decreased the electron transfer of the redox probe couple.
Figure 3. A: Cyclic voltammograms and B: Nyquist plots of 2 mM Fe(CN)63-/4- on the bare GC electrode and GC/GO-IPA.
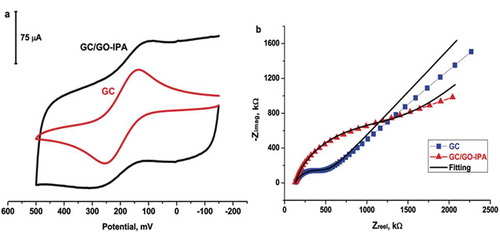
The Nyquist plots of the GC and GC/GO-IPA are shown in . Nyquist plots were obtained at 300,000 Hz/0.1 Hz frequency range using a redox couple of 2 mM Fe(CN)63-/4- redox couple (1:1) in 100 mM KCl media by using EIS. DC potential was determined as 0.18 V by CV. In , the Nyquist plots for 2.0 mM Fe(CN)63-/2.0 mM Fe(CN)64- redox probe in 100 mM KCl at the bare GC and GC/GO-IPA surfaces are shown. The acquired data were fitted to a constant phase element (CPE; Yo and α values) circuit with diffusion (i.e., Randles equivalent circuit with a Warburg impedance element, Zw). The impedimetric values are shown in . The supported electrolyte included solution resistance (Rs) was determined between 129–134 Ω. The charge transfer resistances (Rct) of the redox couple at the GC and GC/GO-IPA were 368.9 ± 11.40 and 1.331 ± 0.06 kΩ, respectively, calculated from fitting the curves to the equivalent circuit. The Rct of redox couple probes for GC/GO-IPA was fitted to a higher valued curve than the bare GC. Fitting of the Nyquist datas of the redox couple gave strong evidence that GC/GO-IPA surface was formed on the GC surfaces, which increase the charge transfer resistant of the redox couple.
Table 1. Fitting values of EIS data for 2 mM Fe(CN)63-/4- redox probe couple on bare GC and GC/GO-IPA in 100 mM KCl supported electrolyte.
Effect of pH, deposition time, and suspension solution volume
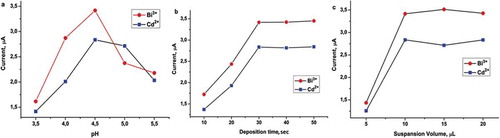
The effect of pH, deposition time and suspension solution volume on the analysis systems was investigated for the range from 3.50 to 5.50, 10–50 s, 5–20 µL, respectively. The results are illustrated in . The maximum signals were observed at pH 4.5 for Cd(II) and Bi(III) analyst (). Optimum deposition time for this method was obtained to be 30 s, and then observed signal was constant by 50 s for all analytes (). Also suspension volumes were performed range of 5–20 and 10 µL suspension volume qualified for maximum signal of Cd (II) and Bi (III) ions (). The optimum parameters were used to remainder experiments.
Influence of different concentrations on the SWASV of Cd (II) and Bi (III)
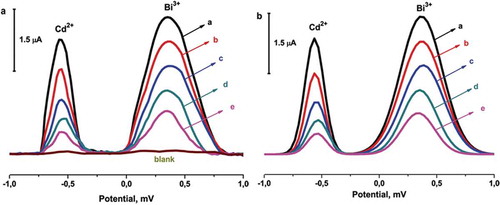
presents SWASV responses of concentrations of Cd2+ and Bi3+ on the GC/GO-IPA surface. The determination limits of Cd (II) and Bi (III) have been supported by SWASV experiments at the on the GC/GO-IPA in 0.1 M acetate buffer (pH 4.5) range of 1×10−8 M – 1×10−12 M Cd2+ and Bi3+, as shown in . The heights of the stripping peaks of Bi3+ were more intensively than that of Cd2+. Hence, the GC/GO-IPA surface exhibited the highest sensitivity and the best stability for assay of Bi3+ and Cd2+. The acetate groups of IPA can electrostatically attract Bi3+ and Cd2+ cations. The oxygen atom can be binding with Bi3+ and Cd2+ ions, and both of two factors affect the GC/GO-IPA-enhanced sensitivity.[Citation56] The authors have not considered which factor predominates the enhanced sensitivity in this study.
Figure 5. SWASV responses of A: 1×10−8 M, B: 1×10−9 M, C: 1×10−10 M, D: 1×10−11 M, and E: 1×10−12 M Cd2+ and Bi3+ on the GC/GO-IPA surface (pulse size: 25 mV, Frequency: 25 Hz.).
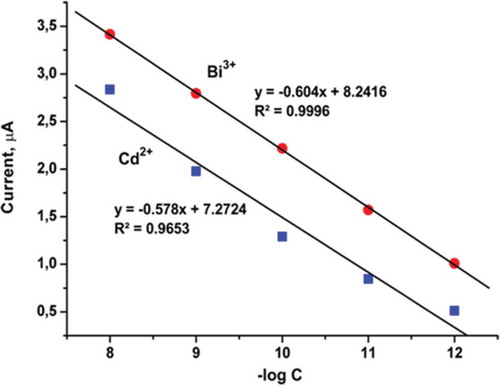
shows calibration curves of different concentrations of Cd2+ and Bi3+ on the GC/GO-IPA. The analysis of Cd2+ and Bi3+ under the optimum conditions was performed by changing the concentration of both of two species. As shown in , the peak current of Cd2+ and Bi3+ are linear with its concentration from 1×10−8 M to 1×10−12 M at a sensitivity (slope) of 0.5–3.5 μA, respectively. The linear curves of Bi3+ and Cd2+ are y = –0.604x + 8.2416 (R2 = 0.9996) and y = –0.578x + 7.2724 (R2 = 0.9653), respectively. The responses of species are practically unaltered with the increase of another species concentration. The detection limits of Cd2+ and Bi3+ were 8.1×10−13 M and 1.06×10−13 M, respectively. Especially, the Bi3+ calibration curve slope is more vertical than that of Cd2+. This is clearly showing that this method is more selectivity for Bi3+ than Cd2+. Some researchers calculated detection limit of Cd2+ species by voltammetry method were 2.1 pM and 1.5 nM,[Citation13,Citation14] respectively. And also someone found detection limit of Bi3+ was 110 ppb.[Citation17] However, this method results are under these limits. These optimum parameters were more advantage according to some literatures as pH, deposition time, and suspension solution volume.[Citation12–Citation14,Citation17,Citation19] The researchers will continue to develop new methods for the growing world.
Analysis of the tap water and milk samples
To determine the effectiveness of our method, recoveries of Bi (III) and Cd (II) experiments were carried out. Therefore, the recoveries of Bi (III) and Cd (II) can be shown that fraction of the Bi (III) and Cd (II) determined after addition of a known amount of the Bi (III) and Cd (II) to a sample.[Citation57] The method was evaluated by the application in the simultaneous determination of Bi3+ and Cd2+ in two tap water samples using standard addition method. The recoveries of Bi3+ and Cd2+ in tap water and milk samples (n = 5) are shown in . GC/GO-IPA electrode was also applied successfully for the simultaneous determination of Bi3+ and Cd2+ in tap water and milk samples. The recoveries of the Bi3+ and Cd2+ were up to 95%, so analytical recovery was obtained for all species and in the real sample. Also, these values were very good as comparison with the literature.[Citation58,Citation59]
Table 2. The recoveries of Bi3+ and Cd2+ in tap water and milk samples (n = 5).
The selectivity of the method for the determination of various metal ions was evaluated for various ions including Bi3+ and Cd2+ solutions. In this study, interferences of equimolar concentrations of Hg2+, Fe2+, Cu2+, and Zn2+ were studied at different pHs. The peak current of Bi3+ and Cd2+ did not affect for equimolar range. The peak current responses of the ions were reduced by about 2% by the presence of 100-fold excess of other heavy metal ions.
Conclusions
The GO was synthesized via electrochemical exfoliation method. It was characterized by using Tem and XRD. IPA attached GO was modified on GC electrode surface under the IR lamp. The modified electrode was electrochemically characterized with CV and EIS techniques assisted with a Fe(CN)63-/4- redox probe couple. An XPS spectrum measurement of the modified surface was shown that it was included presence of the amide’s N1s and functionalized various C1s. The proposed this method, which is using GC/GO-IPA surface, is proven to be an efficient, simple and rapid separation method that can be used for Cd (II) and Bi (III) and tap water and milk samples. Effects of this procedure were investigated for analytical parameters such as pH, deposition time, and suspension solution volume. The detection limits of the method are comparable with the literature.[Citation12–Citation14,Citation17,Citation19,Citation56] Simultaneous Cd (II) and Bi (III) ions by SWASV is one of the most important advantages of the proposed method, which has been applied on tap water and milk samples. And also the recoveries of Bi3+ and Cd2+ were higher than the literature.[Citation60,Citation61] This mean is that GC/GO-IPA electrode show more sensitive for Bi3+ and Cd2+ ions according to literature.[Citation62,Citation63]
Funding
The authors would like to thank to the Research Foundation of Necmettin Erbakan University, Konya, Turkey (BAP-131210012) for financial support of this work.
Additional information
Funding
References
- Jarup, L. Health Effects of Cadmium Exposure—A Review of the Literature and a Risk Estimate. Scandinavian Journal of Work, Environment & Health 1998, 24, 11–51.
- Waalkes, M.P. Cadmium Carcinogenesis. Mutation Research 2003, 533, 107–120.
- Goering, P.L.; Waalkes, M.P.; Klaassen, C.D. Toxicology of Cadmium. In Handbook of Experimental Pharmacology: Toxicology of Metals, Biochemical Effects; Goyer, R.A.; Cherian, M.G.; Eds; Springer-Verlag: New York, NY, 1994; 189–214.
- Bertin, G.; Averbeck, D. Cadmium: Cellular Effects, Modifications of Biomolecules, Modulation of DNA Repair and Genotoxic Consequences (A Review). Biochimie 2006, 88, 1549–1559.
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.B.; Williams, D.J.; Moore, M.R. A Global Presepective on Cadmium Pollution and Toxicity in Nonoccupationally Exposed Population. Toxicology Letters 2003, 137, 65–83.
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An Intracellular Protein to Protect Against Cadmium Toxicity. Annual Review of Pharmacology 1999, 39, 267–294.
- Waalkes, M.P. Cadmium Carcinogenesis in Review. Journal of Inorganic Biochemistry 2000, 79, 241–244.
- Tücks, A.; Beck, H.P. The Photochromic Effect of Bismuth Vanadate Pigments: Investigations on the Photochromic Mechanism. Dyes and Pigments 2007, 72, 163–177.
- Kong, D.; Chen, Y.; Wan, P.; Liu, S.; Khan, Z.U.H.; Men, B. Pre-Plating of Bismuth Film Electrode with Coexisted Sn2+ in Electrolyte. Electrochimica Acta 2014, 125, 573–579.
- Dipalma, J.R. Bismuth Toxicity. American Family Physician 1998, 38, 244–246.
- Ouyang, R.; Zhu, Z.; Tatum, C.E.; Chambers, J.Q.; Xue, Z.L. Simultaneous Stripping Detection of Pb(II), Cd(II) and Zn(II) Using a Bimetallic Hg-Bi/Single-Walled Carbon Nanotubes Composite Electrode. Journal of Electroanalytical Chemistry 2011, 656, 78–84.
- Luo, J.H.; Jiao, X.X.; Li, N.B.; Luo, H.Q. Sensitive Determination of Cd(II) by Square Wave Anodic Stripping Voltammetry With in Situ Bismuth-Modified Multiwalled Carbon Nanotubes Doped Carbon Paste Electrodes. Journal of Electroanalytical Chemistry 2013, 689, 130–134.
- Wei, Y.; Yang, R.; Yu, X.Y.; Wang, L.; Liu, J.H.; Huang, X.J. Stripping Voltammetry Study of Ultra-Trace Toxic Metal Ions on Highly Selectively Adsorptive Porous Magnesium Oxide Nanoflowers. Analyst 2012, 137, 2183–2191.
- Mandil, A.; Pauliukaite, R.; Amine, A.; Brett, C.M.A. Electrochemical Characterization of and Stripping Voltammetry at Screen Printed Electrodes Modified with Different Brands of Multiwall Carbon Nanotubes and Bismuth Films. Analytical Letters 2012, 45, 395–407.
- Afkhami, A.; Ghaedi, H.; Madrakian, T.; Rezaeivala, M. Highly Sensitive Simultaneous Electrochemical Determination of Trace Amounts of Pb(II) and Cd(II) Using a Carbon Paste Electrode Modified with Multi-Walled Carbon Nanotubes and a Newly Synthesized Schiff Base. Electrochimica Acta 2013, 89, 377–386.
- Xu, H.; Zeng, L.; Xing, S.; Xian, Y.; Shi, G.; Jin, L. Ultrasensitive Voltammetric Detection of Trace Lead(II) and Cadmium(II) Using MWCNTs-Nafion/Bismuth Composite Electrodes. Electroanalysis 2008, 20, 2655–2662.
- Ashrafi, A.M.; Vytřas, K. Determination of Trace Bismuth(III) by Stripping Voltammetry at Antimony-Coated Carbon Paste Electrode. International Journal of Electrochemical Science 2012, 7, 68–76.
- Khaloo, S.S.; Ensafi, A.A.; Khayamian, T. Determination of Bismuth and Copper Using Adsorptive Stripping Voltammetry Couple with Continuous Wavelet Transform. Talanta 2007, 71, 324–332.
- Lexa, J.; Stulík, K. Determination of Bismuth by Electrochemical Stripping Analysis; Elimination of Interferences by Using a Mercury Film Electrode Modified with Tri-N-Octylphosphine Oxide and Application to Copper Alloys. Talanta 1986, 33, 11–16.
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W. R. W. Graphene Production Via Electrochemical Reduction of Graphene Oxide: Synthesis and Characterization. Chemical Engineering Journal 2014, 251, 422–434.
- Liu, Q.; Ishibashi, A.; Fujigaya, T.; Mimura, K.; Gotou, T.; Uera, K.; Nakashima, N. Formation of Self-Organized Graphene Honeycomb Films on Substrates. Carbon 2011, 49, 3424–3429.
- Sanghavi, B.J.; Kalambate, P.K.; Karna, S.P.; Srivastava, A.K. 2014. Voltammetric Determination of Sumatriptan Based on a Graphene/Gold Nanoparticles/Nafion Composite Modified Glassy Carbon Electrode. Talanta 2014, 120, 1–9.
- Jung, J.H.; Park, H.J.; Kim, J.; Hur, S.H. Highly durable Pt/Graphene Oxide and Pt/C Hybrid Catalyst for Polymer Electrolyte Membrane Fuel Cell. Journal of Power Sources 2014, 248, 1156–1162.
- Channu, V.S.R.; Ravichandran, D.; Rambabu, B.; Holze, R. Carbon and Functionalized Graphene Oxide Coated Vanadium Oxide Electrodes for Lithium Ion Batteries. Applied Surface Science 2014, 305, 596–602.
- Battumur, T., Ambade, S. B., Ambade, R. B., Pokharel, P., Lee, D. S., Han, S. -H. Lee, W. and Lee, S.-H. Addition of Multiwalled Carbon Nanotube and Graphene Nanosheet In Cobalt Oxide Film for Enhancement of Capacitance in Electrochemical Capacitors. Current Applied Physics 2013, 13, 196–204.
- Lin, Q.; Li, Y.; Yang, M. Tin Oxide/Graphene Composite Fabricated Via a Hydrothermal Method for Gas Sensors Working at Room Temperature. Sensors and Actuators B: Chemical 2012, 173, 139–147.
- Gan, T.; Sun, J.; Huang, K.; Song, L.; Li, Y. A Graphene Oxide–Mesoporous MnO2 Nanocomposite Modified Glassy Carbon Electrode as a Novel and Efficient Voltammetric Sensor for Simultaneous Determination of Hydroquinone and Catechol. Sensors and Actuators B: Chemical 2013, 177, 412–418.
- Demir Mülazımoğlu, A.; Yılmaz, E.; Mülazımoğlu, İ.E. Dithiooxamide Modified Glassy Carbon Electrode for the Studies of Non-Aqueous Media: Electrochemical Behaviors of Quercetin onto the Electrode Surface. Sensors 2012, 12, 3916–3928.
- Mülazımoğlu, İ.E.; Solak, A.O. A Novel Apigenin Modified Glassy Carbon Sensor Electrode for the Determination of Copper Ions in Soil Samples. Analytical Methods 2011, 3, 2534–2539.
- Mülazımoğlu, İ.E. Electrochemical Determination of Copper(II) Ions at Naringenin-Modified Glassy Carbon Electrode: Application in Lake Water Sample. Desalination and Water Treatment 2012, 44, 161–167.
- Zhang, D.; Tong, J.; Xia, B. Humidity-Sensing Properties of Chemically Reduced Graphene Oxide/Polymer Nanocomposite Film Sensor Based on Layer-By-Layer Nano Self-Assembly. Sensors and Actuators B: Chemical 2014, 197, 66–72.
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR Based Fibre Optic Ammonia Gas Sensor Utilizing Nanocomposite Film of PMMA/Reduced Graphene Oxide Prepared by in Situ Polymerization. Sensors and Actuators B: Chemical 2014, 199, 190–200.
- Li, X.; Chen, X.; Yao, Y.; Li, N.; Chen, X. High-Stability Quartz Crystal Microbalance Ammonia Sensor Utilizing Graphene Oxide Isolation Layer. Sensors and Actuators B: Chemical 2014, 196, 183–188.
- Alizadeh, T.; Mirzagholipur, S. A Nafion-Free Non-Enzymatic Amperometric Glucose Sensor Based on Copper Oxide Nanoparticles–Graphene Nanocomposite. Sensors and Actuators B: Chemical 2014, 198, 438–447.
- Liu, H.; Lv, T.; Wu, X.; Zhu, C.; Zhu, Z. 2014. Preparation and Enhanced Photocatalytic Activity of CdS@RGO Core–Shell Structural Microspheres. Applied Surface Science 2014, 305, 242–246.
- He, B.; Shen, Y.; Ren, Z.; Xiao, C.; Jiang, W.; Liu, L.; Yan, S.; Wang, Z.; Yu, Z. Defect-Controlled Synthesis of Graphene Based Nano-Size Electronic Devices Using in Situ Thermal Treatment. Organic Electronics 2014, 15, 685–691.
- Zhou, W.; Sun, C.; Zhou, Y.; Yang, X.; Yang, W. A Facial Electrochemical Approach to Determinate Bisphenol a Based on Graphene-Hypercrosslinked Resin Mn2O2 Composite. Food Chemistry 2014, 158, 81–87.
- Chaudhuri, B.; Bhadra, D.; Mondal, B.; Pramanik, K. Biocompatibility of Electrospun Graphene Oxide–Poly(ε-Caprolactone) Fibrous Scaffolds with Human Cord Blood Mesenchymal Stem Cells Derived Skeletal Myoblast. Materials Letters 2014, 126, 109–112.
- Üstündağ, Z.; Solak, A.O. EDTA Modified Glassy Carbon Electrode: Preparation and Characterization. Electrochimica Acta 2009, 54, 6426–6432.
- Mülazımoğlu, İ.E.; Demir Mülazımoğlu, A. Investigation of Sensitivity Against Different Flavonoid Derivatives of Aminophenyl Modified Glassy Carbon Sensor Electrode and Antioxidant Activities. Food Analytical Methods 2012, 5, 1419–1426.
- Mülazımoğlu, İ.E.; Demir Mülazımoğlu, A.; Yılmaz, E. Determination of Phenol in Tap Water Samples as Electrochemical using 3,3’-Diaminobenzidine Modified Glassy Carbon Sensor Electrode. Desalination 2011, 268, 227–232.
- Li, Y.; Feng, S.; Li, S.; Zhang, Y.; Zhong, Y. A High Effect Polymer-Free Covalent Layer by Layer Self-Assemble Carboxylated MWCNTs Films Modified GCE for the Detection of Paracetamol. Sensors and Actuators B: Chemical 2014, 190, 999–1005.
- Maleh, H. K., Moazampour, M., Gupta, V.K. and Sanati, A. L. Electrocatalytic Determination of Captopril in Real Samples Using NiO Nanoparticle Modified (9,10-Dihydro-9,10-Ethanoanthracene-11,12-Dicarboximido)-4-Ethylbenzene-1,2-Diol Carbon Paste Electrode. Sensors and Actuators B: Chemical 2014, 199, 47–53.
- Demir Mülazımoğlu, A.; Mülazımoğlu, İ.E. Electrochemical Behaviors of 2-Amino-3-Hydroxypyridine onto the Glassy Carbon Sensor Electrode: Simultaneous and Independent Determinations of Quercetin, Galangin, 3-Hydroxyflavone and Chrysin. Food Analytical Methods 2013, 6, 845–853.
- Demir Mülazımoğlu, A.; Mülazımoğlu, İ.E. 2013. Electrochemical Properties of MDA/GC Electrode and Investigation of Usability as Sensor Electrode for Determination of Que, Kae, Lut and Gal using CV, DPV and SWV. Food Analytical Methods 2013, 6, 141–147.
- Demir Mülazımoğlu, A.; Mülazımoğlu, I.E.; Yılmaz, E. Determination of Copper Ions Based on Newly Developed Poly-Chrysin Modified Glassy Carbon Sensor Electrode. Reviews in Analytical Chemistry 2012, 31, 131–137.
- Sanghavi, B.J.; Mobin, S.M.; Mathur, P.; Lahiri, G.K.; Srivastava, A.K. Biomimetic Sensor for Certain Catecholamines Employing Copper(II) Complex and Silver Nanoparticle Modified Glassy Carbon Paste Electrode. Biosensors and Bioelectronics 2013, 39, 124–132.
- Sanghavi, B.J.; Srivastava, A.K. Adsorptive Stripping Differential Pulse Voltammetric Determination of Venlafaxine and Desvenlafaxine Employing Nafion–Carbon Nanotube Composite Glassy Carbon Electrode. Electrochimica Acta 2011, 56, 4188–4196.
- Elyasi, M.; Khalilzadeh, M.A.; Maleh, H.K. High Sensitive Voltammetric Sensor Based on Pt/CNTs Nanocomposite Modified Ionic Liquid Carbon Paste Electrode for Determination of Sudan i in Food Samples. Food Chemistry 2013, 141, 4311–4317.
- Ensafi, A.A. Modified Multiwall Carbon Nanotubes Paste Electrode as a Sensor for Simultaneous Determination of 6-Thioguanine and Folic Acid Using Ferrocenedicarboxylic Acid as a Mediator. Journal of Electroanalytical Chemistry 2010, 640, 75–83.
- Raoof, J.B.; Maleh, H.K.; Hosseinzadeh, R. Electrocatalytic Oxidation and Voltammetric Determination of Hydrazine at Bulk-Modified Carbon Paste Electrode with 1-[-4 (Ferrocenyl Ethynyl Phenyl]-1-Ethanone. Analytical and Bioanalytical Electrochemistry 2014, 6, 91–105.
- Maleh, H.K.; Moazampour, M.; Ahmar, H.; Beitollahi, H.; Ensafi, A.A. A Sensitive Nanocomposite-Based Electrochemical Sensor for Voltammetric Simultaneous Determination of Isoproterenol, Acetaminophen and Tryptophan. Measurement 2014, 51, 91–99.
- Lin, X.; Ni, Y.; Kokot, S. Electrochemical Mechanism of Eugenol at a Cu Doped Gold Nanoparticles Modified Glassy Carbon Electrode and Its Analytical Application in Food Samples. Electrochimica Acta 2014, 133, 484–491.
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing Thermoelectric Properties of Organic Composites Through Hierarchical Nanostructures. Scientific Reports 2013, 3, 3448–3454.
- Kim, H.J.; Bae, I.S.; Cho, S.J.; Boo, J.H.; Lee, B.C.; Heo, J.; Chung, I.; Hong, B. Synthesis and Characteristics of NH2-Functionalized Polymer Films to Align and Immobilize DNA Molecules. Nanoscale Research Letters 2012, 7, 30–36.
- Li, Z.; Chen, L.; He, F.; Bu, L.; Qin, X.; Xie, Q.; Yao, S.; Tu, X.; Luo, X.; Luo, S. Square Wave Anodic Stripping Voltammetric Determination Of Cd2+ and Pb2+ at Bismuth-Film Electrode Modified with Electroreduced Graphene Oxide-Supported Thiolated Thionine. Talanta 2014, 122, 285–292.
- Hesse, P.R. A Textbook of Soil Chemical Analysis. John Murray: London, 1971.
- Cerutti, S.; Escudero, L.A.; Gasquez, J.A.; Olsina, R.A.; Martinez, L.D. On-Line Preconcentration and Vapor Generation of Scandium Prior to ICP-OES Detection. Journal of Analytical Atomic Spectrometry 2011, 26, 2428–2433.
- Gila, R.A.; Cabello, S.P.; Takara, A.; Smichowski, P.; Olsina, R.A.; Martínez, L.D. A Novel on-Line Preconcentration Method for Trace Molybdenum Determination by USN–ICP OES with Biosorption on Immobilized Yeasts. Microchemical Journal 2007, 86, 156–160.
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an Electrochemically Reduced Graphene Oxide Modified Disposable Bismuth Film Electrode and Its Application for Stripping Analysis of Heavy Metals in Milk. Food Chemistry 2014, 151, 65–71.
- Abbasi, S.; Bahiraei, A.; Abbasai, F. A Highly Sensitive Method for Simultaneous Determination of Ultratrace Levels of Copper and Cadmium in Food and Water Samples with Luminol as a Chelating Agent by Adsorptive Stripping Voltammetry. Food Chemistry 2011, 129, 1274–1280.
- Montesinos, P.C.; Cervera, M.L.; Pastor, A.; de la Guardia, M. Determination of Ultratrace Bismuth in Milk Samples by Atomic Fluorescence Spectrometry. Journal of AOAC International 2003, 86, 815–822.
- Feng, P.J.; Jian, W.; Bin, Y.Y. Determination of Trace Heavy Metals in Milk Using an Ionic Liquid and Bismuth Oxide Nanoparticles Modified Carbon Paste Electrode. Chinese Science Bulletin 2012, 57, 1781–1787.