ABSTRACT
The development of the bio-nanocomposite coating materials for eggshells has received more attention because of the decline in interior egg quality. This work evaluated the effects of weight loss, Haugh unit, yolk index, air space and albumen pH, eggshell strength, number of microorganisms on eggshells, contact angle and water vapor permeability of uncoated eggs and eggs coated with soy protein isolate/montmorillonite emulsions (5/0, 5/0.20, 5/0.50, 5/0.80; w/w) during 6 weeks at 25°C. A scanning electron microscope was used to visualize morphologies of soy protein isolate and the soy protein isolate/montmorillonite composite coatings, and the scanning probe microscope was determined. Compared with uncoated and soy protein isolate-coated eggs, the interior egg quality was improved by the increase of Haugh unit, yolk index, air space or eggshell strength, and decrease in weight loss, albumen pH, or the number of microorganisms on eggshells. The higher contact angle indicated the hydrophobicity of coatings. The values of water vapor permeability with 0.2% montmorillonite, 0.5% montmorillonite, and 0.8% montmorillonite were reduced by 21.14, 57.78, and 69.13%, respectively, as compared to those of soy protein isolate coatings. The images by a scanning electron microscope indicated that coatings could cover the cracks of eggshells to preserve the interior egg quality. The experimental values of the scanning probe microscope showed that montmorillonite would not penetrate through the eggshell pores, and it was safe to coat with montmorillonite. This study highlights the use of soy protein isolate/montmorillonite coating to maintain the internal quality and enhance the shelf life of eggs stored at 25°C.
Introduction
Eggs, an excellent source of protein and other nutrients, have played an indispensable role in people’s daily diet. Eggs also have many different functional properties that make them useful in various foods.[Citation1] However, the aging process begins immediately after hens lay eggs, the eggs are perishable immediately, which could alter their chemical, physical, and functional properties. As a result, several problems are encountered in storing eggs, such as weight loss, deterioration of the interior quality, and microbial contamination.[Citation2,Citation3] Through eggshell pores, the eggshell breakages and the loss of moisture and carbon dioxide could become a cause for the quality losses.[Citation4]
Coatings can act as a barrier for moisture, gas, and aroma transfer. To overcome these problems, researchers have paid considerable attention to the development of coating materials from polysaccharides,[Citation5,Citation6] starch,[Citation7] oil,[Citation8–Citation10] protein,[Citation11] and synthetic polymers.[Citation12] Soy proteins have been reported as an effective, low-cost coating material to reduce the weight loss and retard changes in color and pH of egg white and yolk.[Citation13] Wong et al.[Citation14] reported that soy protein coatings could enhance mechanical properties of shell eggs and interior quality. Xie et al.[Citation6] showed that soy protein isolate (SPI), whey protein isolate (WPI), and wheat gluten coatings could heighten mechanical properties of shell eggs and minimize microbial contamination in eggs.
SPI is a commercial form of soy proteins that contain more than 90% of protein. SPI as a raw material has shown advantages over other sources due to its exceptional film-forming properties, barrier properties against oxygen, aromas, or lipids, and its low cost. Soy proteins are also among the most abundant and economic and can be recovered as a recyclable by-product of the vegetable oil industry.[Citation15] Recent studies have included physical, chemical, and enzymatic treatments on SPI or addition of lipids, fiber, nano-reinforcements, and other compounds to activate the films with other properties. Numerous studies have reported that cross-linked montmorillonite (MMT) was beneficial to enhance properties of soy protein hydrogels and films.[Citation16] MMT is a polymer-layered silicate nanocomposite and is environmentally friendly, abundant in nature, and inexpensive. The polymer-layered silicate nanocomposites frequently exhibit improvements in molecular barrier properties and thermal and mechanical characteristics in comparison to pure polymers.[Citation16,Citation17]
SPI-MMT coatings may improve the shell strength to a certain extent and offer a protective barrier against moisture and transfer oxygen across the eggshell, thus extending the shelf life of eggs. SPI-MMT nanocomposite films have been reported to be able to reduce the permeability of water vapor and oxygen, while MMT could increase elastic modulus and tensile strength.[Citation18,Citation19] Recently, Echeverría et al.[Citation28] claimed that MMT acted as a strengthening component when added to soy protein films; it could improve the resistance to mechanical deformation, protein solubility in water, and water vapor permeability (WVP). However, none of the previous studies provides detailed information regarding the quality of eggs after applying such coatings.
Therefore, the present study aims to evaluate the effects on weight loss, Haugh unit (HU), yolk index (YI), air space and albumen pH, eggshell strength, the number of microorganisms on eggshell, CA and WVP of SPI-MMT-coated eggs at room temperature (25°C). At the same time, the morphology and safety of coatings were measured by scanning electron microscope (SEM) and a scanning probe microscope (SPM), respectively.
Materials and methods
Materials
SPI was supplied by Harbin High-tech Company. MMT without organic modification was purchased from Southern Clay Products (USA). Distilled water was used for all sample preparations. All other chemicals were analytical grade. Fresh eggs were purchased from a local eggs producer in Harbin.
Preparation of coating solutions
The SPI coating solution was prepared by mixing 5 g SPI, 3 g glycerol, and 100 mL water with a magnetic stirrer. pH was adjusted to 10.5 using 2.0 N NaOH, and the solution was placed in a water bath at 85°C for 30 min. For the SPI-MMT coating solution, 5 g SPI was dispersed in 80 mL distilled water at room temperature (25 ± 2°C) with a magnetic stirrer, and pH was adjusted to 10.5 with 2.0 N NaOH in triplicate. Likewise, different amounts of the MMT (0.2, 0.5, and 0.8 g) and 3 g glycerol were dispersed in 20 mL distilled water for 1 h, respectively, by sonication with a Sonics Vibra Cell model VCX 750 at 80% amplitude (Sonics & Materials, Inc, USA). The dispersions of SPI and MMT-glycerol were mixed under stirring for 1 h at room temperature, and then the solutions were stirred at 80°C for 30 min. Finally, the solutions were centrifuged at 3500 r/min for 5 min at room temperature to eliminate bubbles.
Coating applications
After washing with water, 60 dozen eggs were immersed individually in coating solutions for 1 min, and then the eggs were wiped to remove excess solution and dried in an incubator at a temperature of 45°C for 5 min (there was no effect on the quality of eggs). The previous procedure was repeated once more. After coating, all eggs were placed in cardboard egg racks and stored in an incubator at room temperature and 50 ± 5% relative humidity (RH) for 6 weeks. Ten eggs were randomly taken at 1-week intervals for the determination of weight loss, HUs, YI, albumen pH, air space, and the number of microorganisms, and three measurements were made and averaged for each sample.[Citation20,Citation21]
Weight loss
All eggs were weighted at specific time intervals, the weight loss was calculated by subtracting the final weight from the initial weight of sample with coating and then dividing by the initial weight of samples.[Citation22] Egg weights were measured with a digital electronic balance (Kern PFB, Kern & Sohn Gmbh, Germany) and recorded to within 0.01 g. For uncoated samples, weights of non-coated eggs were used for calculation.
HU and YI
The height of albumen and yolk and the width of yolk were measured with a tripod micrometer (AMES S-6428, Waltham, Mass, USA) and a digital caliper (CD-20B, Mitutoyo, Japan), respectively. Then, the HU and YI were calculated by the following equations:[Citation23]
where is the height of the albumen (mm);
is the weight of the egg (g);
is the height of the yolk (mm), and
is the diameter of the yolk (mm).
Air space
The depth of the air space was measured by using a digital caliper after marking the outline of air space, and the depth is the distance from the top of air space to its bottom.[Citation23] The depth of the air space was measured and recorded to within 0.01 mm.
Albumen pH
After measuring the HU and YI, the albumen was separated from the yolk for further testing. The thin and thick albumen were blended with a glass rod before being measured for pH with a pH meter (IQ150, IQ Scientific Instruments, San Diego, CA, USA).[Citation24]
Shell strength
Shell strength measurement was performed on eggs using a texture analyzer (TA; TA.XT2, Texture Technologies Crop, Scarsdale, NY). Each egg was mounted on a platform and eggshells were punctured at the top (small end), bottom (large end), and middle of the eggshells using a 2 mm die probe at 5 mm/s constant speed with a 30 kg.[Citation7] The force required to puncture an eggshell (as kgf) was recorded and expressed as the shell strength of the eggshell.
Microorganism
The total number of microorganisms was used to test the antimicrobial activities of the different coatings using the contact plate method, the strain was isolated from the surface of the eggshells. Each egg was wiped with a sterile cotton swab dipped in the sterile saline, and the cotton swab was blended in the 10 mL sterile saline. The contact plate method was consistent with the method of Leleu et al.[Citation25]
Contact angle (CA)
The CA measurements were determined at room temperature by a homebred CA goniometer system (model OCA 20) using the distilled water as the solvent. Both the left and right sides were tested and then averaged.[Citation26]
WVP
WVP was measured using a modified cup method (ASTM Standard E96M-10, 2010) with an analytical balance (ML204, Mettler Toledo, Barcelona, Spain).[Citation26] The contents of eggs punched a 10 mm diameter hole in the middle of the top by a mini drill were removed, and then, after getting dry with a fan, the eggshells were coated by coating solutions. These eggshells were then filled with 20 g CaCl2 before being packaged with gauze, and conditioned for 24 h at 25°C and 65% RH to reach equilibrium. The variation of the eggshell was recorded after 24 h. WVP was calculated by using the following equation:
where is the water vapor transmission rate through a coated eggshell with coating (g/m2·s);
is the coating thickness (m), and
is the partial water vapor pressure difference across two sides of the coated eggshell (Pa).
SEM
Microstructures of the fracture surface (cross-sectional surface) of eggshells were examined by a SEM (FEI Quanta 200; Netherlands) operating at 12.5 kV. Small pieces (0.5 × 0.5 cm) of bio-nanocomposite eggshells were frozen in liquid nitrogen, cut using a sharp razor blade, and mounted on specimen stubs with a double-sided carbon tape. Before the measurement of the SEM, the surfaces of eggshells were sprayed with a thin layer of gold.[Citation26]
SPM
The energy spectrum of the eggshells was examined to ensure the safety of the SPI-MMT coated eggs by a SPM (ICON3100, Bruker, Germany).[Citation27] The surface and the pores of eggshells were measured to determine the content of each element.
Statistical analysis
Experimental values were analyzed by using OriginPro 8.5 and SPSS 13.0 software, and expressed as mean ± standard deviation (SD). The significance level used was 0.05.
Results and discussion
Weight loss
shows the weight loss over time, and exhibits that the weight loss of eggs increased when stored at room temperature because of water loss, loss of carbon dioxide from albumen through the eggshell and storage environment. After 1-week of storage, the weight loss of uncoated eggs was significantly more than that of eggs under other treatments. Eggs coated with SPI exhibited a significant weight loss after 2 weeks of storage. After 6 weeks of storage, the eggs coated with SPI-0.2% MMT, SPI-0.5% MMT, and SPI-0.8% MMT had weight losses of 7.13, 8.56, and 8.91%. respectively, whereas the uncoated and SPI eggs had losses of 14.19 and 10.45%, respectively. Therefore, SPI-MMT acted as a more effective measure in maintaining the weight of eggs during short-term storage (6 weeks). According to the report of Echeverría et al., due to the addition of MMT to the formulation, a diminution in WVP upon addition of MMT to the protein matrix can be attributed to a decrease in both the water solubility coefficient and the diffusion of water throughout the film, with the latter effect being the more pronounced.[Citation28] Similar observations were reported by Lee et al.[Citation29]
Table 1. Effect of different coatings on weight loss during 6 weeks of storage.
There was no significant difference in weight loss of all eggs coated with SPI-MMT after 1 week of storage, but weight loss increased over time progressively and began to be remarkable (p ≤ 0.05) among different SPI-MMT-coated eggs. After 3 weeks of storage, a significant weight loss was observed among the eggs coated with SPI-0.2% MMT, SPI-0.5% MMT, and SPI-0.8% MMT. On the 6th week of storage, significant differences were observed between eggs coated with SPI-0.2% MMT and those coated with other treatments. It implied that the use of a higher percentage of MMT might not be advantageous in minimizing weight loss than a lower percentage, and the addition of MMT to the formulation would produce a drop in weight loss, especially for MMT contents of 2 g per 100 g SPI (at which concentration a decline of 2% in the WVP was observed). Bhale et al.[Citation30] reported that weight loss in 1 and 2% chitosan-coated eggs was in a range of 4.40–6.31%, after 28 days of storage at 25°C. It is similar to the weight loss of SPI-MMT-coated eggs. Therefore, SPI/MMT coatings on eggs have great application prospects.
HU
HUs are the primary index of quality in the egg industry. The higher the HU value is, the better the quality of albumen is. Most fresh eggs leaving the farm should be between 85 and 75 HU,[Citation30] while an older yolk would have a lower HU value.[Citation31,Citation32] It attributes to the decomposition of organic compounds. According to , at 1 week, no significant differences were observed among treatments. Significant HUs differences were observed between uncoated eggs and eggs under other treatments after 2 weeks of storage. Significant differences were exhibited between uncoated or SPI eggs and SPI-MMT eggs after 3 weeks of storage. After 6 weeks of storage, the eggs with SPI-0.2% MMT (64.43) and SPI-0.5% MMT (62.95) had the higher HUs without significant differences, and SPI-0.2% MMT, SPI-0.5% MMT, and SPI-0.8% MMT eggs maintained an average AA (HU > 72) quality for 4, 3, and 3 weeks, respectively. This means that eggs coated with SPI-MMT emulsion could preserve the albumen quality for at least 3 weeks compared with uncoated eggs and SPI-MMT coatings were more advantageous than SPI coatings. However, a higher percentage of MMT might not provide better protective barriers compared with a lower percentage after 6 weeks of storage at room temperature. In comparison with other published data, Herald et al.[Citation33] reported that the uncoated and oil-coated eggs changed in quality from Grade A to B after 1 week of storage, whereas the wheat-gluten-coated eggs remained at Grade A throughout 4 weeks of storage at room temperature.[Citation33] It is not better than coatings with SPI/MMT. Bhale et al.[Citation30] Indicated that non-coated and 1% acetic acid eggs changed from Grade AA to B after 1 week; for SPI/MMT-coated eggs, this change occurred after more than 6 weeks of storage.
Table 2. Effect of different coatings on Haugh unit during 6 weeks of storage.
YI
The YI is an indirect measure of the vitelline membrane strength and spherical shape of the yolk,[Citation34] and can be used as an indication of freshness.[Citation30] The spherical nature of egg yolk can be expressed as YI by measuring the height and width of the yolk. The YI of fresh, high-quality eggs is about 0.45, while an older egg will have a lower YI. Hence, the higher the YI is, the better the yolk quality.[Citation35] However, the storage time has a significant effect on YI.[Citation32]
The YI significantly decreased over time. Uncoated eggs and eggs going through other treatments did not exhibit a significant difference in YI until 3 weeks (). There was a significant difference between SPI-MMT-coated eggs and SPI coated eggs after 5 weeks. After 6 weeks, decreases in YI of 0.12, 0.14, and 0.14 were observed in SPI-0.2% MMT, SPI-0.5% MMT, and SPI-0.8% MMT, respectively. These reductions are significantly less than those of uncoated eggs and SPI coated eggs. It showed the combined effects of SPI-MMT coating in preserving the quality of yolk stored for a long time, as they effectively reduced the rate of water and CO2 loss from the albumen through the eggshell to inhibit albumen liquefaction and water uptake of the yolk. Compared with previous study by Suresh et al.,[Citation24] the YI of SPI/MMT-coated eggs (0.30–0.32) was higher than that of chitosan-coated eggs during the 5-week storage period at room temperature. Therefore, the SPI/MMT coating had a positive effect on the YI.
Table 3. Effect of different coatings on Yolk index during 6 weeks of storage.
Air space
Air space is an important indicator to evaluate the quality of eggs, and the fresh eggs tend to have a small air space. When eggs have been preserved for a long time, moisture and carbon dioxide continue to lose through the pores, which lead to a large air space. shows the change of air space under different treatments. Air space increased over time, and after 1-week of storage, the air space of uncoated eggs was significantly more than its under other treatments. Eggs coated with SPI exhibited a significant larger air space after 3 weeks. At 6 weeks of storage, the air spaces of SPI-0.2% MMT, SPI-0.5% MMT, and SPI-0.8% MMT were 5.26, 5.52, and 5.96 mm, respectively, and there were significant differences between them. As the SPI/MMT-coated eggs aged, the loss of moisture and carbon dioxide through the pores was less than that of other eggs. In the present study, Lee et al.[Citation29] also reported that SPI-MMT could have lower permeability to water vapor. Therefore, SPI-MMT was an effective measure to maintain the air space, especially for MMT contents of 2 g per 100 g SPI. It was similar to the conclusions of weight loss.
Table 4. Effect of different coatings on air space during 6 weeks of storage.
Albumen pH
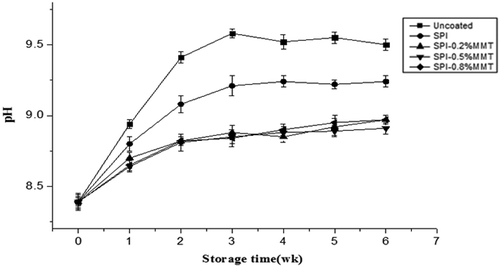
Albumen pH can also be an important indicator of egg freshness and quality. An increase in albumen pH during storage can be attributed to the CO2 loss through eggshell pores that occurs naturally in the albumen after the egg is laid, resulting in changes in the bicarbonate buffer system.[Citation36,Citation37] A weak solution of carbonic acid held in the egg white breaks down to supply more CO2 and water as the egg ages, resulting in the thinning of egg white and an increase in the pH of the albumen.[Citation34]
Freshly laid eggs have an albumen pH range of 6.0–7.7, but the albumen pH increases to 9.0–9.7 within a short time due to the release of CO2. Freshly laid eggs contain 1.44–2.05 mg CO2 per 1 g albumen,[Citation38] which is rapidly lost after being laid. Therefore, the slightly higher initial albumen pH value (8.39) observed in this study was possibly due to the time lapse between the egg being laid and when it was coated. However, this is not unusual in egg-coating experiments.[Citation37,Citation39]
The albumen pH of uncoated and SPI-coated eggs gradually increased over time (). Eggs coated with SPI-MMT exhibited a significantly lower pH than going through other eggshell treatments after 6 weeks of storage. These data suggest that the SPI-MMT coatings may control the rate at which CO2 is transferred through the eggshells. The lower albumen pH of eggs coated with emulsion may have been due to the excellent sealing and hydrophobic properties of SPI and MMT, which was effective at minimizing the loss of additional CO2 and water at eggshell surface during long-term storage.[Citation35]
After 3 weeks of storage, the pH of albumen in SPI-MMT-coated eggs increased slowly, and no significant differences were observed among different SPI-MMT-coated eggs. The SPI-MMT coating can work as a barrier to CO2 diffusion during storage, helping to maintain the quality of the albumen, and Lee et al.[Citation29] came to the same conclusions that the barrier properties of SPI/MMT films were improved.
Shell strength
Eggshell breakage is directly related to the quality of the shell. Improved shell strength will result in reduced breakage during handling and storage.[Citation24] Mechanical properties of coatings help enhance eggshell strength under conditions of stress that would occur during handling and storage.
Shell strength is the maximum stress at break. There were decreases in shell strength after 6 weeks of storage. The top of the eggshell has a higher puncture strength than the bottom. Uncoated eggshells exhibited significantly lower puncture strength than SPI-MMT-coated eggshells at the middle, top and bottom (). The puncture strength of eggshells coated with SPI-MMT was significantly greater than that of uncoated and SPI-coated eggshells. However, no significant differences have been found among the different SPI-MMT coatings. This clearly indicates the potential application of SPI-MMT coating in minimizing eggshell breakage. Kumer et al.[Citation18] had reported that MMT could enhance the mechanical properties of the SPI films, so the investigation results show that MMT coating could improve the shell breaking strength.
Table 5. Effect of different coatings on shell strength after 6 weeks of storage.
Microorganism
Eggshell can prevent microbial invasion, but this function will decline gradually over time. It is easy to invade the inside of eggs after long time storage and the coatings can enhance the eggshell function. shows that there were significant differences between uncoated or SPI-coated eggs and SPI-MMT-coated eggs after 1 week of storage. The total number of microorganisms was uncountable after 5 weeks. After 6 weeks of storage, SPI-0.8% MMT-coated eggs had the best antibacterial activity to prevent microbial invasion. It may be attributed to the cross-linking between SPI and MMT, which made the coatings compact enough to prevent microbial invasion. Although nano-MMT itself does not have the ability of antibacterial ability, MMT has special structure with unsaturated negative charge, as well as ion-exchange capacity, which has strong adsorption and fixation on Escherichia coli, Staphylococcus aureus or rotavirus, so as to achieve the antibacterial purpose. In the report of Rhim et al.,[Citation40] SPI/MMT films showed a better antibacterial activity than SPI films, but no better antibacterial activity of nano-silver Ag-Ion.
Table 6. Effect of different coatings on the total number of microorganism during 6 weeks.
CA
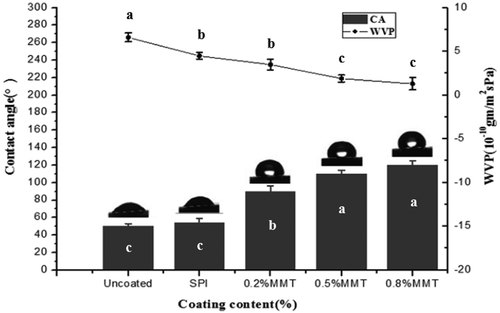
Generally, the water CA can reflect the hydrophilicity or hydrophobicity of materials, and the wettability of eggshells is strong when the water CA is high. Therefore, CA measurement was used to investigate the surface hydrophilicity and hydrophobicity of the coatings. shows the changes in the CA of eggshells. There were significant differences between uncoated or SPI-coated eggshells and SPI-MMT-coated eggshells. It can be obviously found that SPI-coated eggshells had a lower CA than SPI-MMT-coated eggshells, which may be attributed to the hydrophilicity and water sensitivity of SPI. The SPI-MMT-coated eggshells showed a higher value with an increase in the percentage of the MMT and the value of the SPI-0.8% MMT-coated eggshells was 115.5°. It may be attributed to the affinities between the clay and the soy protein or the glycerol, which leave fewer available interaction sites for the retention of water.[Citation22] Therefore, SPI-MMT-coated eggshells showed a higher hydrophobicity. In the present study, Zhang et al.[Citation26] reported the CA of the glycosylation-SPI film was about 95°, which was less smaller than that of SPI-MMT-coated eggs. Thus it can be seen that SPI/MMT coatings had better hydrophobicity, which would be beneficial to the preservation of eggs.
WVP
The quality of eggs can be affected by water vapor transmission, and the WVP values of SPI-MMT-coated eggs and uncoated eggs are shown in . The WVP of uncoated eggs was 7.45×10−10gm/m2sPa, which was higher than those of coated eggs. Coatings containing MMT had significantly (p < 0.05) lower WVP than SPI coatings. WVP for coatings with 0.2% MMT, 0.5% MMT, and 0.8% MMT reduced by 21.14, 57.78, and 69.13%, respectively. It can be attributed to the chain cross-linking, and SPI-MMT can bind water migration through the eggshells. Kumar et al.[Citation18] reported that WVP reduced by as much as 42.9% as the MMT content increased from 0 to 15%. Zeng et al.[Citation41] explained that the reduction in WVP by MMT had been attributed to the creation of a tortuous pathway for water vapor to diffuse out of the bionanocomposite matrix. This increases the effective path length for diffusion of water vapor molecules, thus reducing WVP.
SEM
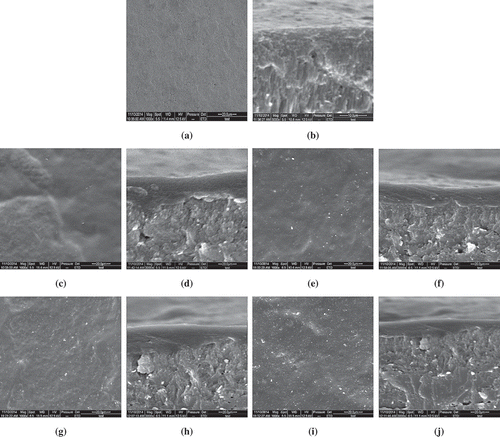
The morphology of coatings is a very important characteristic as it could ultimately determine many properties of biodegradable materials. shows the SEM images of eggshells under different treatments. and show the morphology of uncoated eggshells, from which obvious cracks could be seen, and from through 3g, it could be seen that the cracks of eggshell were covered by coatings. and indicate a smoother structure of SPI-coated eggshells than others (–) and different morphology in cross-section and surface was observed after MMT was added into SPI films. SEM images of SPI/MMT composite films (–) demonstrated a smoother surface morphology at 0.2%MMT as compared to 0.5 or 0.8% MMT. It may be attributed to the good distribution of MMT in SPI matrix, and this also indicated that intercalated nano materials filled the SPI polymer, which is consistent with the significant (p < 0.05) WVP of SPI/MMT composite films. Similar observations were seen in the study of Lee et al.[Citation29]
SPM
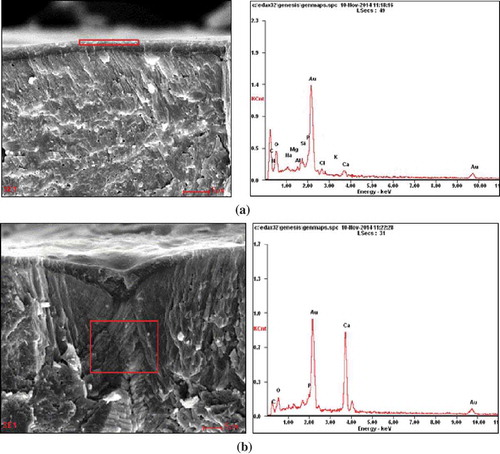
Eggshells have a lot of pores, which are channels for CO2 and H2O. Coatings can fill these pores, but they also can permeate through the eggshells and pollute the internal quality, which would influence the safety of eggs. Therefore, it is important to observe whether the MMT can enter into the interior of eggs. shows the energy spectrum of SPI-MMT-coated eggs surface and eggshell pores. It can be seen that the higher content elements of the surface were C, N, O, Na, Mg, Al, Si, P, Cl, K, and Ca, because the molecular formula of MMT is Ex (H2O)4 {(Al2-xMgx) [Si4O10] (OH)2} (E=Ca, Na; ). The higher content elements in the eggshell pores were C, N, O, P, and Ca, which were the main elements of SPI and the eggshell (); Na, Mg, Al, and Si were not detected. On the one hand, it indicates that the MMT did not penetrate through the eggshell pores; on the other hand, it shows that MMT has been dispersed in the SPI. Therefore, MMT is desirable for potential applications in egg coatings, and it did not penetrate into the interior of eggs, which would affect the safety of the eggs.
Conclusions
This study indicated that bio-nanocomposite coatings with different percentages of SPI-MMT solutions were effective in preserving the interior quality (weight loss, HU, YI, air space, and albumen pH) of eggs and in extending their shelf life during storage at 25°C. Eggs coated with SPI/MMT would have lower weight loss, higher HU, higher YI, lower air space, lower albumen pH, higher eggshell strength, fewer number of microorganisms, larger CA, and less WVP during 6 weeks of storage at 25°C, which showed the positive effect on extending the shelf life. The SPI-0.2% MMT-coated maintained AA quality for 4 weeks compared with only 2 weeks for uncoated eggs. The images of SEM indicated that coatings could cover the cracks of eggshells to preserve the interior quality of eggs. In addition, the experimental values of SPM showed that MMT would not penetrate through the eggshell pores, and it is safe to coat with MMT. The prolongation of eggs shelf life is extremely benefit to large egg production.
Funding
This study was supported by the National Natural Science Foundation of China (Young Scholars No. 31101386); National Natural Science Foundation of China (General Program No. 31271961); Northeast Agricultural University Scholars Program: Northeast Agricultural University Academic Backbone Project (16XG21); Northeast Agricultural University Foundation for Dr. (2012RCB02). The author also gratefully acknowledge the financial support from Scientific and Technological Achievements Industrialization Prophase Research Project of Heilongjiang Province University (1253CGZH26); Northeast Agricultural University Science and Technology Innovation Foundation for Post Graduate (yjscx14066).
Additional information
Funding
References
- Freeland-Graves, J.H.; Peckman, G.C. Eggs. Foundation of Food Preparation. Macmillan Publishing Company: New York, 1987, 415–440.
- Lucisano, M.; Hidalgo, A.; Comelli, E.M.; Rossi, M. Evolution of Chemical and Physical Albumen Characteristics During the Storage of Shell Eggs. Journal of Agricultural and Food Chemistry 1996, 44(5), 1235–1240.
- Stadelman, W.J. The Preservation of Quality in Shell Eggs. Egg Science and Technology Press: Macmillan Education, UK, 1986.
- Stadelman, W.J. Quality Identification of Shell Eggs. Egg Science and Technology Press: Macmillan Education, UK, 1986.
- Kim, S.H.; No, H.K.; Prinyawiwatkul, W. Effect of Molecular Weight, Type of Chitosan, and Chitosan Solution pH on the Shelf‐Life and Quality of Coated Eggs. Journal of Food Science 2007, 72(1), S044–S048.
- Xie, L.; Hettiarachchy, N.S.; Ju, Z.Y.; Meullenet, J.; Wang, H.; Slavik, M.F.; Janes, M.E. Edible Film Coating to Minimize Eggshell Breakage and Reduce Post‐Wash Bacterial Contamination Measured by Dye Penetration in Eggs. Journal of Food Science 2002, 67(1), 280–284.
- Kim, K.; Daeschel, M.; Zhao, Y. Edible Coatings for Enhancing Microbial Safety and Extending Shelf Life of Hard‐Boiled Eggs. Journal of Food Science 2008, 73(5), M227–M235.
- Jirangrat, W. Effects of Mineral Oil Coating on Internal Quality of Chicken Eggs Under Refrigerated Storage. International Journal of Food Science & Technology 2010, 45(3), 490–495.
- Wardy, W. Chitosan-Soybean Oil Emulsion Coating Affects Physico-Functional and Sensory Quality of Eggs During Storage. LWT–Food Science and Technology 2011, 44(10), 2349–2355.
- Nongtaodum, S. Oil Coating Affects Internal Quality and Sensory Acceptance of Selected Attributes of Raw Eggs During Storage. Journal of Food Science 2013, 78(2), S329–S335.
- Caner, C. Whey Protein Isolate Coating and Concentration Effects on Egg Shelf Life. Journal of the Science of Food and Agriculture 2005, 85(13), 2143–2148.
- Meyer, R.; Spencer, J.V. The Effect of Various Coatings on Shell Strength and Egg Quality. Poultry Science 1973, 52(2), 703–711.
- Rhim, J.W.; Weller, C.L.; Gennadios, A. Effects of Soy Protein Coating on Shell Strength and Quality of Shell Eggs. Food Science and Biotechnology 2004, 13(4), 455–459.
- Wong, Y.; Herald, T.; Hachmeister, K. Evaluation of Mechanical and Barrier Properties of Protein Coatings on Shell Eggs. Poultry Science 1996, 75(3), 417–422.
- Park, S. Mechanical Properties and Water-Vapor Permeability of Soy-Protein Films Affected by Calcium Salts and Glucono-δ-Lactone. Journal of Agricultural and Food Chemistry 2001, 49(5), 2308–2312.
- Chiou, B.S. Extruded Starch–Nanoclay Nanocomposites: Effects of Glycerol and Nanoclay Concentration. Polymer Engineering & Science 2007, 47(11), 1898–1904.
- Cyras, V.P. Physical and Mechanical Properties of Thermoplastic Starch/Montmorillonite Nanocomposite Films. Carbohydrate Polymers 2008, 73(1), 55–63.
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D.; Gorga, R.E. Preparation and Characterization of Bio-Nanocomposite Films Based on Soy Protein Isolate and Montmorillonite Using Melt Extrusion. Journal of Food Engineering 2010, 100(3), 480–489.
- Lee, H.H.; Hong, S.I.; Kim, D. Microbiological and Visual Quality of Fresh-Cut Cabbage As Affected by Packaging Treatments. Food Science and Biotechnology 2011, 20(1), 229–235.
- Lee, S.; No, H.; Jeong, Y. Effect of Chitosan Coating on Quality of Egg During Storage. Journal of the Korean Society of Food and Nutrition (Korea Republic) 1996 25(2), 288–293.
- Wardy, W. Viscosity Changes of Chitosan Solution Affect Physico-Functional Properties and Consumer Perception of Coated Eggs During Storage. LWT–Food Science and Technology 2014, 55(1), 67–73.
- Suppakul, P.; Jutakorn, K.; Bangchokedee, Y. Efficacy of Cellulose-Based Coating on Enhancing the Shelf Life of Fresh Eggs. Journal of Food Engineering 2010, 98(2), 207–213.
- Garnjanagoonchorn, W. Eggs and Egg Products. Food Science and Technology 1996, 449(127C), 230–242.
- Suresh, P.V.; Raj, K.R.; Nidheesh, T.; Pal, G.K.; Sakhare, P.Z. Application of Chitosan for Improvement of Quality and Shelf Life of Table Eggs Under Tropical Room Conditions. Journal of Food Science And Technology 2015, 52(10), 6345–6354.
- Leleu, S.; Herman, L.; Heyndrickx, M.; De, R.K.; Michiels, C.W.; De Baerdemaeker, J.; Messens, W. Effects on Salmonella Shell Contamination and Trans-Shell Penetration of Coating Hens’ Eggs with Chitosan. International Journal of Food Microbiology 2011, 145(1), 43–48.
- Zhang, H.J.; Wang, S.N.; Zheng, X.Z.; Jiang, L.Z.; Lv, X.P.; Shi, Y.J.; Li, L. Effect of Glycosylation on the Mechanical Properties of Edible Soy Protein Packaging Film. European Food Research and Technology 2014, 238(6), 1049–1055.
- Figueiredo, T.C.; Assis, D.C.S.; Menezes, L.D.M.; Oliveira, D.D.; Lima, A.L.; Souza, M.R.; Heneine, L.G.D.; Cancado, S.V. Effects of Packaging, Mineral Oil Coating, and Storage Time on Biogenic Amine Levels and Internal Quality of Eggs. Poultry Science 2014, 93(12), 3171–3178.
- Echeverría, I.; Eisenberg, P.; Mauri, A.N. Nanocomposites Films Based on Soy Proteins and Montmorillonite Processed by Casting. Journal of Membrane Science 2014, 449, 15–26.
- Lee, J.E.; Kim, K.M. Characteristics of Soy Protein Isolate-Montmorillonite Composite Films. Journal of Applied Polymer Science 2010, 118(4), 2257–2263.
- Bhale, S.; No, H.K.; Prinyawiwatkul, W.; Farr, A.J.; Nadarajah, K.; Meyers, S.P. Chitosan Coating Improves Shelf Life of Eggs. Journal of Food 2003, 68(7), 2378–2383.
- Caner, C. The Effect of Edible Eggshell Coatings on Egg Quality and Consumer Perception. Journal of the Science of Food and Agriculture 2005, 85(11), 1897–1902.
- Yuceer, M.; Caner, C. Antimicrobial Lysozyme–Chitosan Coatings Affect Functional Properties and Shelf Life of Chicken Eggs During Storage. Journal of the Science of Food and Agriculture 2014, 94(1), 153–162.
- Herald, T.J.; Gnanasambandam, R.; McGuire, B.H.; Hachmeister, K.A. Degradable Wheat Gluten Films: Preparation, Properties and Applications. Journal of Food Science 1995, 60(5), 1147–1150.
- Mountney, G. Reduced Sodium Usage in Poultry Muscle Foods. Food Technology 1976, 7, 60.
- Wardy, W.; Torrico, D.D.; Corredor, J.A.H.; No, H.K.; Zhang, X.M.; Xu, Z.M.; Prinyawiwatkul, W. Soybean Oil-Chitosan Emulsion Affects Internal Quality and Shelf-Life Of Eggs Stored at 25 and 4 Degrees C. International Journal of Food Science and Technology 2013, 48(6), 1148–1156.
- Keener, K.; LaCrosse, J.; Babson, J. Chemical Method for Determination of Carbon Dioxide Content in Egg Yolk and Egg Albumen. Poultry Science 2001, 80(7), 983–987.
- Wardy, W.; Torrico, D.D.; No, H.K.; Prinyawiwatkul, W. Edible Coating Affects Physico‐Functional Properties and Shelf Life of Chicken Eggs During Refrigerated and Room Temperature Storage. International Journal of Food Science & Technology 2010, 45(12), 2659–2668.
- Biladeau, A.; Keener, K. The Effects of Edible Coatings on Chicken Egg Quality Under Refrigerated Storage. Poultry Science 2009, 88(6), 1266–1274.
- Torrico, D.D.; No, H.K.; Prinyawiwatkul, W.; Janes, M.; Corredor, J.A.H.; Osorio, L.F. Mineral Oil–Chitosan Emulsion Coatings Affect Quality and Shelf‐Life of Coated Eggs During Refrigerated and Room Temperature Storage. Journal of Food Science 2011, 76(4), S262–S268.
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. Journal of Agricultural & Food Chemistry 2006, 54(16), 5814–5822.
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q.; Paul, D.R. Clay-Based Polymer Nanocomposites: Research and Commercial Development. Journal of Nanoscience and Nanotechnology 2005, 5, 1574–1592.