ABSTRACT
Hulless barley starch was extracted and further acetylated using acetic anhydride at different level (3.75, 7.5, 11.25 g/100 g) in this study. The structure changes and functional properties of acetylated hulless barley starches comparing to the native starch were evaluated and analyzed. The shape of granules remained unaltered with cracks formed after modification. Small- (1 μm) and large-sized (20 μm) were observed in four kinds of starches while granule particle sizes distribution changed dramatically. Four hulless barley starches presented A-type x-ray diffraction pattern, with relative crystallinity of 25.6, 27.1, 26.2, and 24.8% for native and acetylated starches. The infrared ratio of 1045/1024 and 1025/995 cm−1, indicated the difference in long-range order of crystallinities and short-range order of double helices. Results observed in swelling power, gelatinization parameters, pasting viscosities, and in vitro digestibility indicated acetylated hulless barley starch’s potential as a functional food additive and a healthy ingredient.
Introduction
Hulless barley, known in Chinese as qingke, is an economical crop widely grown in the highland of China with multiple applications in the food industry, such as low alcohol liquors, pastas, and in bakering.[Citation1] In terms of nutrient composition, hulless barley is comparable with commonly consumed cereals due to its high content of β-glucan and various trace elements.[Citation2]
Starch, the major component of hulless barley ranged from 58 to 67 g/100 g in different genotypes,[Citation3] has possessed diverse characteristics. Different properties are depended mainly on botanic resources which has been extensively studied and discussed.[Citation4–Citation7] The limit applications of native starches have encouraged researchers to explore many methods of modifications to broad the utilization. Acetylation is one of the most well studied modifications with acetyl percentage (Ac%) of less than 2.5 g/100 g recommended by the U.S. Food and Drug Administration (FDA) for food application.[Citation8] The acetylated starch is commonly obtained by esterification of native starch with acetic anhydride in the presence of an alkaline catalyst. Recently, studies have shown that iodine is an excellent acetylation catalyst and attracts large attention.[Citation9,Citation10] Many researches on improved freeze-thaw stability, decrease in gelatinization temperature, resistance to digestibility, and decline in viscosity by acetylation have been done.[Citation11,Citation12]
Most research[Citation1–Citation3] was focused on investigating isolation, composition, and physicochemical properties of hulless barley starch. However, information of the acetylation mechanism and the corresponding structural and functional properties are limited. Moreover, hulless barley starch is of great potential to be an alternative starch due to its cheap price and wide resource. Thus, acetylated hulless barley starch at different acelyl content level were prepared for further evaluation of structure changes and functional properties compared to the native starch in this study. The relationship between molecular order and physicochemical properties were analyzed and the potential applications in the food and non-food industries were predicted.
Materials and methods
Materials
Hulless barley grains were kindly provided by Highland Barley Resources Co., Ltd, Yunnan Province, China. Amylose (A0847), amylopectin (10120), and α-amylase (A3176, Type VI-B from porcine pancreas, 28 U/mg) were purchased from Sigma Chemical Co. (USA) while amyloglucosidase (3260 U/mL) and glucose oxidase/peroxidase (GOPOD) kit were purchased from Megazyme International Ireland Ltd. (Ireland).
Hulless barley starch isolation
Starch was isolated from hulless barley by the method of Singh et al.[Citation3] with modifications. Hulless barley grain was ground into hulless barley powder which was then sieved through 60-mesh screen. The hulless barley powder (120 g) was steeped in 400 mL of NaOH (0.1%, w/v) at 40°C water base for 8 h. The slurry was filtered through 100-mesh screen and then the filtrate was centrifuged at 2000 × g for 15 min. The top gray-colored, protein-rich layer was removed away and the substratum was the target starch which was then resuspended by excess water for centrifugation for several times until no protein layer could be found. The starch was washed and dried for 24 h at 40°C. The isolated starch from hulless barley showed approximately 99% purity (11% moisture, 0.3% protein, 0.5% fat, and 0.2% ash).
Preparation of acetylated starches
The acetylation reaction was conducted using the method described by Singh et al.[Citation13] with modifications. Starch (100 g, dry basis) was dispersed with 200 mL deionized water, stirred at 25°C for 30 min. Acetic anhydride(3.75, 7.5, 11.25 g) was added dropwise to the slurry within 20 s. During this course, the pH should be maintained at the range of 8.0~8.4 using 0.5 M NaOH. After 40 min of continuous stirring for acetylation, the reaction was stopped by adjusting the pH to 4.5 using 0.5 M HCl. The final suspension was centrifuged for 5 min at 2000 × g and the sediment was washed with ethyl alcohol and then distilled water to remove any remaining acetic anhydride and then dried at 40°C to 11% moisture.
Determination of Ac% and degree of substitution (DS)
The Ac% for calculating DS was determined by titration method as described by Bartz.[Citation14] Starch (1 g, dry basis) was incubated with 50 mL of 75% (v/v) ethanol in the water bath at 50°C for 30 min. The slurry was cooled to ambient temperature and then 40 mL of 0.5 M KOH was added. After constant stirring for 72 h at ambient temperature, excess alkali was titrated by 0.5 M HCl with phenolphthalein as an indicator. Then the solution was continually stirred for another 2 h and extra alkali which may have leached out was titrated as well and the native hulless barley starch was conducted in the same way as a blank.
DS is defined as the average number of sites per glucose unit that possess a substituent group.[Citation14]
Morphological properties
Scanning electron microscopy (JSM-6360LV, Jeol Ltd., Tokyo, Japan) was applied to describe the morphological properties of native and acetylated starches according to the method of Halal.[Citation15] Samples were dispersed by ethanol to acquire 1% (w/v) suspension and then were maintained in ultrasound for 10 min. A small quantity of each sample was spread on the stub. All samples were coated with gold and were examined at an acceleration voltage of 15 kV at 10,000×, 3000×, and 500× magnification.
Particle size distribution
The particle size distribution of starches were measured by laser particle size analyzer (LS230, Beckman Corporation, Massachusetts, USA). Starch (0.05 g) was suspended with 5 mL distilled water and a feed rate of 45 r/min was applied. One milliliter of sample was added to reach an obscuration of 40%.[Citation16] The size distribution was expressed in terms of the volumes of equivalent spheres while numbers and surface areas were calculated according to Utilla et al.[Citation17]
Crystalline structure analysis (x-ray diffraction)
The x-ray diffraction patterns of starched were obtained with an x-ray diffractometer (D/max2550V, Japan Rigaku Corporation, Tokyo, Japan) and the relative crystallinity was calculated as described by Huang[Citation18] with the following equation:
where Ac is the area of crystalline peak and At is total area measured from the baseline between 3 and 40°. The diffractometer was operated at voltage of 40 kV, current of 100 mA and scanning speed of 1°/min with a step of 0.02° ranging from 3 to 50°.
Iodine binding analysis and amylose content
The iodine binding analysis was determined using a colorimetric method under ultraviolet (UV)/visible spectrophotometer (UV-754N, Alpsh Intrument Co., Ltd., Shanghai, China). An iodine regent was acquired by adding 200 mg of I2 and 2 g of KI into 100 mL of deionized water. Each starch sample (50 mg, dry basis) was dispersed with 10 mL deionized water and 0.5 mL of starch suspension was added into 1.5 mL of iodine regent. The absorbance spectra and the wavelength of maximum absorption (λmax) were measured over a wavelength scan of 500~800 nm. The amylose content was determined according to Zhang.[Citation19]
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of starches were obtained by a FTIR spectrometer (IRAffinity-1, Shimadzu Corporation, Kyoto, Japan) equipped with an attenuated total reflectance (ATR) single reflectance cell in the range of 4000~400 cm−1. The original spectras were deconvoluted in the region from 1200 to 800 cm−1 and the ratios of absorbance at 1045/1024 and 1024/995 were used to estimate the short-range ordered structure of starches as described by Liu.[Citation20]
Thermal properties
Thermal properties of starches were acquired by using a differential scanning calorimetry (DSC; DSC-60, Shimadzu Corporation, Kyoto, Japan). Three milligrams (dry basis) of each sample was place in an aluminium pan with 9 μL of deionized water added. The pan was sealed and allowed to stand for 12 h at room temperature before analysis. The pan was heated from 30 to 90°C at a heating rate of 3°C/min with an empty pan as a reference. Retrogradation was determined using the same gelatinized sample which had been stored at –18°C for a week.
Pasting properties and swelling power
Brabender visco analyzer (Micro-Visco-amylograph, Duisburg, Germany) was applied to measure the pasting behavior of starches according to the method of Li[Citation21] with some modification. Starch slurries (8%, dry basis) were heated to 95°C, and then were held at 95°C for 5 min before cooling to 50°C, and then were held at 50°C for 1 min. Both heating and cooling rate were 7.5°C/min. The swelling power of starch granules was determined at temperature ranging from 50 to 90°C in 10°C intervals with starch/water (w/v) ratio of 1:100 according to Wang[Citation22] with some modifications. Starch swelling power was calculated as the ratio of the weight of sedimented swollen starch to the initial dry weight of starch.
In vitro enzymatic digestibility
In vitro enzymatic digestibility was measured by AACC approved method according to Ambigaipalan P.[Citation23] and the concentration of glucose which was determined by Megazyme GOPOD kit. Three kinds of starches were classified based on the rate of hydrolysis including rapidly digested starch (RDS, digested within 20 min), slowly digested starch (SDS, digested between 20 and 120 min), and resistant starch (RS; undigested starch after 120 min).
Statistical analysis
Analytical data were conducted in triplicate and standard deviations were reported for all of the data collected except for x-ray diffraction. One-way analysis of variance (ANOVA) was performed and the mean separation were performed by Tukey’s test at 5% significance level using SPSS Statistical Software Program (Version 19.0, SPSS Inc., Chicago, USA)
Results and discussion
Ac% and DS
The DS and Ac% of acetylated hulless barley starches were 0.029, 0.059, 0.085, and 0.765, 1.53, 2.20 with the acetic anhydride addition of 3.75, 7.5, 11.25 g/100 g, respectively. There was a significant effect between DS and acetic anhydride addition (R2, 0.998). The Ac% of all samples were under the maximum permissible level of 2.5% permitted for food formulation.[Citation4,Citation13] The acetylated starch were classified as low DS (<0.1), medium DS (0.1–1.0) and high DS (>1.0) acetates according to DS.[Citation4] The Ac% and DS could be the reason to reagent type, concentration, pH, reaction time, catalyst presence, catalyst type, botanical origin of starch and characteristics of size and structure of starch granules.
Morphology and size distribution of starch granule
The scanning electron micrographs (SEM) and particle size distribution of native and acetylated hulless barley starches are shown in . Hulless barley starch has small- (1 μm) and large-sized (20 μm) granules with lenticular and irregular shapes. Little difference of distribution in number ratio would be more distinguished through volume distribution () and surface area distribution () showing that the second peak of DS0.029 was much weaker than others. An unanticipated peak appearing around 100 μm for DS0.059 () could be explained by starch aggregation during acetylation and the agglomerated starch group of DS0.059 was observed through SEM while other samples have not been found ( did not give). These results agree with Singh[Citation13] who verified that granules surface of acetylated starch was less smooth than in native one, but the starch granules still kept a relatively complete particle structure. The aggregation of granules in the present study may be attributed to the introduction of hydrophilic groups which increased non-covalent binding between surface chains. Nevertheless, bi-modal size distribution characteristic was determined except tri-modal found in DS0.059. while multiple studies displayed diverse results showing uni-modal in ginkgo, potato, rice, maranta starches[Citation24,Citation25] bi-modal in wheat starch,[Citation26] and tri-modal in banaba starch.[Citation17] Interestingly, the deepest substituted sample DS0.085 performed most closely to the native one in particle size distribution, with DS0.029 having largest proportion of B-type granules, followed by DS0.059.
Figure 1. Morphology and particle size distribution of four starch granules. A: 10,000×; B: 3000×; C: 500×; and A: number ratio; B: volume ratio; C: surface area ratio, respectively.
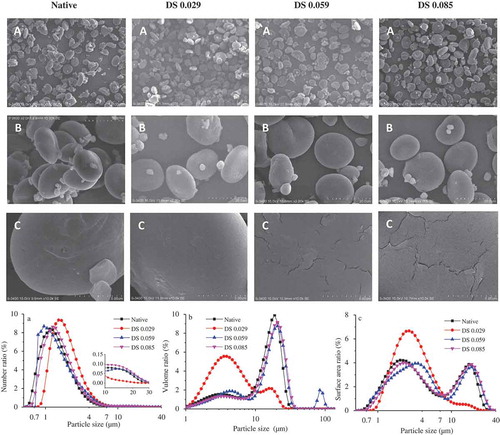
The shape and surface characteristic of starches were evaluated using SEM. There were almost two types of starch size, which was measured through particle size distribution as well. Namely A-type granules possessed big, smooth, and round or oval characteristics while B-type granules were the smaller ones with irregular edges. A-type granules were considered to possess higher amylose content, lower gelatinization temperature, and higher transition enthalpy being compared to B-type granules.[Citation26] Small particles were apt to stick to the big particles in all samples. More free small particles were found in DS0.059, which could be the reason for largest proportion of small particle size. In addition, more cracks and fissures were found with more advanced acetylation. However, no significant fusion,[Citation13] pores,[Citation27] or wrinkles[Citation14] was found in the present work which is recognized as common acetylation destruction and have been observed in many studies.
Both morpholohical performance and particle distribution revealed the effects of acetylation which inevitably conducts destruction to the surface of the starch granules. While disproportionate changes of particle size distribution caused by increased substituted degrees could be accounted for interactions of two aspects. Acetylation made B-type granules free from A-type granules that increased small particles while aggregation after acetylation could decrease B-type granule content. To some extent, particle properties including morphology and size are of great significance for further studies on rheology, function, and nutrition of starches.
Molecular structure of acetylated starch
Native and acetylated starches presented the similar diffraction patterns with strong peaks at 2θ = 15, 17, 18, 23°, indicating an A-type crystalline pattern. In addition, an increase in the intensity of peak at 2θ = 19° () was attributed to the formation of a V-type complex which was a consequence of the inserting of bulky group into starch chains according to Bartz.[Citation14] V-type crystal consists of amylose, emulgator, and fatty lipid,[Citation26] indicating that hulless barley starch especially acetylated one has potential for emulsifier application. Starch crystallites are considered to be developed by ordered arrangement of single and double helices which are formed by unbranched or slightly branched fractions of amylopectin with more than 10 glucose units.[Citation22,Citation26] The relative crystallinity was 25.6, 27.1, 26.2, and 24.8% for native starch, DS0.029, DS0.059, and DS0.085, respectively (). Many factors including degree of polymerization, substituent groups and water content could explain the crystallinity in addition to amylopectin content. Several researches[Citation13–Citation15] reported no significant reduction in crystallinity was found with low DS but reverse result was obtained with high DS, indicating that the acetyl groups were concentrated primarily in the amorphous region of starch with excess acetyl groups binding to crystal region. Low DS (all three samples) took place in present work that only reduction of the crystallinity can be found in the sample DS0.085, which displayed the most severe damages through SEM. The unexpected increase in the crystallinity of sample DS0.029 and DS0.059 could be explained by the size distribution performance. That is to say, small granules possess more compact structure. The collapse of acetylated starches could also be observed by iodine binding analysis (). The absorbance at λmax of starch varied accordingly to the degree of polymerization (DP).[Citation28,Citation29] The absorbance was 0.28, 0.28, 0.28, and 0.20, respectively, for native starch, DS0.029, DS0.059, and DS0.085 with λmax shifting from 640 to 620 nm gradually.
Figure 2. Molecular structure of four starches. A: XRD diffraction patterns; B: iodine binding analysis over wavelength scan of 500~800 nm; C: FTIR spectroscopy; D: deconvoluted FTIR spectroscopy from 1200 to 800 cm−1.
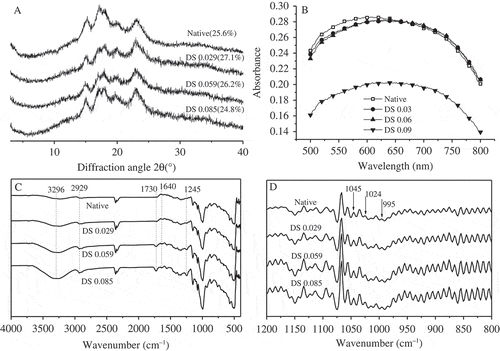
The FTIR spectra of native and acetylated starches with the wave number ranged from 400 to 4000 cm−1 (). The “fingerprint region” for polysaccharides is from 1000 to 1220 cm−1.[Citation14] Compared to native starch, acetylated starches exhibited similar patterns with some differences in some bond area. New peaks at 1245 and 1730 cm−1 were evidences of acetylation to starch, which were accepted to correspond to the stretching vibration of the C(=O)-O and C=O, respectively. As DS increased, certain peaks displayed more intense. A peak at 2929 cm−1 derived from the stretching vibration of the -CH groups was observed, and an intense peak at 1640 cm−1 relative to vibration of O-H of water absorbed in the amorphous domain of starch was recorded along with a peak at 3296 cm−1, which is resulted from the vibration of the hydrophilic –OH groups.[Citation27] That is to say, introduction of acetyl moiety during modification made starch molecular more hydrophilic, which might be attributed to more space for water binding after bulky acetyl group inserting. And the repulsion between adjacent starch molecules caused by negatively charged acetyl groups could be another explanation for increasing the level of hydrated molecules.
Deconvoluted ATR-FTIR spectrum () is widely used in diverse researches to show sensitive changes in structure on a molecular level (short-range order).[Citation22] The so-called short-range order reflects double helical order while XRD is responsive to the packing of double helices. The absorbances at 1045 and 1024 cm−1 have been confirmed to be separately related with ordered and amorphous structure of starch, and another band at 995 cm−1 is associated to the hydrated crystalline. Consequently, two ratios 1045/1024 cm−1 and 1024/995 cm−1 can be applied to qualify the short-range order of starch. The ratio of 1045/1024 cm−1 was 1.191, 1.239, 1.248, and 1.246, respectively, for native starch, DS0.029, DS0.059, and DS0.085 with no significant differences observed in ratio of 1022/995 cm−1, indicating that a tiny increase in molecular order occured with acetylation. The 1045/1024 cm−1 for potato and corn starches were 1.503 and 1.058, respectively. It can be inferred that the short-range order of starch largely depends on botanical resources and starches from hulless barley showed a moderate position between potato and corn starches in short-range order. Various studies exhibited positive correlation between crystallinity (detected by XRD) with short-range order[Citation24,Citation26] while no obvious relationship was found in present work, which could be attributed to two explanation. First, not all of the helical ordered starch molecules are arranged into crystals according to Liu.[Citation20] And second, the penetration depth of the IR beam in the ATR-FTIR system is about 2 μm. Thus, organisation investigated by ATR-FTIR is limited to the regions near the granule surface leading to incomplete measurement inside the granule, where is thought to be rich in ordered structure.[Citation23]
Thermal and other functional properties of starch
During the gelatinization process, a decline trend in transition temperatures could be observed with the increased acetyl content, showing the introduction of voluminous hydrophilic groups to the chain enhances structure flexibility. It will be vulnerable for water binding and leads to premature rupture of double helices during heating.[Citation13,Citation21] Transition temperatures represent crystal stability while ΔH reflects the melting enthalpy of both packing crystal and amylopectin based double helix and ΔH was as well confirmed to be positively correlated to be relative crystallinity in many studies.[Citation20,Citation24,Citation25] However, change in ΔH by acetylation in present work was not significantly effect on crystallinity (R2, 0.156) and the similar results in other researches[Citation14,Citation17,Citation26,Citation30]were found, and it could be explained by the incomplete swelling of starches due to a starch/water ratio of 1:3 during DSC measurement. Therefore, starch ΔH of gelatinization depends primarily on short-range order (R2 [with 1047/1022cm−1], 0.908) which is consistent with the results of Yu et al.[Citation26,Citation30,Citation31] To investigate the retrogradation of starch gels, gelatinized starch gels after DSC were stored at 4°C for 7 days and then were scanned for a second time. No significant endothermic peak of cooled gels, indicated that retrogradation of starch gels was too little to be measured by DSC ().
Figure 3. Thermal and pasting properties of four starches. A: Thermogram of gelatinization and retrogradation; B: Pasting curves measured by Brabender Viscograph.
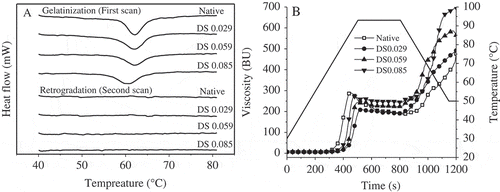
Swelling power increased with the increasing of temperature while swelling power after acetylation was not proportional to the insertion of acetyl groups (). Specifically, a significant decreasing in swelling power with low DS was observed and then an increasing tendency was predicted. It is widely accepted that swelling of granule is the first step in pasting procedure and plays a vital role in various characteristics of diverse botanic starches. However, many researchers got different results of acetylated starches. Halal[Citation15] found reduced swelling power after acetylation of barley starch while Colussi[Citation4] observed the opposite trend in rice starch.
Table 1. Functional properties of four starches.
Pasting viscosity properties including peaking viscosity (PV) and final viscosity (FV) are supposed to have significant effect on swelling power and additionally, the higher viscosity could be attributed to its larger particle size, which makes sense in present work. The high pasting temperature (PT) and low viscosity in DS 0.029 could due to its large proportion of B-type particle and high amylose content. The acetylated starches exhibited lower breakdown viscosity (BD) which represents stability of hot starch paste under the shear force comparing to native starch. While increased setback (SB) viscosity was measured with increasing of acetyl groups inserting, indicating an susceptibility to retrogradation after acetylation.
Both DSC and Brabender Viscograph (BV) are widely accepted to detect the parameters of interaction of starch granules and water, but inconsistent results by two instruments were determined in present work. DSC is commonly used where there is a phase transition. However, the small sample size may cause quite a large error in enthalpy, especially in the case when the peak of the endotherm is not so regular. BV can reflect the process of either swelling or reintegrating of starch. Both DSC and BV are possible when only a small sample size is available and rapidity is required.[Citation32]
Nutritional fractions
RDS, SDS, and RS of native and acetylated starches were determined by in vitro digestibility with a mixture of porcine pancreatic α-amylase and amyloglucosidase (). Digestibility is of nutritional value which is affected by granular form, molecular structure, and processing conditions. Granular form including particle size and surface characteristics (for example, pores, cracks, or fusions) determines the speed of amylase’s early binding. Molecular structure factors consisting of amylose/amylopectin ratio, amylopectin structure, type and degree of crystallinity, phosphorus content could be the main causes of diverse digestibility. And the processing by heat, cool, or moisture treatment could also have effect on digestibility as many studies have reported.[Citation33–Citation35]
Table 2. In vitro enzymatic digestibility of four starches.
The largest RS content was found in DS0.029 and the biggest SDS content was native hulless barley starch. Foods in high content of SDS and RS possess low glycemic indices.[Citation36,Citation37] A positive correlation between RS content and amylose content (R2; 0.905) and a close relation between SDS content and ΔH detected by DSC (R2, 0.653) in the study similar to Yao[Citation33] with amylose was the molecular basis of RS and amylopectin played a key role in the structure of SDS and was the main constituent of SDS. The different RDS levels reflects the interplay between the surface characteristics and the extent of molecular order at the granule surface while the SDS and RS differences can be accounted for crystalline stability and inner molecular order. The SDS and RS contents of potato starch (9.65 and 30.94%, respectively) and corn starch (6.93 and 16.94%, respectively) were determined as well for comparison. Naive potato starch with long amylose chain are resistant to enzymatic digestion and similar to hulless barley starch. It indicated hulless barley starch especially acetylated starches is of great nutritional potential as a dietary fiber. Starches from bananas and[Citation17,Citation31] peas[Citation33] showed even higher resistance while some starches from botanical source like ginkgo,[Citation24,Citation30] pearl millet,[Citation34] waxy maize[Citation22] were much more susceptible to enzymatic digestion.
Botanical origin is considered to be a major source of variability in digestibility. B-type or C-type crystallites starch shows much more resistance to enzyme hydrolysis than A-type[Citation24,Citation34] while hydrolysis speed of α-1,4 linkage is 30 times greater than that of α-1,6 linkage.[Citation28,Citation29] A-type crystallites is weaker because its α-1,6 branch points is present in the crystalline region with B-type starch’s α-1,6 branch points being present solely in the amorphous regions (Ambigaipalan P.).[Citation23]
Conclusion
The acetylated hulless barley starches with different levels which was confirmed by the changes observed in the FTIR bands and peaks, had significant differences in their functional properties. However, diverse characteristics were not linearly affected by the increasing acetyl groups. Analysis by XRD, FTIR, DSC, and iodine binding analysis indicated the starch molecule suffered different modification after acetylation. While the digestibility depended on the DS and the integrity of starch granules. The acetylated starches had higher FV comparing to native starch, indicating a potential of thickening power after heating to be used as dehydrated soup and sauces. The obvious low BD revealed a resistance to shear and heat, which could be applied in heating process. The acetylation decreased gelatinization temperatures and enthalpy which was important to industry due to low power applied to the process to reduce costs. The increased RS content after acetylation proved its nutritional value as a low glycemic index food. However, the applications of acetylated hulless barley starch still needs further study including its eating quality and safety evaluation.
References
- Liu, Z.F.; Yao, Z.J.; Zhi, J.; Yu, C.Q.; Zhong, Z.M. Assessing Crop Water Demand and Deficit for the Growth of Spring Highland Barley in Tibet, China. Journal of Integrative Agriculture 2013, 12(3), 541–551.
- Hadado, T.T.; Rau, D.; Bitocchi, E.; Papa, R. Genetic Diversity of Barley (Hordeum Vulgare L.) Landraces from the Central Highlands of Ethiopia: Comparison Between the Belg and Meher Growing Seasons Using Morphological Traits. Genetic Resources and Crop Evolution 2009, 56(8), 1131–1148.
- Gao, J.; Vasanthan, T.; Hoover, R. Isolation and Characterization of High-Purity Starch Isolates from Regular, Waxy, and High-Amylose Hulless Barley Grains. Cereal Chemistry 2009, 86(2), 157–163.
- Colussi, R.; Halal, S.L.M.E.; Pinto, V.Z.; Bartz, J. Acetylation of Rice Starch in An Aqueous Medium for Use in Food. LWT–Food Science and Technology 2015, 62(2), 1076–1082.
- Wu, Y.; Lin, Q.L.; Cui, T.; Xiao, H.X. Structural and Physical Properties of Starches Isolated from Six Varieties of Millet Grown in China. International Journal of Food Properties 2014, 17(10), 2344–2360.
- Thirathumthavorn, D.; Trisuth, T. Gelatinization and Retrogradation Properties of Native and Hydroxypropylated Crosslinked Tapioca Starches with Added Sucrose and Sodium Chloride. International Journal of Food Properties 2008, 11(4), 858–864.
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Source. Food Chemistry 2003, 81(2), 219–231.
- Ashogbon, A.O.; Akintayo, E.T. Recent Trend in the Physical and Chemical Modification of Starches from Different Botanical Sources. Starch/Stärke 2014, 66(1–2), 41–57.
- Diop, C.I.K.; Li, H.L.; Xie, B.J.; Shi, J. Impact of the Catalytic Activity of Iodine on the Granule Morphology, Crystalline Structure, Thermal Properties and Water Solubility of Acetylated Corn (Zea Mays) Starch Synthesized Under Microwave Assistance. Industrial Crops and Products 2011, 33(2), 302–309.
- Sánchez-Rivera, M.M.; Almanza-Benitez, S.; Bello-Perez, L.A. Acetylation of Banana (Musa Paradisiaca L.) and Corn (Zea Mays L.) Starches Using a Microwave Heating Procedure and Iodine As Catalyst: II. Rheological and Structural Studies. Carbohydrate Polymers 2013, 92(2), 1256–1261.
- Yu, S.X.; Mu, T.H.; Zhang, M.; Ma, M.M.; Zhao, Z.K. Effects of Retrogradation and Further Acetylation on the Digestibility and Physicochemical Properties of Purple Sweet Potato Flour and Starch. Starch/Stärke 2015, 67(9–10), 892–902.
- Yadav, D.K.; Patki, P.E. Effect of Acetyl Esterification on Physicochemical Properties of Chick Pea (Cicer Arietinum L.) Starch. Food Science and Technology 2015, 52(7):4176–4185.
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and Functional Properties of Acetylated Sorghum Starch. International Journal of Food Properties 2012, 15(1–2), 312–325.
- Bartz, J.; Goebel, J.T.; Giovanaz, M.A.; Zavareze, E.D.A. Acetylation of Barnyard Grass Starch with Acetic Anhydride Under Iodine Catalysis. Food Chemistry 2015, 178, 236–242.
- Halal, S.L.M.; Collssi, R.; Pinto, V.Z.; Bartz, J. Structure. Morphology and Functionality of Acetylated and Oxidised Barley Starches. Food Chemistry 2015, 168, 247–256.
- Rodriguez, H.M.P.; Acevedo, E.A.; Montealvo, G.M. Effect of Acid Treatment on the Physicochemical and Structural Characteristics of Starches from Different Botanical Sources. Starch/Stärke 2012, 64(2), 115–125.
- Utrilla-Coello, R.G.; Rodriguez-Huezo, M.E.; Carrillo-Navas, H. In Vitro Digestibility, Physicochemical, Thermal and Rheological Properties of Banana Starches. Carbohydrate Polymers 2014, 101, 154–162.
- Huang, J.R.; Henk, A.S.; Jeroen, J.G. Physicochemical Properties and Amylopectin Chain Profiles of Cowpea, Chickpea and Yellow Pea Starches. Food Chemistry 2007, 101(4), 1338–1345.
- Zhang, H.X.; Zhang, W.X.; Xu, C.Z. Studies on the Rheological and Gelatinization Characteristics of Waxy Wheat Flour. International Journal of Biological Macromolecules 2014, 64, 123–129.
- Liu, Y.; Chen, W.Y.; Chen, C.; Zhang, J. Physicochemical Property of Starch-Soluble Dietary Fiber Conjugates and Their Resistance to Enzymatic Hydrolysis. International Journal of Food Properties 2015, 18(11), 2457–2471.
- Li, G.L.; Zeng, J.; Gao, H.Y.; Li, X.H. Characterization of Phosphate Monoester Resistant Starch. International Journal of Food Properties 2011, 14(5), 978–987.
- Wang, S.J.; Wang, J.R.; Zhang, W.; Li, C.L.; Yu, J.L. Molecular Order and Functional Properties of Starches from Three Waxy Wheat Varieties Grown in China. Food Chemistry 2015, 181, 43–50.
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Starch Chain Interactions Within the Amorphous and Crystalline Domains of Pulse Starches During Heat-Moisture Treatment at Different Temperatures and Their Impact on Physicochemical Properties. Food Chemistry 2014, 143, 175–184.
- Cai, J.W.; Cai, C.H.; Man, J.M.; Xu, B.; Wei, C.X. Physicochemical Properties of Ginkgo Kernal Starch. International Journal of Food Properties 2015, 18(2), 380–391.
- Valencia, G.A.; Moraes, L.C.F.; Lourengo, R.V. Physicochemical Properties of Maranta (Maranta Arundinacea L.) Starch. International Journal of Food Properties 2015, 18(9), 1990–2001.
- Yu, X.R.; Yu, H.; Zhang, J.; Shao, S.S.; Zhou, L.; Xiong, F.; Wang, Z. Comparison of Endosperm Starch Granule Development and Physicochemical Properties of Starched from Waxy and Non-Waxy Wheat. International Journal of Food Properties 2015, 18(11), 2409–2421.
- Xu, S.S.; Zhang, L.B.; Li, B.; Li, J.; Zhou, B.; Yan, J.J.; Song, R.K. Preparation and Physical Characteristics of Resistant Starch (Type 4) in Acetylated Indica Rice. Food Chemistry 2012, 134, 149–154.
- Miao, M.; Xiong, S.S.; Jiang, B.; Jiang, H.; Cui, S.W.; Zhang, T. Dual-Enzymatic Modification of Maize Starch for Increasing Slow Digestion Property. Food Hydrocolloids 2014, 38, 180–185.
- Miao, M.; Li, R.; Huang, C.; Ye, F.; Jiang, B.; Zhang, T. Structural Modification and Characterisation of a Sugary Maize Soluble Starch Particle after Enzyme Treatment. Carbohydrate Polymers 2015, 122, 101–107.
- Zheng, Y.; Zhang, H.X.; Yao, C.; Hu, L.L.; Peng, Y.J.; Shen, J. Study on Physicochemical and in-Vitro Enzymatic Hydrolysis Properties of Ginkgo (Ginkgo Biloba) Starch. Food Hydrocolloids 2015, 48, 312–319.
- Zhang, P.Y.; Hamaker, B.R. Banana Starch Structure and Digestibility. Carbohydrate Polymers 2012, 87(2), 1552–1558.
- Qian, J.Y.; Kuhn, M. Evaluation on Gelatinization of Buckwheat Starch: A Comparative Study of Brabender Viscoamylography, Rapid Visco-Analysis, and Differential Scanning Calorimetry. European Food Research and Technology 1999, 29(3), 277–280.
- Yao, W.R.; Liu, C.G.; Xi, X.J.; Wang, H.Y. Impact of Process Conditions on Digestibility of Pea Starch. International Journal of Food Properties 2010, 13(6), 1355–1363.
- Suma, F.S.; Urooj, A. Isolation and Characterization of Starch from Pearl Millet (Pennisetum Typhoidium) Flours. International Journal of Food Properties 2015, 18(12), 2675–2687.
- Mar, N.N.; Umemoto, T.; Akmar, S.N.; Maziah, M. Chain Length Distribution of Amylopectin and Physicochemical Properties of Starch in Myanmar Rice Cultivars. International Journal of Food Properties 2015, 18(8), 1719–1730.
- Kiatponglarp, W.; Tongta, S.; Rolland-Sabate, A.; Buleon, A. Crystallization and Chain Reorganization of Debranched Rice Starches in Relation to Resistant Starch Formation. Carbohydrate Polymers 2015, 122, 108–114.
- Menon, R.; Padmaja, G.; Sajeev, M.S. Ultrastructural and Starch Digestibility Characteristics of Sweet Potato Spaghetti: Effects of Edible Gums and Fibers. International Journal of Food Properties 2015, 18(6), 1231–1247.
