ABSTRACT
The objective of the present research work was to evaluate the antioxidant, antimicrobial and antimalarial activities of essential oil and various extracts from O. sanctum. Gas chromatography-mass spectrometry analysis identified the following major compounds with their quantification as: eugenol (22.0%), β-elemene (19.2%), β-caryophyllene (19.1%), and Germacrene D (5.03%). HPLC analysis of O. sanctum extracts revealed that gallic acid, chlorogenic acid, p-hydroxy benzoic acid, caffeic acid, vanillic acid, p-coumeric acid, sinapic acid, and ferulic acid were the important phenolic acids. The methanol extract exhibited highest level of total phenolic (1.36 g/100 g dry plant material) and total flavonoid (0.67 g/100 g dry plant material) followed by ethanol and n-hexane extracts. The oil and extracts exhibited excellent free radical scavenging potential as measured by 2,2-diphenyl-1-picrylhydrazyl radical free radical-scavenging ability, and antioxidant activity as measured by inhibition of linoleic acid oxidation. Essential oil, n-hexane, methanol, and ethanol extracts exhibited moderate antimalarial potential in term of anti-haem biocrystallization activity. In the resazurin microtitre plate and disc diffusion assays, the essential oil of O. sanctum showed better antibacterial activity than various extracts. The results of the present investigation demonstrated significant (p < 0.05) variations in the antioxidant, antimicrobial, and antimalarial activities of essential oil and extracts from O. sanctum.
Introduction
Population-based epidemiological evidence has helped to clarify the role of diet in preventing and controlling morbidity and premature mortality resulting from chronic diseases.[Citation1] Diet and nutrition are vital aspects in the endorsement and maintenance of good health throughout the entire life course. Poor diet is a major contributor to the leading causes of chronic and fatal diseases including coronary heart disease, cancer, and strokes. Research and development in food science is helping to identify many functional foods ingredients, because they can provide health benefits by reducing risk and enhancing the body’s ability to manage certain chronic diseases.[Citation2,Citation3] The functional properties of plants/foods depend on the presence of specific chemical compounds, mainly secondary metabolites such as phenolics and flavonoids.[Citation4–Citation6] The presence of such bioactive compounds promotes the therapeutic uses of medicinal plants also.[Citation5,Citation7]
The Ocimum is a big genus of family Lamiaceae and covering more than 160 species that are cultivated all over the temperate regions of world. The species Ocimum sanctum (O. sanctum) is commercially cultivated in several countries of East Asia, America, Europe, and Australia for food and isolation of essential oils.[Citation6,Citation8] O. sanctum is generally present in two types, i.e., vanya which is found in wild form while other type is called as gramya which is grown in homes. Both types have similar uses, however, the leaves of the former are little darker. Traditionally, O. sanctum has been extensively utilized in the food and perfume industries as well as in traditional herbal medicine to treat a number of ailments including carminative, stomachic, malaria, wounds, and microbial infections.[Citation8–Citation13]
The chemical composition of the O. sanctum, essential oil might vary depending upon the origins, environmental conditions, and types of cultivars.[Citation9,Citation10] The antioxidant and other biological properties of the O. sanctum essential oils and extracts have been of great interest in both the academia and food industries because of their antioxidant and antimicrobial potentials. Even though few reports on the antimicrobial and antioxidant activities of the O. sanctum essential oil and extracts are available separately.[Citation10,Citation12] But to the best of our knowledge, the potential antimalarial activity of O. sanctum essential oil has never been investigated before. In continuation of our phytochemical and bioactivity studies on plants of Pakistani flora, we now report on the potential antimicrobial, antimalarial, and antioxidant activities of essential oil and extracts of O. sanctum from temperate region of Pakistan. Characterization data essential oil and extract determined by gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) have also been reported.
Materials and methods
Collection and pretreatment of plant materials
The aerial parts of the cultivated O. sanctum at the flowering stage were harvested in July–August, from three different sites in the periphery of Faisalabad region (latitude 31°–26’ N; longitude 73°–06’ E and altitude 184.4 m above mean sea level). The mean data for minimum and maximum temperatures (°C) for the months of July–August in the Faisalabad region were 28.1 ± 2.6, 38.1 ± 3.4 (average 33.1), respectively. The average relative humidity and total rainfall of the months of July–August in Faisalabad region were 46.6 ± 14.6% and 79.2 mm, respectively. The plant specimen was further authenticated and identified by Dr. Qasim Ali, Assistant Professor, Department of Botany, GC University Faisalabad, Pakistan (Voucher No 005, Herbarium of Qauid-e-Azam University, Islamabad). The collected materials were then dried at 35°C for extraction of essential oil and various extracts.
Reference compounds, reagents, and chemicals
Standards, C9-C24 n-alkanes and reference chemicals used in this study including gallic acid, chlorogenic acid, ferulic acid, vanillic acid, p-coumeric acid, sinapic acid, p-hydroxy benzoic acid, caffeic acid, catechin, linoleic acid (60–74%), Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), Tween 80, butylated hydroxytoluene (BHT), butylated hydroxylanisole (BHA), ciprofloxacin, MTT [3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide] were obtained from Sigma Chemical Co. (St Louis, MO, USA). All other chemicals (analytical grade), i.e., ferrous chloride, ammonium thiocyanate, hydrochloric acid, chloroform, hexane, ethanol, and methanol used in this study were purchased from Merck (Darmstadt, Germany), unless stated otherwise. Sterile resazurin tablets were obtained from BDH Laboratory Supplies (Poole, UK). Sterile isosensitest broth was purchased from Oxoid Ltd. (Hampshire, UK) and sterile isosensitest agar was obtained by Southern Group Laboratory (SGL).
Isolation of essential oil
The dried and finely ground (80 mesh) aerial parts of O. sanctum were subjected to hydro-distillation for 4 h using a Clevenger-type apparatus.[Citation6] The isolated essential oil was dried over anhydrous sodium sulfate (Merck), filtered and stored at –4°C until analyzed.
Preparation of extracts
Three different solvents systems, i.e., n-hexane, ethanol, and methanol were selected for the preparation of different polarity O. sanctum extracts. Briefly, the ground plant materials (100 g for each sample) were extracted with 500 mL of each of the solvent in a Soxhlet unit (1000 mL capacity) for 4–6 h.[Citation14] The extracts were then filtered through Whatman filter paper (No. 1). The solvents were removed under reduced pressure, using a rotary evaporator (EYELA, SB-651, Rikakikai Co. Ltd. Tokyo, Japan). The dried, crude, concentrated extracts were weighed to calculate the yield and stored in a refrigerator (–4°C), until used for analyses.
Analysis of essential oil
Gas chromatography
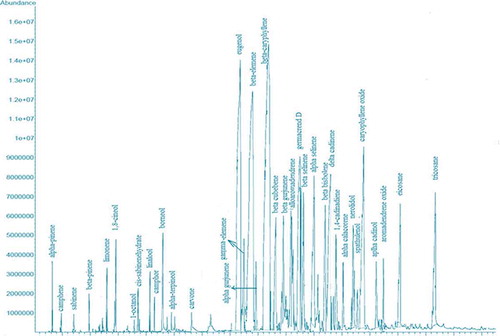
A Perkin-Elmer gas chromatograph (model 8700), with flame ionization detector (FID) and capillary column (HP-5MS) having following dimension 30 m × 0.25 mm, film thickness 0.25 µm was used for the chemical analysis of the O. sanctum essential oil. The temperatures of the injector and detector were set at 220 and 290°C, respectively. The column thermostat temperature was started from 80°C and raised to 220°C at the rate of 4°C min−Citation1, whereas initial and final temperatures were held for 3 and 10 min, respectively. The carrier gas was helium with a flow of 1.5 mL min−Citation1. A sample of 1.0 µL was injected (split ratio 100:1). For quantification purposes a built-in data-handling program of the equipment (Perkin-Elmer) was used. The essential oil composition was reported as a relative percentage of the total peak area. Furthermore, the main components of the essential oils like eugenol, β-elemene, β-caryophyllene, and germacrene D were quantified by means of the internal standard addition method.
GC-MS analysis
GC-MS analysis of the O. sanctum essential oil was performed using an Agilent-Technologies (Little Falls) 6890N Network gas chromatographic system, equipped with an Agilent-Technologies mass selective detector (model 5975 inert XL) and 7683B series auto-injector. The essential oil chemical constituents were separated using a “HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 µm; Little Falls).” A sample of 1.0 µL was injected (split ratio 100:1). For GC-MS detection, an electron impact ionization mode, with ionization energy of 70 eV, was used. The column oven temperature program and the carrier gas (helium) flow rate were the same as employed for the GC analysis. Mass range was 50–550 m/z while the injector and MS transfer line temperatures were set at 220 and 290ºC, respectively.
Compounds identification
The O. sanctum essential oil compounds identification was based on the comparison of their retention indices in relation to (C9-C24) n-alkanes with those of data published or with authentic standards.[Citation6,Citation15] The compounds were also identified by comparing their MS data with those from the National Institute of Standards and Technology (NIST) mass spectral library and available mass spectra and, wherever promising, by means of co-injection of authentic standards.[Citation16,Citation17]
Analysis of extract
Hydrolysis of sample
The hydrolysis of O. sanctum extract was done as reported previously.[Citation18] Briefly, 10 mL of 50% aqueous methanol solution containing 1.2 M HCl and 0.04 % (w/v) ascorbic acid as antioxidant was added to 1000 mg of crude extract. The hydrolysis was performed at 80°C under reflux for 2 h. After refluxing, the extracts were allowed to cool and were made up to 10 mL with methanol. The extracts were filtered through 0.45 µm non-pyrogenic filter (Minisart, Satorius Stedim Biotech GmbH) prior to injection.
Preparation of calibration curves
Stock solutions of the standards (gallic acid, p-hydroxy benzoic acid, chlorogenic acid, caffeic acid, vanillic acid, p-coumeric acid, sinapic acid, ferulic acid) were freshly prepared by dissolving authentic compounds in methanol (100 µg/mL). Working standards solutions were made by gradual dilution with methanol to the required concentration 0.4 to 100 µg/mL. The calibration curve was constructed for each standard by plotting the concentration of standard against peak area.
Chromatographic conditions
The HPLC analysis was performed with Shimadzu CBM-20A system (Shimadzu Corporation) equipped with gradient model LC-20AD pumps system, a SPD 20A ultraviolet (UV)/Visible detector, CTO-10AS VP column oven, an auto injection (SIL-20AHT) and degasser (DGU-20A5) systems. A hypersil GOLD C18 column (250 × 4.6 mm internal diameter, 5 µm particle size; Thermo Fisher Scientific Inc.) and a non-linear gradient consisting of solvent A (acetonitrile:methanol, 70:30) and solvent B (water with 0.5% glacial acetic acid). Following gradient program was used for the separation of phenolic acids and flavonoids; 10–15% A from 0 to 5 min; 15–20% A from 5 to 18 min; 20–40% A from 18 to 40 min and kept at 40% A from 40 to 45 min; 40–10% A from 45 to 50 min and kept at 10% A from 50 to 55 min). UV spectra were recorded at 275 nm. The analytes were identified by matching the retention times and spiking samples with standards and quantification was based on an external standard method.
Antioxidant activity
Determination of total phenolics (TP) and total flavonoids (TF) contents
Amounts of TP and TF in the O. sanctum extracts were determined using Folin–Ciocalteu reagent method and aluminum chloride colorimetric assay, respectively, as reported previously.[Citation18]
DPPH radical scavenging assay
The free radical scavenging activity of the O. sanctum essential oil and extracts was tested by appraising their capacity to scavenge 2, 2׳-diphenyl-1-picrylhydrazyl (DPPH) stable radicals. The DPPH assay was performed as described earlier.[Citation6,Citation18] Briefly, the samples (from 0.5 to 500.0 µg mL−Citation1) mixed with 1 mL of 90 µM DPPH solution, were made up with 95% MeOH to a final volume of 4 mL. BHT, a synthetic antioxidant, was used as a positive control. After 1 h incubation at room temperature, the absorbance was read at 515 nm. Percent radical scavenging concentration was worked out using the following formula:
where “Ablank is the absorbance of the control (containing all reagents except the test essential oils/compounds), and Asample is the absorbance of the test essential oils/compounds.” IC50 values, which represent the concentration of essential oil that caused 50% scavenging of DPPH radicals, was calculated from the curve drawn by plotting percent scavenging versus concentration.
Percent inhibition in linoleic acid system
The O. sanctum essential oil and extracts was also evaluated for their antioxidant activity by measuring their ability to inhibit linoleic acid peroxidation following the method described previously.[Citation6] The essential oil or main components (5 mg) were added to 0.13 mL linoleic acid, 10 mL 99.8% ethanol and 10 mL of 0.2 M sodium phosphate buffer (pH 7) solution mixture. The mixture was then diluted to 25 mL with distilled water and incubated at 40 °C for 175 h. The magnitude of oxidation was measured by peroxide value following the colorimetric method. Briefly, 10 mL of 75% ethanol, 0.2 mL of an aqueous solution of ammonium thiocyanate (30%) and 0.2 mL of ferrous chloride solution (20 mM in 3.5% HCl) were sequentially added to 0.2 mL sample solution. The absorbance was noted after 3 min of stirring, at 500 nm using spectrophotometer. A control was performed without essential oils while BHT was used as a positive control. Percent inhibition of linoleic acid oxidation was calculated as follows:
Percent inhibition of linoleic acid oxidation = 100 – ([increase in absorbance of sample at 175h /increase in absorbance of control at 175h] × 100)
Haem biocrystallization and inhibition assay for potential antimalarial activity
The potential antimalarial activity of the O. sanctum essential oil and extracts was evaluated using the assay protocol with modification as reported previously.[Citation7] Briefly, 100 μL of essential oils at a concentration of 0.01–10 mg/mL in 10% Dimethyl Sulfoxide (DMSO) were incubated with 100 μL of 3mM Hematin (freshly dissolved in 0.1 M NaOH), 10 mM lipid, 10 μL HCl and sodium acetate buffer of pH 5 to make of volume of 1000 μL. Chloroquine diphosphate was used as a positive control while tube containing all the things except drug/essential oils was considered as negative control. The incubation was done for 4 h with gradual shaking/inverting of each tube. Centrifugation of samples was done at 14,000 rpm for 10 min. After removing the supernatant one mL of sodium dodecyl sulphate (SDS) solution was added to each tube, aspirating the pellet with 1 mL pipette tip. Each tube was sonicated for 30 min and centrifuge (10 min, 14,000 rpm). After carefully removing supernatant, the above step was repeated until supernatant is clear (usually 3–5 times over a 48 h period). Finally, the pellets were washed with 0.1 M bicarbonate solution as in above step. Then, haem polymer was dissolved in 0.1 M NaOH for at least 2 h. The absorbance was taken at 400 nm and % inhibition of haem polymerization inhibition (HPI) was calculated by the following formula:
Antimicrobial activity
Collection of strains
The O. sanctum essential oil and extracts were tested individually against a panel of pathogenic and food-borne microorganisms including six strains of bacteria: Staphylococcus aureus NCTC 6571, Bacillus subtilis NCTC 10400, Bacillus cereus NCTC 7464, Bacillus pumilis wild type, Pasturella aeruginosa NCTC 1662, and Escherichia coli ATCC 8739 and two strains of fungi: Candida krusei ATCC 6258, and Aspergillus flavous QC 6158. The strains were collected from the Microbiology Laboratory, School of Biomedical Sciences, University of Ulster, Coleraine, Northern Ireland, UK. Bacterial strains were cultured overnight (16 h) in nutrient agar (NA, Oxoid) at 37ºC, while the fungal strains cultured overnight (16 h) in potato dextrose agar (PDA, Oxoid) at 30ºC.
Measurement of inhibition zones (IZ)
The antimicrobial activity of the O. sanctum essential oil and extracts in terms of IZ were determined by disc diffusion method, as reported previously.[Citation6] Briefly, 100 µL of suspension containing 108 colony-forming units (CFU)/mL of bacteria cells and 104 spores/mL of fungi were spread on NA and PDA media, respectively. The paper discs (6 mm in diameter) were separately impregnated with O. sanctum essential oil (30 µL/disc) and extracts (30 µg/disc) and placed on the agar which had previously been inoculated with the selected test microorganism. Standard antibiotics, ciprofloxacin (30 µg/disc) and fluconazole (30 µg/disc) were used as a positive reference for bacteria and fungi, respectively, while the discs without samples were used as a negative control. Plates were incubated at 37ºC for 24 h for bacteria and at 30°C for 48 h for fungal strains. Antimicrobial activity was assessed by calculating the diameter of IZ in millimeters (including disc diameter of 6 mm) for the test organisms comparing to the controls.
Measurement of minimum inhibitory concentration (MIC)
For the measurement of MIC of O. sanctum essential oil and extracts, a modified resazurin microtitre-plate assay was used as reported previously.[Citation19] Briefly, essential oil and extract solutions (5 mg/mL, w/v in 10% DMSO) and standard antibiotic (5.0 mg/mL in 10% DMSO) was pipetted into the first row of the 96 well plates. To all other wells 50 μL of nutrient broth was added. Two fold serial dilutions were performed using a multi-channel pipette such that each well had 50 μL of the test material in serially descending concentrations. 30 μL of 3.3× strength isosensitised broth and 10 μL of resazurin indicator solution (prepared by dissolving 270 mg tablet in 40 mL of sterile distilled water) were added in each well. Finally, 10 μL of bacterial suspension was added to each well to achieve a concentration of approx 5 × 105 cfu/mL. Each plate was wrapped loosely with cling film to ensure that bacteria did not become dehydrated. Each plate had a set of controls: a column with a ciprofloxacin as positive control, a column with all solutions with the exception of the test compound, a column with all solutions with the exception of the bacterial solution adding 10 μL of nutrient broth instead and a column with 10% DMSO (v/v) solution as a negative control. The plates were prepared in triplicate, and incubated at 37°C for 24 h. The color change was then assessed visually. The growth was indicated by color changes from purple to pink or colorless. The lowest concentration at which color change occurred was taken as the MIC value.
Statistical analysis
All the analyses were carried out in triplicate and the data presented as mean values ± standard deviation for triplicate determinations. Analysis of variance (ANOVA) was performed by using STATISTICA 5.5 (Stat Soft Inc, Tulsa, OK, USA) software and a probability value of p ≤ 0.05 was reflected to represent a statistical significance difference.
Results and discussion
The essential oil yield of O. sanctum was 0.52 g/100 g () while the n-hexane, methanol, and ethanol extracts yields were 3.66, 14.6, and 10.3 g/100 g, respectively. The chemical composition of the essential oil of each O. sanctum is reported in . Thirty-five essential oil components were identified representing 96.1% of the total (). The major compounds in O. sanctum essential oil were eugenol (22.0%), β-elemene (19.2%), β-caryophyllene (19.1%), and Germacrene D (5.03%). On quantitative basis, the amounts, determined using calibrated curves with pure standards compounds, of the main components of O. sanctum essential oil were eugenol (20.9 g/100 g), β-elemene (18.7 g/100 g), β-caryophyllene (18.5 g/100 g), and Germacrene D (4.9 g/100 g; ).
Table 1. Chemical composition of O. sanctum essential oil.a
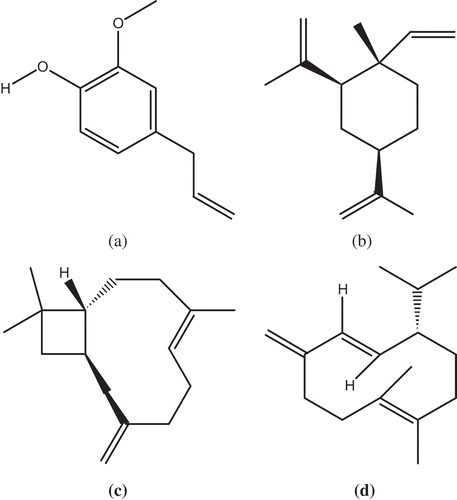
Figure 2. Structure and quantity (g/100 g) of some major compounds of O. sanctum essential oil: A: Eugenol (20.9 g/100 g); B: β-elemene (18.7 g/100 g); C: β-caryophyllene (18.5 g/100 g); D: Germacrene D (4.9 g/100 g).
shows the amount (mg/100 g of dry plant material) of eight phenolic acids including gallic acid, chlorogenic acid, p-hydroxy benzoic acid, caffeic acid, vanillic acid, p-coumeric acid, sinapic acid, and ferulic acid in different extracts of O. sanctum. The reverse-phase (RP)-HPLC analysis of O. sanctum extracts revealed the presence of ferulic acid, vanillic acid, sinapic acid, p-coumeric acid, gallic acid, p-hydroxy benzoic acid and chlorogenic acid, quercetin and myricetin being the most prominent polyphenolic components. Chlorogenic acid was found to be major phenolic acid in all three extracts. The highest concentration of chlorogenic acid was found in methanol extract (6.16 mg/100 g of dry plant material). Similarly chlorogenic acid was the major phenolic acid identified (5.85 mg/100 g of dry plant material) in the ethanol extract followed by gallic acid (3.50 mg/100 g of dry plant material). Significant (p < 0.05) variations were observed in the contents of phenolic acids with respect to different O. sanctum extracts
Table 2. Phenolic acids contents identified from different extracts of Ocimum sanctum.
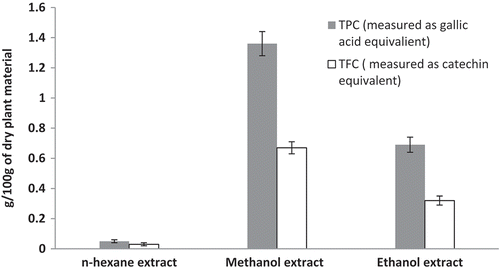
Amounts of TP and TF are given in . The highest TP was found in methanol extract (1.36 g/100 g dry plant material, measured as gallic acid equivalent) and the lowest in hexane extract (0.05 g/100 g dry plant material, measured as gallic acid). Similarly, the amount of TF in n-hexane, methanol, and ethanol extracts was found to be 0.03, 0.67, and 0.32 g/100 g of dry plant material, measured as catechin equivalent).
Figure 3. Total phenol and total flavonoid contents of n-hexane, methanol, and ethanol extracts of O. sanctum. Total phenolic contents, g/100 g of dry plant material, measured as gallic acid equivalent. Total flavonoid contents, g/100 g of dry plant material, measured as catechin equivalent.
The free radical scavenging activity of the O. sanctum essential oil and various extracts was assessed using DPPH radical scavenging assay. The values for 50% scavenging (IC50) are given in . An ANOVA showed a significant (p < 0.05) variation in the radical scavenging activity of essential oil and different extracts. The methanol extract of O. sanctum presented good radical scavenging activity (3.98 µg/mL) followed by ethanol extract (5.68 µg/mL), essential oil (11.1 µg/mL), and n-hexane extract (15.5 µg/mL). shows the percent inhibition of linoleic acid oxidation exhibited by the O. sanctum essential oil and extracts. Maximum inhibition was observed by methanol (84.7%) and ethanol (79.3%) extracts. The essential oil and n-hexane showed 74.0 and 46.5% antioxidant activity, respectively.
Table 3. Antioxidant and antimalarial activities of essential oil and various extracts of O. sanctum.a
This assay was employed to evaluate the antimalarial potential of the O. sanctum essential oil and various extract (). All three O. sanctum extracts reveled poor inhibition even at 10 mg/mL concentration showed poor anti-haem biocrystallization activity. The O. sanctum essential oil exhibited inhibition 58.9% showed moderate antimalarial activity. The inhibitory effect of O. sanctum essential oil on cell viability ranged from 83–96% at 500 µg mL−Citation1 (). The extract showed poor activity thus only essential oil was selected for further study to calculate the IC50 value.
Ocimum sanctum essential oil was found to be comparatively more active against the bacterial and fungal strains tested than tested O. sanctum extracts (). The activity was more pronounced against Gram positive strains than Gram negative strains. The IZ were ranged from 25.1–27.2 mm and 19.4–20.8 mm against Gram positive and Gram negative bacterial, respectively, by O. sanctum essential oil. MIC of against Gram positive, Gram negative and fungal strains were 776–840, 1449–1719, and 911–1103 µg mL−Citation1, respectively. The extracts showed much higher MIC than essential oil (930–2500 µg mL−Citation1).
Table 4. Antimicrobial activity of O. sanctum essential oil and various extracts.
The essential oil yield reported in the present study was comparable to reports by Agniseka et al.,[Citation20] who found the 0.59–0.83% essential oil yield from O. sanctum at different growth stages. However, Zheljazkov et al.[Citation8,Citation9] reported 0.07–0.34% (wt/wt % of oil in air-dry herbage) essential oil contents from O. sanctum cv. local United States. Whereas, Joshi[Citation12] reported 1.20% yield of essential oil from O. sanctum from Belgaum. Such variations in the essential oil content of O. sanctum across countries might be attributed to the varied agroclimatic conditions of the regions.
Literature showed high content of methyl chavicol (44.63%) and linalool (21.84%) in the O. sanctum essential oil from India.[Citation21] In another study reported from India, eugenol (61.30%) was found as major component in the essential oil of O. sanctum followed by β-caryophyllene (11.89%) and germacrene D (9.14%).[Citation22] Joshi[Citation12] also reported that mehyl eugenol is detected as major (92.4%) component of O. sanctum essential oil from Belgaum. The results of the present study also showed eugenol (22.0%) as major component but the percentage is less than reported in this study. Such variations in the chemical composition of essential oil across countries might be attributed to the varied agroclimatic (climatical, seasonal, geographical) conditions of the regions, stage of maturity, genetic differences, and adaptive metabolism of plants. The amounts of the major components of O. sanctum essential oil were also determined on quantitative basis using calibrated curves with pure standards compounds.
The antioxidant property of plant extracts depend on the amount of flavonoids and phenolic acids of extracts.[Citation23] The release secondary metabolites including phenolic acids and flavonoids from plants may vary from plant to plant and species to species. The effect of different solvent systems on the amount of TP and TF extracted was significant (p < 0.05) in the present study. Methanol has been proven as effective solvent to extract phenolic compounds.[Citation24] Some previous reports in the literature reveal the variation in the antioxidant potential of different O. sanctum around the world.[Citation10,Citation12,Citation25] The O. sanctum analyzed in the present study exhibited better antioxidant activity than that reported in the literature.[Citation12] The variation in the antioxidant activity might be ascribed to the varied phenolic profiles of Ocimum sanctum specie native to Pakistan.
The capability of any drug/compound to inhibit the biocrystallization of ferriprotoporhyrin IX (FPIX) is believed to be directly connected to their antimalarial activity.[Citation26] The haem biocrystallization assay is efficient, less costly and easy to perform. It is based on observation that during the intraerythrocytic development, malaria parasites degrade large amounts of hemoglobin within a specialized organelle, the digestive vacuole to supply amino acids for growth and development.[Citation7] A toxic by-product, FPIX, is produced during the catabolism of hemoglobin. The parasite detoxifies this FPIX through the formation of insoluble hemozoin crystals. Thus, agents that inhibit the conversion of FPIX to hemozoin may cause death from the lytic effects of haem or the haem drug complex may mediate parasite death.[Citation27] Moreover, in vitro hematin at acidic pH, leads to β-hematin, a compound presumed to be identical to hemozoin. Hence, bioactive compounds or plant extracts able to inhibit the biocrystallization of hematin at this pH, may possess antiplasmodial properties.[Citation7,Citation27] This assay was employed to assess the potential antimalarial activity of the O. santum essential oil for the first time. The ability of any compound to inhibit the biocrystallization of FPIX is believed to be directly connected to their antimalarial activity.[Citation28]
Both bacteria and fungi were more sensitive against Ocimum sanctum essential oil than extracts. The results of antimicrobail potential of the O. sanctum essential oil are better than those shown by other works,[Citation12,Citation29,Citation30] and confirms its traditional uses. In contrary to findings of the present study, the rate of inhibition in most of the reports was greater against Gram positive bacteria than that observed against Gram negative bacteria.[Citation12,Citation30] However, some reports were also available showing higher antibacterial activity against Gram positive bacteria than Gram negative bacteria also available.[Citation31] Overall the fungi, especially C. krusei, showed more resistance against O. sanctum essential oils than bacteria strains. C. krusei causes infection, mainly in critically ill patients, and is most often isolated in hematology patients from hospitals with severe neutropenia.[Citation32]
The Gram-positive and Gram-negative microorganisms differ in several aspects other than with respect to the structure of their cellular walls, mainly with regard to the presence of lipoproteins and lipopolysaccharides in Gram-negative bacteria that form a barrier to hydrophobic compounds.[Citation33] Some reports showed that there is a relationship between the chemical structures of the most abundant in the tested essential oil and the antimicrobial activity. The better essential oil activity was might be due to presence of eugenol and caryophyllene.[Citation12] The efficacy of O. sanctum essential oil and extracts against bacteria and fungi may provide a scientific ground for the application of the herb in the prevention and treatment of infections caused by micrroganisms such as Staphylococcus aureus and Escherichia coli, which have developed resistance to antibiotics. The results of study present the O. sanctum herb as a good candidate to explore new alternative antibacterial agents to combat pathogenic microorganisms.
Conclusion
Overall, the data generated on the biological activities of the O. sanctum essential oil and n-hexane, methanol, and ethanol extracts revealed significant differences (p < 0.05) due to a different nature of the compounds present. Methanol and ethanol extracts of Ocimum sanctum exhibited excellent antioxidant activity and free radical scavenging capacity followed by essential oil and hexane extract. O. sanctum essential oil showed good antimicrobial activities and moderate antimalarial activity. High TP and TF contents and antioxidant potential of O. sanctum extracts and excellent antimicrobial activity of essential oil lead to its possible use as a food preservative. Moreover, they may be used in pharmaceutical and natural therapies for treatment of oxidative stress. Further studies should also be done in order to evaluate the practical effectiveness of essential oil and extracts using specific food substrates under local environmental, storage, and food processing conditions.
Funding
This work is a part of research project funded (Grant No. 20-1584/R&D/09/2167) by the Higher Education Commision (HEC), Islamabad, Pakistan.
Additional information
Funding
References
- Shahidi, F.; Wanasundara, PK. Phenolic Antioxidants. Critical Reviews of Food Science and Nutrition 1992, 32, 67–103.
- Dallak, M.; Al-Khateeb, M.; Abbas, M.; Elessa R.; Al-Hashem, F.; Bashir, N.; Khali, M. In Vivo, Acute, Normo-Hypoglycemic, Antihyperglycemic, Insulinotropic Actions of Orally Administered Ethanol Extract of Citrullus Colocynthis (L.) Schrab Pulp. American Journal of Biochemistry and Biotechnology 2009, 5, 119–126.
- Uddin, M.N.; Mitra, K.; Haque, M.Z.; Comparative Bio-Active Compounds Determination and in Vitro Antioxidant Properties of Newly Developed Soy Mixed Wheat Flour and Traditional Wheat Flour. International Journal of Food Properties 2016, DOI:10.1080/10942912.2015.1110163
- Ali, S.; Chatha, S.A.S.; Ali, Q.; Hussain, A.I.; Hussain, S.M.; Parveen, R. Oxidative Stability of Cooking Oil Blend Stablized with Leaf Extract of Eucalyptus Citriodora. International Journal of Food Properties 2016, 19, 1556–1565.
- Lee, O.H.; Lee, B.Y. Antioxidant and Antimicrobial Activities of Individual and Combined Phenolics in Olea Europaea Leaf Extract. Bioresource Technology 2010, 101, 3751–3754.
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum Basilicum) Essential Oils Depends on Seasonal Variations. Food Chemistry 2008, 108, 986–995.
- Hussain, A.I.; Anwar, F.; Rasheed, S.; Nigam, P.S.; Janneh, O.; Sarker, S.D. Composition, Antioxidant and Chemotherapeutic Properties of the Essential Oils from Two Origanum Species Growing in Pakistan. Revista Brasileira de Farmacognosia 2011, 38, 943–952.
- Zheljazkov, V.D.; Cantrell, C.L.; Tekwani, B.; Khan, S.I. Content, Composition, and Bioactivity of the Essential Oils of Three Basil Genotypes as a Function of Harvesting. Journal of Agricultural and Food Chemistry 2008, 56, 380–385.
- Zheljazkov, V.D.; Cantrell, C.L.; Evans, W.B.; Ebelhar, M.W.; Coker, C. Yield and Composition of Ocimum Basilicum L. and Ocimum Sanctum L. Grown at Four Locations. Hortscience 2008, 43, 737–741.
- Beatović, D.; Jelačić, S.; Oparnica, Č.; Krstić-Milošević, D.; Glamočlija, J.; Ristić, M.; Šiljegović, J. Chemical Composition, Antioxidative and Antimicrobial Activity of Essential Oil Ocimum Sanctum L. Hemijska Industrija 2013, 67(3), 427–435.
- Das, S.K.V.; Tulsi, D.M. The Indian Holy Power Plant. Natural Product Radiance 2006, 5, 279–283.
- Joshi, R.K. Chemical Composition, in Vitro Antimicrobial and Antioxidant Activities of the Essential Oils of Ocimum Gratissmum, O. Sanctum and Their Major Constituents. Indian Journal of Pharmaceutical Sciences 2013, 75, 457–462.
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T.A. Hand Book of Medicinal Plant, 1st Ed; Agrobios, VEDIC Book: New Delhi, India, 2003.
- Hussain, A.I.; Chatha, S.A.S.; Noor, S.; Arshad, M.U.; Ali, Z.; Rathore, H.A.; Sattar, M.Z.A. Effect of Extraction Techniques and Solvent Systems for the Extraction of Antioxidant Components from Peanut (Arachis Hypogaea L.) Hulls. Food Analytical Methods 2012, 5, 890–896.
- Vagionas, K.; Graikou, K.; Ngassapa, O.; Runyoro, D.; Chinou, I. Composition and Antimicrobial Activity of the Essential Oils of Three Satureja Species Growing in Tanzania. Food Chemistry 2007, 103, 319–324.
- Adam, R.P. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corp.: Carol Stream, IL, 2001.
- Massada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. John Wiley & Sons: New York, NY, 1976.
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.A.; Chatha, S.A.S.; Ahmad, F.U.D.; Ahmad, A.; Johns, E.J. Phenolic Profile and Antioxidant Activity of Various Extracts from Citrullus Colocynthis (L.) from the Pakistani Flora. Industrial Crops and Products 2013, 45, 416–422.
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Sarker, S.D.; Moore, J.E.; Rao, J.R.; Mazumdar, A. Antibacterial Activity of Some Lamiaceae Essential Oils Using Resazurin as an Indicator of Cell Growth. LWT–Food Science and Technology 2011, 44, 1199–1206.
- Agnieska, K.; Anna, K.; Danuta, K. Composition of the Essential Oil of Ocimum Sanctum L. Grown in Poland During Vegetation. Journal of Essential Oil Research 2005, 17, 217–219.
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousaf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum Sanctum Essential Oil and Its Active Principles Exerted Their Antifungal Activity by Disrupting Ergosterol Biosynthesis and Membrane Integrity. Research in Microbiology 2010, 161, 816–823.
- Kumar, A.; Dubey, N.K.; Srivastva, S. Antifunfgal Evaluation of Ocimum Sanctum Essential Oil Against Fungal Deterioration of Raw Material of Rauvolfia Serpentine During Storage. Industrial Crops and Products 2013, 45, 30–35.
- Selvam, K.; Rajinikanth, R.; Govarthanan, M.; Paul, A.; Selvankumar, T.; Sengottaiyan, A. Antioxidant Potential and Secondary Metabolities In Ocimum Sanctum L. at Various Habitats. Journal of Medicinal Plants Research 2013, 7, 706–712.
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa Oleifera Lam.) Leaves. Journal of Agricultural and Food Chemistry 2003, 51, 2144–2155.
- Trevisan, M.T.S.; Silva, M.G.V.; Pfundstein, B.; Spiegelhalder, B.; Owen, R.W. Characterization of the Volatile Pattern and Antioxidant Capacity of Essential Oils from Different Species of the Genus Ocimum. Journal of Agricultural and Food Chemistry 2006, 54, 4378–4382.
- Raynes, K.; Foley, M.; Tilley, L.; Deady, L.W. Novel Bisquinoline Antimalarials. Synthesis, Antimalarial Activity, and Inhibition of Haem Polymerization. Biochemical Pharmacology 1996, 52, 551–559.
- Egan, T.J.; Marques, H.M. The Role of Haem in the Activity of Chloroquine and Related Antimalarial Drugs. Coordination Chemistry Reviews 1999, 192, 493–517.
- Raynes, K.; Foley, M.; Tilley, L.; Deady, L.W.; Novel Bisquinoline Antimalarials-Synthesis, Antimalarial Activity, and Inhibition of Haem Polymerization. Biochemical Pharmacology 1996, 52, 551–559.
- Mishra, P.; Mishra, S. Study of Antibacterial Activity of Ocimum Sanctum Extract Against Gram Positive and Gram Negative Bacteria. American Journal of Food Technology 2011, 6, 336–341.
- Rathnayaka, R.M.U.S.K. Antibacterial Activity of Ocimum Sanctum Extracts Against Four Food-Borne Microbial Pathogens. Scholars Journal of Applied Medical Sciences 2013, 1, 774–777.
- Mann, C.M.; Cox, S.D.; Markham, J.L. The Outer Membrane of Pseudomonas Aeruginosa NCTC 6749 Contributes to Its Tolerance to the Essential Oil of Melaleuca Alternifolis (Tea Tree Oil). Letters in Applied Microbiology 2000, 30(4), 294–297.
- Samaranayake, Y.H.; Samaranayake, L.P. Candida Krusei: Biology, Epidemiology, Pathogenecity and Clinical Manifestations of an Emerging Pathogen. Journal of Medical Microbiology 1994, 41, 295.
- Mazutti, M.; Mossi, A.J.; Cansian, R.L.; Corazza, M.L.; Dariva, C.J.; Oliveira, V. Chemical Profile and Antimicrobial Activity of Boldo (Peumus Boldus Molina) Extracts Obtained by Compressed Carbon Dioxide Extraction. Brazilian Journal of Chemical Engineering 2008, 25, 427–434.