ABSTRACT
Antidiabetic, anti-Alzheimer, general toxicity, and antioxidant activities of Salvia syriaca were evaluated. Phytochemical composition of the essential oil and methanolic extract of the plant were identified using gas chromatography-mass spectrometry and reverse-phase high-performance liquid chromatography-diode array detector techniques, respectively. Essential oil of S. syriaca exhibited strong cytotoxicity, antioxidant, α-amylase, and α-glucosidase inhibitory activities. Gas chromatography-mass spectrometry analysis showed that spathulenol (87.4%), isospathulenol (7.6%), and bornyl acetate (2.7%) are the major compounds in essential oil. High-performance liquid chromatography analysis indicated that rutin, quercetin, apigenin, rosmarinic acid, and ferulic acid are the most abundant phenolic components. S. syriaca could be considered as a valuable source of bioactive natural compounds for functional foods, medical, and pharmaceutical applications.
Introduction
The genus Salvia is the largest member of Lamiaceae family comprising more than 1000 species distributed worldwide.[Citation1] Salvia species have been traditionally used as folk medicine for the management of various diseases such as colds, antileishmanial, aches, infections, bronchitis, Alzheimer’s disease (AD), anthelmintic, antispasmodic, carminative, anti-inflammatory, sedative, and haemorrhage.[Citation2–Citation4] Additionally, these plants are economically important due to their broad uses in food industries such as flavor, spices, food preservation, and wine production.[Citation5–Citation7] Interest in the medicinal properties of Salvia has been increased by the discovery of novel terpenoid and phenolic compounds possessing wide bioactivities such as antioxidant, antiproliferative, anticholinesterase, anti-inflammation, antiviral, and antimicrobial.[Citation8,Citation9] The leaves and flowers of Salvia plants commonly are rich in flavonoid and terpenoid components.[Citation10,Citation11] Also, aerial parts contain volatile compounds including monoterpenoids and sesquiterpenoids, while the roots are rich in diterpenoids.[Citation12–Citation14] Salvia plants are commonly called ada çayı in Turkey, sage in English, and maryam Goli in Iran.
Salvia syriaca is used as forage.[Citation15] Previous phytochemical studies on S. syriaca reported the isolation of some flavonoids,[Citation16] one sesterterpenoid salvisyriacolide,[Citation17] a new sesquiterpene named syriacine,[Citation18] and four novel seco-ursadiene triterpenoids[Citation18,Citation19] from the aerial parts of the plant. Three triterpenoids, sitosterol, and daucosterol with acetylcholinesterase inhibitory activity,[Citation20] and some di- and triterpenoids and one flavonoid were isolated from the roots of the plant with cardiovascular activities.[Citation21] Also, there are some studies on the essential oil composition of S. syriaca which reported germacrene B, germacrene D, and bicyclogermacrene as major volatile compounds.[Citation15,Citation22]
Although many studies have described the phytochemistry and pharmacology of Salvia species, there is not any literature available about the application of S. syriaca as pharmaceuticals or functional foods. Thus, this study was planned to determine antioxidant properties and enzyme inhibitory activities of S. syriaca linked to AD, diabetes mellitus, and skin disorders for the first time. We also characterized the chemical composition of the essential oil and the phenolic profile of the methanol extract using high-performance liquid chromatography-diode array detector (HPLC-DAD). Our findings indicated the great potential of S. syriaca for possible applications in the food and pharmaceutical industries.
Materials and methods
Plant material
Aerial parts of Salvia syriaca were collected during the flowering stage in May 2015 at Urmia locality, West Azerbaijan province (Northwest Iran). The species was identified by taxonomist Mr. Shahram Bahadori and a voucher specimen was deposited for the plant (USPH-104) in the herbarium of Urmia School of pharmacy, Urmia, Iran.
Preparation of extracts
The extracts were prepared from whole aerial parts by maceration method. Fifty grams of the powdered plant bodies were successively extracted using 500 mL of n-hexane, dichloromethane, and methanol. The extraction was carried out during 72 h at room temperature with shaking. Subsequently, extracts were filtered through a filter paper and the solvent of the extracts were evaporated using a rotary evaporator under reduced pressure and 40°C.
Essential oil isolation
The plant material was grounded and the essential oil was extracted using hydrodistillation method during 3 h by a clevenger type apparatus according to the British pharmacopoeia. The sample was stored at 4°C until analysis.
Essential oil analysis
Gas chromatography-mass spectrometry (GC-MS) analysis was carried out using a Thermoquest Finnigan instrument employing the following conditions: DB-5 column (30 m × 0.32 mm i.d., film thickness 0.25 µm) and helium as carrier gas (1.1 mL/min). Temperatures of the detector and the injector were 250 and 240°C, respectively. The column temperature programming was 60–250°C at the rate of 5°C/min, and at 250°C for 10 min. The injection volumes were 1 µL. The obtained oils were diluted in dichloromethane and 0.5 µL of the solution was injected into the GC-MS apparatus with a split ratio of 1:100. Electron ionization-mass spectrometry (EI-MS) measurements were carried out with ionization voltage of 70 eV. Mass range was scanned from 35 to 456 amu. Afterward, identification of compounds was performed based on GC retention indices of the compounds relative to n-alkanes (C6-C24). Components of the oils were identified by matching of their mass spectra with Wiley, The National Institute of Standards and Technology (NIST) and Adams Mass Spectral libraries as well as by comparison of retention indices with those published in the literature. Relative area percentages obtained from GC were used for quantification of the components.
Determination of total phenolic and flavonoid contents
The amounts of phenolic and flavonoid compounds (total phenolic compound [TPC] and flavonoid compound [TFC]) in the extracts were determined through Folin–Ciocalteu and aluminium chloride colorimetric methods, respectively.[Citation23] TPCs are expressed as milligrams of gallic acid equivalents (GAE) per gram of dry extracts (mg GAE/g) using the calibration curve of gallic acid. Also, TFCs are expressed as milligrams of quercetin equivalents per gram of dry extracts (mg QE/g) using the calibration curve of quercetin as standard reagent. All analyses were carried out in triplicates and the results are expressed as average value ± standard error mean (SEM).
Measurement of the free radical scavenging capacity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity of the essential oil and solvent extracts of S. syriaca was determined.[Citation24] Butylated hydroxy toluene (BHT) was used as standard antioxidant. The results are expressed as average value ± SEM of three independent experiments.
Ferric reducing power
The ferric reducing power of the essential oil and extracts of S. syriaca was evaluated.[Citation25] The increased reducing power of the plant samples was indicated with increased absorbance of the reaction mixture. Ascorbic acid was used as standard reagent and the results are expressed as average value ± SEM of observed absorbance values from three parallel experiments.
α-Amylase inhibition assay
α-Amylase inhibitory activity assays were carried out by Caraway-Somogyi iodine/potassium iodide (IKI) method with some modifications.[Citation26] The sample solutions (25 µL) were mixed with α-amylase solution (50 µL) in phosphate buffer (pH = 6.9 with 6 mM sodium chloride) in 96-well microplates and incubated for 10 min at 37°C. After pre-incubation, the reaction was initiated with the addition of starch solution (50 µL, 0.05%). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (α-amylase) solution. The reaction mixture was incubated 10 min at 37°C. The reaction was then stopped with the addition of HCl (25 µL, 1 M). This was followed by addition of the iodine-potassium iodide solution (100 µL). The sample and blank absorbances were read at 630 nm (Thermo MultiSkan Go). The absorbance of the blank was subtracted from that of the sample and the α-amylase inhibitory activity was expressed as IC50 values (mg/mL).
α-glucosidase inhibition assay
α-glucosidase inhibitory activity was performed using a previously published method with some modifications.[Citation27] The sample solution (50 µL) was mixed with glutathione (50 µL), α-glucosidase solution (50 µL) in phosphate buffer (pH = 6.8) and 4-Nitrophenyl β-D-glucopyranoside (pNPG; 50 µL) in a 96-well microplate and incubated for 15 min at 37°C. Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (α-glucosidase) solution. The reaction was then stopped with the addition of sodium carbonate (50 µL, 0.2 M). The sample and blank absorbances were read at 400 nm. The absorbance of the blank was subtracted from that of the sample and the α-glucosidase inhibitory activity was expressed as IC50 values (mg/mL).
Acetylcholinesterase and butyrylcholinesterase inhibition assays
The cholinesterase inhibitory potential of the plant samples was evaluated using Ellman method with some modification.[Citation28] Briefly, 50 µL of sample solutions were mixed with 125 µL 5,5′dithiobis nitro benzoic acid (DTNB) and 25 µL tested enzyme solution in Tris–HCl buffer (pH = 8.0) and incubated for 15 min at room temperature. A sample of 25 µL of butyrylthiocholine chloride (BTCl)/acetylthiocholine iodide (ATCI) was added to initiate of the reaction. A mixture of the reagents without enzymes was used as the blank sample. After a 10 min incubation, absorbance values were read at 405 nm. The enzyme inhibitory activities were expressed as IC50 values (mg/mL).
Tyrosinase inhibition assay
The anti-tyrosinase activity of the extracts and the essential oil of the S. syriaca was investigated using a published method with modification.[Citation29] A sample of 25 µL of samples were mixed with 40 µL of enzyme solution and 100 µL phosphate buffer (pH = 6.8). Then the mixture was incubated at room temperature for 15 min. Thereafter, the reaction was initiated by addition of 40 µL L-DOPA. Also, a blank was made with all of the reagents without tyrosinase. After a 10 min incubation, absorbance values were read at 492 nm using a microplate reader.
General toxicity
The toxicity experiments were carried out against Brine shrimp (Artemia salina) larva according to a previously published method with some modifications.[Citation30] Test samples were prepared in dimethyl sulfoxide (DMSO). LC50 values were determined from the linear equation. Podophyllotoxin was used as positive control, and pure DMSO as untreated control. Final DMSO concentration in the test solutions was 0.5%.
Quantification of phenolic compounds by HPLC-DAD
Phenolic components were characterized using reversed-phase HPLC (Shimadzu Scientific Instruments, Tokyo, Japan). Detection and quantification were performed by a LC-10ADvp pump, DAD, CTO-10Avp column heater, SCL-10Avp system controller, DGU-14A degasser and SIL-10ADvp auto sampler (Shimadzu Scientific Instruments, Columbia, MD). Separation process was conducted at 30°C on Agilent® Eclipse XDB C-18 reversed-phase column (250 mm × 4.6 mm length, 5 µm particle size). Phenolic metabolites of the methanolic extract were characterized using a previously published modified method.[Citation31] Standard phenolic and flavonoid compounds including gallic acid, protocatechuic acid, (+)-catechin, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, (-)-epicatechin, syringic acid, vanillin, p-coumaric acid, ferulic acid, sinapic acid, benzoic acid, o-coumaric acid, rutin, naringin, hesperidin, rosmarinic acid, eriodictyol, trans-cinnamic acid, quercetin, luteolin, kaempferol, and apigenin were used for the analysis (Sigma-Aldrich, United States). Determination of phenolics and quantitative analysis were carried out by comparison with standard agents. The identification of the compounds was carried out by separate injection of each standard solution and also with the injection of the stock solution containing all standards. Thus, for each compound the resolution peak and the run time were determined. When all standards were eluted with good resolution, a chromatographic run was performed with the extracts spiked with the standard solution, to verify the correct identification of all compounds, as also the elution order. The quantity of each phenolic compound is expressed as µg/g of extract using external calibration curves, which were obtained for each phenolic standard individually.
Statistical analysis
All of the analysis were carried out in triplicates. The results are expressed as mean value ± SEM. Statistical comparisons were investigated by one-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test for multiple comparisons with control. Statistical analyses were performed using SPSS 16.0 software. A value of p < 0.05 was considered to indicate the statistical significance.
Results and discussion
Chemical composition of the essential oil
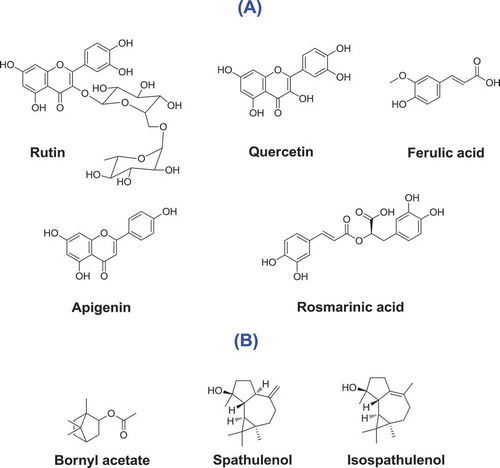
Essential oils contain many diverse volatile compounds. These complex and bioactive mixtures are widely used in the perfume, food, and pharmaceutical industries. Also, essential oils have broad ethno-botanical uses. In the present study, the chemical composition of the aerial parts of S. syriaca was determined (). This is the first report of essential oil composition of S. syriaca from Urmia. The plant is widely distributed in mountains and hills around the city. Previous studies reported germacrene B, germacrene D, and bicyclogermacrene as the main components of S. syriaca oil which are hydrocarbon sesquiterpenes. Interestingly, spathulenol and other oxygenated sesquiterpenes were identified as major components of the oil in this study (). The isolated oil had pale yellow color with strong odor. The oil was obtained in yield of 0.4% (v/w). GC-MS analysis led to the identification of 12 compounds comprising 98.9% of essential oil. Main components were identified as spathulenol (87.4%), isospathulenol (7.6%), and bornyl acetate (2.7%) as shown in . The presence of spathulenol as the major volatile compound in this study was also confirmed using standard spathulenol (Sigma-Aldrich). This sample has totally different composition from previous studies. It could be due to plant environment condition such as humidity, temperature, altitude, etc. Spathulenol as the most abundant volatile compound has several pharmacological activities such as immunoinhibitory, anti-inflammatory,[Citation32] anti-cancer, and apoptosis inducer.[Citation33] Due to the high percent of spathulenol and its biological properties, further pharmacological studies on the essential oil of S. syriaca (from Urmia) is warranted.
Table 1. Chemical composition of the essential oil from the aerial parts of Salvia syriaca.
Total bioactive compounds
Phenolic and flavonoid compounds are important natural agents because of their wide pharmacological effects such as antioxidant, anti-inflammation, anti-cancer, and antimicrobial. TPCs and TFCs from extracts of S. syriaca were determined according to the equations as gallic acid and QE (mg/g extract), respectively (y = 0.0096 × gallic acid (mg) + 0.1799, r2 = 0.989 and y = 0.0049 × quercetin (mg) + 0.0522, r2 = 0.998). TPCs of different extracts showed considerable differences ranging from 86 to 255 mg GAE/g dry extract ().The highest amount of TPC was observed in the methanolic extract. Similarly, the methanolic extracts had the highest TFCs (). TPCs and TFCs of S. syriaca are higher than previously studied Salvia species.[Citation34,Citation35]
Table 2. Antioxidant activity, total bioactive compounds, and toxicity of the essential oil and extracts of S. syriaca.a
Radical scavenging activity
Antioxidant compounds and radical scavengers are valuable dietary supplements which could protect the human health against several diseases. DPPH radicals scavenging test is a reliable method for the investigation of antioxidant capacity of natural or synthetic compounds. At the present work, radical scavenging activities of the extracts and essential oil of S. syriaca were evaluated using DPPH assay at different concentrations (0.05–4 mg/mL). As shown in the essential oil of S. syriaca showed the strongest antiradical activity (IC50 value of 45 µg/mL) followed by methanolic extract (IC50 value of 70 µg/mL). Essential oil was more effective than BHT in scavenging DPPH radicals. It may be due to presence of high amounts of spathulenol (87%) in the oil composition because of its proton donor and electron transfer abilities.
Ferric reducing capacity
The metal reducing capability of natural products is considered as an important indicator of their antioxidant activities. The ferric reducing antioxidant power (FRAP) of S. syriaca was examined (). The amount of conversion of Fe3+ cyanide complex to the ferrous form is measured spectroscopically, via the change of the yellow color of the solution to green. Higher absorbance of the reaction mixture at 700 nm shows increased reducing ability of the tested sample. The order of decreasing potency in FRAP assay of S. syriaca samples was EO> MeOH > n-hexane > DCM. The slopes of the trend lines (SRP) were calculated for samples which clearly shows the ferric reducing power.[Citation25] The results are similar to those obtained from the DPPH assay.
Cholinesterases inhibitory activity
AD is responsible for neurodegeneration which results in cognitive illnesses and finally death. Today, the inhibition of cholinesterases is regarded as an efficient strategy for management of AD.[Citation36] The genus Salvia is known in European folkloric medicine as memory enhancer herb.[Citation37] Moreover, Salvia species have been found to be effective on other central nervous system disorders.[Citation38] So, we aimed to evaluate the anti-Alzheimer’s potential of S. syriaca using enzyme inhibitory assays. The enzyme inhibitory potential of the tested essential oil and extracts in comparison with galantamine (standard anti-Alzheimer’s drug) were weak (). Dichloromethane extract was the most active sample. Findings showed S. syriaca has moderate cholinesterase inhibitory activity in comparison with previous studies on Salvia species.[Citation39,Citation40]
Table 3. Enzyme inhibitory activity of the extracts and essential oil of Salvia syriaca.a
α-Amylase and α-glucosidase inhibitory activity
One important strategy for treatment of diabetes mellitus is the inhibition of α-glucosidase and α-amylase. There are several classes of natural and synthetic compounds which have α-glucosidase and α-amylase inhibitory effects.[Citation3,Citation41] These enzyme inhibitor drugs could manage type 2 diabetes by reducing the glucose level of blood. In this work, all of the tested extracts and essential oil showed strong α-glucosidase inhibitory activity and moderate α-amylase inhibitory activity (). Acarbose was used as the standard antidiabetic drug (IC50 = 9.6 mg/mL and 1.33 mg/mL in α-glucosidase and α-amylase assays, respectively). In both of assays the essential oil exhibited the strongest activity (IC50 = 1.18 mg/mL and 1.54 mg/mL in α-glucosidase and α-amylase assays, respectively). Regarding to the results, further phytochemical studies for discovery of new antidiabetic constituents is recommended.
Tyrosinase inhibitory activity
The tested samples in this study did not show remarkable anti-tyrosinase activity in comparison with standard drug kojic acid (). Therefore, the extracts and essential oil of S. syriaca do not contain active metabolites to bond to the active site of tyrosinase.
Toxicity
Brine shrimp lethality assay is a simple test for preliminary evaluation of toxicity of natural compounds.[Citation30] The eggs of brine shrimp (Artemia salina) are available in market. The results of brine shrimp lethality assay could be correlated well with cytotoxicity potential of tested compounds against human cancer cells.[Citation42] In this study different extracts of S. syriaca together with its essential oil were tested for their brine shrimp lethality potential. LC50 values could be seen in . The essential oil showed high toxicity with LC50 value of 85 µg/mL. Also, the dichloromethane extract was relatively active (LC50 = 285 µg/mL). Other extracts showed weak toxicity. Plant derived extracts with LC50 values less than 1000 µg/mL could be considered active.[Citation43] Accordingly, the dichloromethane extract and the essential oil of S. syriaca could be regarded as good candidates for discovery of new anti-cancer natural agents.
Phenolic compounds profile
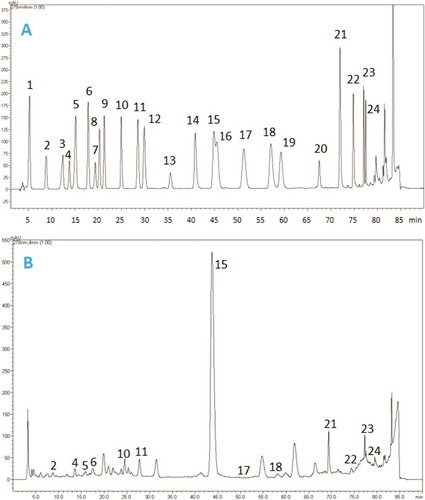
The methanolic extract of S. syriaca contains high phenolic and flavonoid contents due to TPC and TFC measurements (). We could find a good correlation between TPC and TFC of methanolic extract and antioxidant activities of this extract (DPPH and FRAP). In this context, we aimed to investigate the profile of phenolic components of this extract to have an insight on its bioactive compounds. Identification and quantification of phenolic metabolites were carried out by RP-HPLC–DAD (). Analytical characteristics (linearity range, limits of detection [LOD] and quantification [LOQ]) were determined to validate the HPLC method for quantification of phenolic components (). Phenolic components of S. syriaca were studied and quantified according to the mentioned method. As shown in , 24 standard phenolic compounds were used in analysis and the presence of 13 of them were confirmed in the extract (). Rutin, rosmarinic acid, ferulic acid, apigenin, and quercetin were the main phenolics. Chemical structures of major phenolic components detected in S. syriaca could be seen in . These metabolites found to be responsible for several pharmacological activities such as antioxidant, antimicrobial, enzyme inhibitory, antimalarial and anticancer.[Citation44,Citation45] The most abundant compound was characterized as rutin (9425 µg/g extract). A broad spectrum of significant pharmacological activities have been reported for rutin including anti-oxidation, anti-diabetic, anti-inflammation, neuroprotective, anti-adipogenic, and hormone therapy in vitro and in vivo.[Citation46,Citation47] Accordingly, the previously mentioned phenolics could be responsible for observed high DPPH radical scavenging capacity, ferric reducing activity and enzyme inhibitory effects of the methanolic extract. These results indicate that S. syriaca methanolic extract could be considered for several applications as pharmaceuticals and functional foods. To the best of our knowledge this is the first report on the phenolic compounds of S. syriaca.
Table 4. Quantitative results for determination of phenolic components in the methanol extract of S. syriaca.
Figure 2. HPLC chromatograms of A: standard phenolic compounds; and B: the methanolic extract of S. syriaca. Standard phenolics used for analysis are 1: gallic acid, 2: protocatechuic acid, 3: (+)-catechin, 4: p-hydroxybenzoic acid, 5: chlorogenic acid, 6: caffeic acid, 7: (-)-epicatechin, 8: syringic acid, 9: vanillin, 10: p-coumaric acid, 11: ferulic acid, 12: sinapic acid, 13: benzoic acid, 14: o-coumaric acid, 15: rutin, 16: naringin, 17: hesperidin, 18: rosmarinic acid, 19: eriodictyol, 20: trans-cinnamic acid, 21: quercetin, 22: luteolin, 23: kaempferol, and 24: apigenin.
Conclusion
This study was performed to investigate the pharmacological activities of S. syriaca and for identification of its bioactive phytoconstituents. Several extracts and the essential oil of the plant exhibited promising antidiabetic and antioxidant activities. Moreover, moderate anti-AD potential was observed for S. syriaca. GC-MS and HPLC analysis revealed that S. syriaca is rich in pharmacologically active natural products. These results showed that this species could be regarded as a valuable source of pharmaceuticals and nutriceuticals for possible food and medical applications.
Funding
Financial support by the Urmia University of Medical sciences is gratefully acknowledged (Project number: 1394-01-36-2104).
Additional information
Funding
References
- Moridi Farimani, M.; Bahadori, M.B.; Ahmadi Koulaei, S.; Salehi, P.; Nejad Ebrahimi, S.; Khavasi, H.R.; Hamburger, M. New Ursane Triterpenoids from Salvia Urmiensis Bunge: Absolute Configuration and Anti-Proliferative Activity. Fitoterapia 2015, 106, 1–6.
- Bahadori, M.B.; Valizadeh, H.; Asghari, B.; Dinparast, L.; Moridi Farimani, M.; Bahadori, S. Chemical Composition and Antimicrobial, Cytotoxicity, Antioxidant and Enzyme Inhibitory Activities of Salvia Spinosa L. Journal of Functional Foods 2015, 18(10), 727–736.
- Eskandani, M.; Bahadori, M.B.; Zengin, G.; Dinparast, L.; Bahadori, S. Novel Natural Agents from Lamiaceae Family: An Evaluation on Toxicity and Enzyme Inhibitory Potential linked to Diabetes Mellitus. Current Bioactive Compounds 2016, 12(1), 34–38.
- Bahadori, M.B.; Valizadeh, H.; Moridi Farimani, M. Chemical Composition and Antimicrobial Activity of the Volatile Oil of Salvia Santolinifolia Boiss. From Southeast of Iran. Pharmaceutical Sciences 2016, 22(1), 42–48.
- Topçu, G. Bioactive Triterpenoids from Salvia Species. Journal of Natural Products 2006, 69(3), 482–487.
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chemical Reviews 2012, 112(11), 5967–6026.
- Bahadori, M.B.; Mirzaei, M. Cytotoxicity, Antioxidant Activity, Total Flavonoid and Phenolic Contents of Salvia Urmiensis Bunge and Salvia Hydrangea DC. ex Benth. Research Journal of Pharmacognosy 2015, 2(2), 27–32.
- Moridi Farimani, M.; Nejad Ebrahimi, S.; Salehi, P.; Bahadori, M.B.; Sonboli, A.; Khavasi, H.R.; Zimmermann, S.; Kaiser, M.; Hamburger, M. Antitrypanosomal Triterpenoid with An ε-Lactone E-Ring from Salvia Urmiensis. Journal of Natural Products 2013, 76(9), 1806–1809.
- Ulubelen, A. Cardioactive and Antibacterial Terpenoids from Some Salvia Species. Phytochemistry 2003, 64(2), 395–399.
- Kafil, V.; Eskandani, M.; Omidi, Y.; Nazemiyeh, H.; Barar, J. Abietane Diterpenoid of Salvia Sahendica Boiss and Buhse Potently Inhibits MCF-7 Breast Carcinoma Cells by Suppression of the PI3K/AKT Pathway. RSC Advances 2015, 5(23), 18041–18050.
- Moridi Farimani, M.; Abbas-Mohammadi, M. Two New Polyhydroxylated Triterpenoids from Salvia Urmiensis and Their Cytotoxic Activity. Natural Product Research 2016, 30(23), 2648–2654.
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive Phytochemicals from Shoots and Roots of Salvia Species. Phytochemistry Reviews 2016, 15(5), 829–867.
- Bautista, E.; Toscano, R.n.A.; Ortega, A. 5, 10-Seco-Neo-Clerodanes and Neo-Clerodanes from Salvia Microphylla. Journal of Natural Products 2014, 77(4), 1088–1092.
- Alimpić, A.; Pljevljakušić, D.; Šavikin, K.; Knežević, A.; Ćurčić, M.; Veličković, D.; Stević, T.; Petrović, G.; Matevski, V.; Vukojević, J. Composition and Biological Effects of Salvia Ringens (Lamiaceae) Essential Oil and Extracts. Industrial Crops and Products 2015, 76, 702–709.
- Flamini, G.; Cioni, P.L.; Morelli, I.; Bader, A. Essential Oils of the Aerial Parts of Three Salvia Species from Jordan: Salvia Lanigera, S. Spinosa and S. Syriaca. Food Chemistry 2007, 100(2), 732–735.
- Hatam, N.A.; Yousif, N.J. Flavonoids from Salvia Syriaca. International Journal of Pharmacognosy 1992, 30(2), 109–111.
- Rustaiyan, A.; Sadjadi, A. Salvisyriacolide, a Sesterterpene from Salvia Syriaca. Phytochemistry 1987, 26(11), 3078–3079.
- Al-Jaber, H.I.; Abrouni, K.K.; Al-Qudah, M.A.; Abu Zarga, M.H. New Terpenes from Salvia Palaestina Benth. and Salvia Syriaca L. Growing Wild in Jordan. Journal of Asian Natural Products Research 2012, 14(7), 618–625.
- Al-Aboudi, A.M.; Abu Zarga, M.H.; Abu-Irmaileh, B.E.; Awwadi, F.F.; Khanfar, M.A. Three New Seco-Ursadiene Triterpenoids from Salvia Syriaca. Natural Product Research 2015, 29(2), 102–108.
- Bahadori, M.B.; Dinparast, L.; Valizadeh, H.; Moridi Farimani, M.; Nejad Ebrahimi, S. Bioactive Constituents from Roots of Salvia Syriaca L.: Acetylcholinesterase Inhibitory Activity and Molecular Docking Studies. South African Journal of Botany 2016, 106, 1–4.
- Ulubelen, A.; Oksüz, S.; Kolak, U.; Birman, H.; Voelter, W. Cardioactive Terpenoids and a New Rearranged Diterpene from Salvia Syriaca. Planta Medica 2000, 66(7), 627–629.
- Baser, K.; Demircakmak, B.; Ermin, N. Essential Oil of Salvia Syriaca L. Journal of Essential Oil Research 1996, 8(1), 105–106.
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Chemical and Biological Approaches on Nine Fruit Tree Leaves Collected from the Mediterranean Region of Turkey. Journal of Functional Foods 2016, 22, 518–532.
- Zengin, G. A Study on in Vitro Enzyme Inhibitory Properties of Asphodeline Anatolica: New Sources of Natural Inhibitors for Public Health Problems. Industrial Crops and Products 2016, 83, 39–43.
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of Antioxidant Activities and Total Phenolic Contents of Typical Malting Barley Varieties. Food Chemistry 2008, 107(1), 296–304.
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Antioxidant Potential and Inhibition of Key Enzymes Linked to Alzheimer’s Diseases and Diabetes Mellitus by Monoterpene-Rich Essential Oil from Sideritis Galatica Bornm. Endemic to Turkey. Records of Natural Products 2016, 10(2), 195–206.
- Ceylan, R.; Zengin, G.; Uysal, S.; Ilhan, V.; Aktumsek, A.; Kandemir, A.; Anwar, F. GC-MS Analysis and in Vitro Antioxidant and Enzyme Inhibitory Activities of Essential Oil from Aerial Parts of Endemic Thymus Spathulifolius Hausskn. et Velen. Journal of Enzyme Inhibition and Medicinal Chemistry 2016, 31(6), 983–990.
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant Potentials and Anticholinesterase Activities of Methanolic and Aqueous Extracts of Three Endemic Centaurea L. Species. Food and Chemical Toxicology 2013, 55, 290–296.
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum Myrtifolium Boiss. Endemic to Turkey and Its Inhibitory Potential Against Key Enzymes Involved in Alzheimer, Skin Diseases and Type II Diabetes. Industrial Crops and Products 2014, 53, 244–251.
- Valizadeh, H.; Sonboli, A.; Mahmoodi Kordi, F.; Dehghan, H.; Bahadori, M.B. Cytotoxicity, Antioxidant Activity and Phenolic Content of Eight Fern Species, from North of Iran. Pharmaceutical Sciences 2015, 21(1), 18–24.
- Caponio, F.; Alloggio, V.; Gomes, T. Phenolic Compounds of Virgin Olive Oil: Influence of Paste Preparation Techniques. Food Chemistry 1999, 64(2), 203–209.
- Ziaei, A.; Ramezani, M.; Wright, L.; Paetz, C.; Schneider, B.; Amirghofran, Z. Identification of Spathulenol in Salvia Mirzayanii and the Immunomodulatory Effects. Phytotherapy Research 2011, 25(4), 557–562.
- Martins, A.; Hajdú, Z.; Vasas, A.; Csupor-Löffler, B.; Molnár, J.; Hohmann, J. Spathulenol Inhibit the Human ABCB1 Efflux Pump. Planta Medica 2010, 76(12), 608.
- Loizzo, M.R.; Abouali, M.; Salehi, P.; Sonboli, A.; Kanani, M.; Menichini, F.; Tundis, R. In Vitro Antioxidant and Antiproliferative Activities of Nine Salvia Species. Natural Product Research 2014, 28(24), 2278–2285.
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, Antioxidant and Antimicrobial Activities and Phenolic Contents of Eleven Salvia Species from Iran. Iranian Journal of Pharmaceutical Research 2013, 12(4), 801–810.
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum Vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Industrial Crops and Products 2015, 70, 178–184.
- Çulhaoğlu, B.; Yapar, G.; Dirmenci, T.; Topçu, G. Bioactive Constituents of Salvia Chrysophylla Stapf. Natural Product Research 2013, 27(4–5), 438–447.
- Karami, M.; Gohari, A.R.; Naghshvar, F. Comparison effects of methanolic extracts of Salvia macrosiphon and Withania Coagulans on withdrawal syndrome in mice. Pharmaceutical Sciences 2013, 18(3), 183–186.
- Loizzo, M.R.; Tundis, R.; Conforti, F.; Menichini, F.; Bonesi, M.; Nadjafi, F.; Frega, N.G.; Menichini, F. Salvia Leriifolia Benth (Lamiaceae) Extract Demonstrates in Vitro Antioxidant Properties and Cholinesterase Inhibitory Activity. Nutrition Research 2010, 30(12), 823–830.
- Kolak, U.; Hacibekiroğlu, I.; Öztürk, M.; Özgökçe, F.; Topçu, G.; Ulubelen, A. Antioxidant and Anticholinesterase Constituents of Salvia Poculata. Turkish Journal of Chemistry 2009, 33(6), 813–823.
- Dinparast, L.; Valizadeh, H.; Bahadori, M.B.; Soltani, S.; Asghari, B.; Rashidi, M.-R. Design, Synthesis, α-Glucosidase Inhibitory Activity, Molecular Docking and QSAR Studies of Benzimidazole Derivatives. Journal of Molecular Structure 2016, 1114, 84–94.
- Anderson, J.; Goetz, C.; McLaughlin, J.; Suffness, M. A Blind Comparison of Simple Bench‐Top Bioassays and Human Tumour Cell Cytotoxicities As Antitumor Prescreens. Phytochemical Analysis 1991, 2(3), 107–111.
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Medica 1982, 45(5), 31–34.
- Lee, C.-C.; Shen, S.-R.; Lai, Y.-J.; Wu, S.-C. Rutin and Quercetin, Bioactive Compounds from Tartary Buckwheat, Prevent Liver Inflammatory Injury. Food & Function 2013, 4(5), 794–802.
- Deschner, E.E.; Ruperto, J.; Wong, G.; Newmark, H.L. Quercetin and Rutin As Inhibitors of Azoxymethanol-Induced Colonic Neoplasia. Carcinogenesis 1991, 12(7), 1193–1196.
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. Journal of Ethnopharmacology 2013, 150(3), 805–817.
- La Casa, C.; Villegas, I.; De La Lastra, C.A.; Motilva, V.; Calero, M.M. Evidence for Protective and Antioxidant Properties of Rutin, a Natural Flavone, Against Ethanol Induced Gastric Lesions. Journal of Ethnopharmacology 2000, 71(1), 45–53.