ABSTRACT
Dental erosion is a growing health problem linked to the exceptional increase in the consumption of soft drinks, fruit juices, and sport drinks in many countries including Bangladesh. Dental erosion is the chemical dissolution of the dental hard tissues by acids without the involvement of microorganisms. Hydrogen ions (H+) from acidic solutions can replace the calcium ions (Ca2+) of the enamel, consequently breaking the crystal structure of the enamel and initiating dental erosion. Erosive tooth wear can lead to severe impairment of esthetics along with loss of hardness and functionality. Sources of the erosive acidic challenges can be intrinsic (i.e., gastroesophaegal reflux disease) and/or extrinsic (i.e., exposure from acidic foods and beverages). Continuous intake of drinks or food with pH lower than the critical erosive pH of enamel (5.2–5.5) and root dentin (~6.7) are considered to be responsible for dental erosion. Drinks with low pH and high titratable acidity (TA) have more potential to dissolved enamel and root dentin; on the other hand, drinks with low degree of saturation can stimulate leaching of minerals. In Bangladesh, there is limited scientific information available to assess the potential of dental erosion of the commercially available beverages and drinking water. This research aims to characterize the dental erosion potential of soft drinks, energy drinks, fruit juices, and bottled drinking water available in Bangladesh by determining their pH, TA, calcium (Ca2+), and phosphate (PO43−). The degrees of saturation of the selected samples were calculated from the experimental results of pH, calcium, and phosphate levels. Soft drinks were found to have high erosion potential followed by energy drinks, fruit juices, and bottled drinking water. Most of the beverages locally available were found highly acidic. Phosphate levels were high in black cola drinks. Total TA was highest for the energy drinks, and moderate for soft drinks and fruit juices. Fruit juices contained high level of calcium compared with other beverages. The degree of saturation was moderate for fruit juices, and very low for few of the soft drinks and most of the bottled drinking waters. This study will be useful as a reference line for the health professionals and regulatory authorities for quality control of the beverages and bottled drinking water available in the local market.
Introduction
Dental erosion is the acidic dissolution of enamel and dentin – the major components of dental hard tissue. Erosive tooth wear has progressively become a major concern for detrimental dental health[Citation1,Citation2]. Dental enamel is composed primarily of hydroxyapatite, Ca5(PO4)3(OH), and impurities such as carbonate and fluoride. Dental erosion refers to the dissolution of tooth structure under continuous exposure to low pH, while not being associated with bacterial infection[Citation4]. Replacement of minerals, primarily calcium from enamel or hydroxyapatite, can induce degradation of teeth structure and upon long-term exposure can lead to severe deleterious impacts on dental esthetics and functionality such as loss of strength[Citation1]. Etiological studies report two major classifications of the causes of the dental erosion – intrinsically by the gastroesophegal reflux disease, and extrinsically due to the exposure toward the acidic food and beverages[Citation5–Citation7]. Prolonged contact between either extrinsic or intrinsic acids with tooth surfaces can cause softening and dissolution of surface minerals.
Dental erosion potential from regular diet can be appreciated from the pH and titratable acidity (TA) values of the commonly available drinks and food[Citation8–Citation11]. Erosion of root dentin and enamel happens when pH values reach threshold values of 6.7 and 5.2–5.5, respectively[Citation12,Citation13]. Fruit and vegetable juices, soft drinks, sports and energy drinks, and some bottled drinking water can have pH lower or around critical erosive pH[Citation8–Citation10,Citation14,Citation15]. While pH (or –log[H+]) provides the amount of hydrogen ions (H+) that will be available to cause replacement of minerals (e.g., calcium) from tooth structure, TA provides the amount of alkali required to neutralize the acid, that is, the erosive capacity of the food or beverage. In combination with these parameters, the mineral contents of calcium (Ca2+) and phosphate (PO43−) ions in the food/drinks and saliva provide the degree of saturation[Citation9]. Other parameters dictating the dental erosion potential include consumption frequency, temperature of the drinks, oral hygiene, etc[Citation16,Citation17].
Prevalence of erosion-induced teeth degradation has been increasing among both young and adult population around the world including Bangladesh[Citation2,Citation18]. A recent survey showed that 52% of the 147 participating pregnant women presented with the prevalence of moderate to severe dental erosion[Citation19]. This erosion prevalence may be attributed not only to the increased consumption of low pH drinks (as predicted from market research data) but also from poor oral hygiene practice, lack of dental education, and unaffordable dental care among general population of Bangladesh[Citation20–Citation22]. Thus, identifying the sources of such erosion including the dental erosion potential of locally available soft drinks, energy drinks, fruit juices, and bottled drinking water has become significant.
The present study aimed to experimentally measure dental erosion potential indicating key parameters, such as pH, TA, phosphate (PO43−), and calcium (Ca2+) contents, of the soft drinks, energy drinks, fruit juices, and bottled drinking water available in the local market of Bangladesh, and to evaluate the potential erosive hazard associated with these drinks. The degree of saturation of the above drinks was also calculated and analyzed to characterize their dental erosion potential.
Materials and methods
Sample collection
Locally available and/or produced beverages and bottled drinking water samples were collected from different markets and super shops of Dhaka, Bangladesh. The collected samples were broadly classified into four categories: (a) soft drinks (13 brands), (b) energy drinks (4 brands), (c) fruit juices (12 brands; all were mango juices), and (d) bottled drinking water (8 brands). For each brand, three samples from different production batches were collected.
Chemical analyses
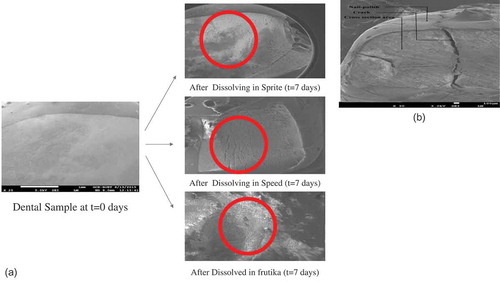
All the chemicals used were reagent grades. Each test was repeated three times (i.e., samples from three batches and three tests for each sample) and the average results were used for further analysis. All the tests were carried out at room temperature. The pH of each sample drink was measured immediately upon opening. A calibrated bench-top pH meter (Hanna HI2211) was used to measure pH of the samples. A HACH Model 44600 Conductivity/TDS Meter was used to measure the TDS of the samples. The TDS of each sample drink was measured immediately upon opening. For carbonated samples, however, the samples were allowed to sit till some of the gas escaped. The TA of each sample was measured by titrating 25 mL of each sample with 0.1 M NaOH, using phenolphthalein as the indicator. For the samples with an intense color (e.g., black cola), the samples were diluted for accuracy to determine the titration endpoint. The calcium (Ca2+) concentrations in the samples were determined using complexometric titration method. Ethylenediaminetetraacetic acid (EDTA) solution was prepared by dissolving 1.4612 g of reagent grade EDTA (Sigma Aldrich) in 500 mL of water to prepare a 0.01 M solution. A pH 11 buffer solution was prepared to maintain an alkaline environment which would help any magnesium precipitate; else the EDTA would simultaneously complex with the magnesium ions. Then, 25 mL of the samples were titrated with the EDTA solution, using Calcon as the indicator. The phosphate content of the beverage samples were measured using spectrophotometer (Hach UV/vis spectro-photometer, DR4000). Now, 10 mL samples of the beverages were taken into cuvettes, diluted appropriately so as to reduce color effect or reduce the phosphate (PO43−) concentration below the maximum threshold of the equipment. Using the preset program for determining total phosphate as orthophosphate, the procedure was carried out. Dental samples were analyzed via scanning electron microscopy (acceleration voltage = 3.0 kV; JSM-7600F SEM). SEM images were taken before and after dissolution of dental samples in beverages (Fanta, Speed, and Fruitika) for 10 days.
Dental erosion potential characterization
Acidity and dissolution
The dental erosion potential of soft drinks and food items can be characterized using pH and TA [Citation8,Citation9,Citation23]. Beverages with pH values below 6.7 and 5.2–5.5 can potentially cause erosive wear to root dentin and enamel, respectively[Citation3,Citation24]. Soft drinks, including carbonated beverages, fruit juices, and energy drinks, are considered highly acidic; therefore, continuous consumption of such drinks can lead to dental erosion[Citation8,Citation9,Citation14,Citation23,Citation25].
High acidity or a low pH implies the presence of high concentration of hydrogen ions (H+) that will be available to cause replacement of minerals (e.g., calcium) from tooth structure. On the other hand, TA indicates the amount of alkali required to neutralize the acid. Therefore, TA is also known as buffering capacity. The greater the buffering capacity (i.e., TA) of the drink, the longer it will take for the saliva to neutralize the acid[Citation24]. TA works as a better indicator of dental erosion than pH. pH gives an indication of the equilibrium concentration of hydrogen (H+) ions but does not provide a measure of the free hydrogen ions that can cause demineralization.
Calcium and phosphate
Calcium (Ca2+) and phosphate (PO43−) contents of beverages are important factors influencing erosive potential. Different research groups demonstrated that the enamel loss is lower for the people who consume the calcium fortified drinks than for the people drinking conventional orange drinks[Citation26,Citation27]. Scientists have suggested that fortifying dentally erosive drinks with calcium and phosphate reduces the erosive potential of the drink on tooth surface[Citation26,Citation28,Citation29]. Added calcium and phosphate saturate the drink with respect to hydoxyapatite mineral [Ca5(PO4)3OH] of the enamel[Citation26].High levels of calcium in the solution blocks the release of calcium ions (Ca2+) from the enamel surface[Citation30].However, a high concentration of calcium in beverages may cause an unpleasant metallic taste[Citation6].
Degree of saturation
The degree of saturation is the driving force for the dissolution process. This parameter is a potential indicator to show how far the mineral composition is from equilibrium[Citation31]. A high degree of saturation inhibits demineralization of the enamel and dentin. The loss of minerals and re-mineralization of enamel is a constant process. The equilibrium between the tooth enamel and the fluid surrounding the tooth surface is influenced by the degree of saturation. A critical pH value of a beverage controls the dissolution of minerals from tooth. However, this critical pH is not a fixed value, and it depends on the concentration of other ions such as calcium and phosphate. The pH of saliva is higher than the critical pH for tooth, and is supersaturated with respect to enamel. Due to its mineral content of calcium, phosphate, and fluoride, saliva can possess a reparative effect on early enamel erosion which is characterized by surface softening and slight subsurface mineral loss[Citation4]. The degree of saturation could be calculated with respect to hydroxyapatite by determining the pH, calcium, and phosphate contents of the beverages[Citation4]. For hydroxyapatite:[Citation32]
where I is ion activity product of hydroxyapatite (mol9 L–9), [Ca2+] is concentration of calcium in the solution, [PO43−] is concentration of phosphate in the solution, [OH–] is concentration of hydroxyl ions in the solution. The degree of saturation S is calculated using the following equation:
where KSP is solubility product of enamel (mol9 L–9), n is total number of ions in the formula unit (n = 9 in the case of hydroxyapatite). Researchers demonstrated that the solubility of apatite increases logarithmically with decreasing pH[Citation14], for example, the solubility of apatite increases from 75 g/L at pH 3 to 400 g/L at pH 2.5. Besides the presence of ions and degree of saturation, other factors such as frequency of consumption, buffering capacity of saliva, temperature of the drinks, and oral hygiene have shown to impact the absolute erosion caused by the drinks[Citation16,Citation33].
Results
The mean values of the experimental results of pH, TA, phosphate and calcium contents, and the calculated values of degree of saturation of soft drinks, energy drinks, fruit juices, and bottled drinking water are shown in , and are graphically presented in –. The degree of saturation was calculated using Eq. (2) and average values of the related parameters.
Table 1. pH, TA, phosphate, calcium, and degree of saturation of soft drinks, energy drinks, fruit juices, and bottled drinking water.
Figure 1. Dental erosion indicating parameters of soft drinks. (a) pH, (b) TA, (c) phosphate, (d) calcium, (e) degree of saturation; error bars indicate standard deviation for (a–d).
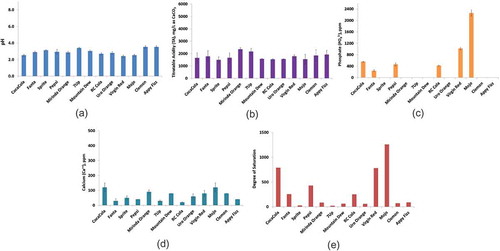
Figure 2. Dental erosion indicating parameters of energy drinks. (a) pH, (b) TA, (c) phosphate, (d) calcium, (e) degree of saturation; the low values of phosphate are shown in the in site picture of (c); error bars indicate standard deviation for (a–d).
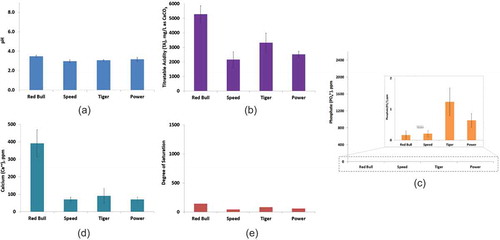
Figure 3. Dental erosion indicating parameters of fruit juices. (a) pH, (b) TA, (c) phosphate, (d) calcium, (e) degree of saturation; the low values of phosphate are shown in the in site picture of (c); error bars indicate standard deviation for (a–d).
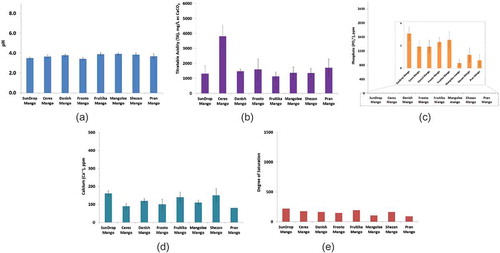
Figure 4. Dental erosion indicating parameters of bottled drinking water. (a) pH, (b) TA, (c) phosphate, (d) calcium, (e) degree of saturation; the low values of TA and phosphate contents are shown in the in site picture of (b) and (c), respectively; error bars indicate standard deviation for (a–d).
Soft drinks
Prolonged exposure of teeth to food or drinks with pH values below critical dental erosive pH values can lead to dissolving of potential dental hard tissue[Citation24,Citation34,Citation35]. Most of the soft drink samples had low pH values, with the black cola drinks having the minimum: below pH 3 (). The TA of soft drink samples varied from 1490.3 to 2356.5 ppm (). Low pH and high level of TA in soft drinks can dissolve protective protein layers deposited on teeth by salivary fluid[Citation24]. After dissolving the protective protein layers, the drinks can diffuse inside the enamel and can cause leaching of minerals.
Most of the soft drink samples contained moderate level of calcium (). Fanta and black cola drinks, which include CocaCola, Pepsi, RC Cola, Virgin Red, and Mojo, contained high levels of phosphate (). White and colored soft drinks such as Sprite, 7Up, Mirinda Orange, Mountain Dew, Uro Orange, Clemon, and Appy Fizz contained very low level of phosphate, ranging from 0.1 to 2.3 ppm ().
Degree of saturation of a drink is directly proportional to its calcium and phosphate contents, and pH value. Because of very low phosphate contents, the degree of saturation of white and colored soft drinks were less than that of Fanta and black cola drinks (). Low degree of saturation indicates the soft drinks under saturated with respect to hydoxyapatite mineral, and therefore, more likely to cause dental erosion[Citation26].
Energy drinks
The energy drink samples had slightly higher pH than the soft drinks: 3.0–3.5 ( and ), and TA values were much higher than other beverages and bottled drinking water (, , , , and ). Low pH and high level of TA indicate that the energy drinks may possess high dental erosion potential. All the energy drink samples contained moderate amount of calcium (). However, the phosphate levels in energy drinks were found to be very low, ranging from 0.2 to 1.2 ppm (); therefore, the calculated degree of saturation of energy drink samples were found lower than that of fruit juices, Fanta, and black cola drinks (, , , and ). Low degree of saturation indicates the energy drinks likely to cause dental erosion through leaching minerals. Other studies carried out on sport and energy drinks show that they have a high erosion potential relative to other drinks, which is consistent with studies from other countries[Citation23,Citation25].
Fruit juices
Fruit juices were also found acidic in nature (pH ranging from 3.4 to 3.9), and contained high level of TA as soft drinks ( and ), which indicates high erosive potential of the fruit juices. The concentration of phosphate content of fruit juice samples were found to be very low (). The calcium contents of the fruit juices were higher compared with that of soft drinks and energy drinks (, , , and ). Ceres Mango was found to be more acidic with higher TA (), while Sundrop exhibited the highest calcium and phosphate content among all the juice samples ( and ). All the fruit juices homogeneously exhibited moderate degree of saturation (). This high calcium/phosphate ratio and moderate degree of saturation can reduce the erosive potential of the drink[Citation30].
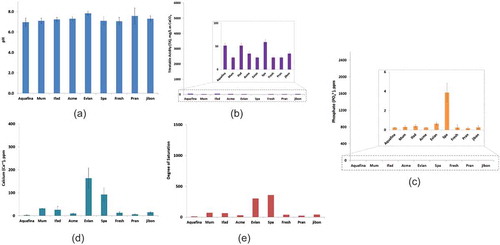
Bottled drinking water
The experimental results show that all the bottled water samples had a pH around 7 (ranging from 7.0 to 7.8), and negligible amount of TA (ranging from 0.0 to 59.2 ppm) (; and ). Low TA and neutral pH range indicates that bottled drinking water samples are unlikely to dissolve dental enamel and root dentin. However, all the bottled drinking water contained very low amount of phosphate (PO43−), and various range of calcium (Ca2+) contents (ranging from 0.0 to 163.3 ppm) ( and ). Among the samples, Spa and Evian showed high calcium content. With the exception of Evian and Spa, the other water samples had low degree of saturation (), which indicates those bottled drinking water samples have the potential to stimulate leaching of minerals from dental enamel and root dentin.
SEM image of dental sample
SEM images were taken before and after dissolution of dental samples in beverages for 10 days (). It was found that dental samples exposed to Fanta, Speed, and Tiger were eroded and lost the shiny upper layer (). shows that exposure of dental sample to Sprite for 10 days caused the tooth surface eroded and cracked. The reason could be the dissolution of enamel of dental sample in beverage.
Discussion
The experimental results show that most of the beverages locally available were highly acidic, with the soft drinks category having the lowest pH values, followed by the energy drinks, and the fruit juices. The bottled drinking water samples had pH values close to 7. It is noticeable that all of the beverage samples had a pH below 4.0, which is way below to the critical erosive pH values for enamel (5.2–5.5) and dentin (~6.7). Total TA was the highest for the energy drinks, moderate for soft drinks and fruit juices, and the lowest for bottled drinking water. High TA values indicate high buffering capacity of the beverages. Low pH and high TA drinks require high amount of alkali to neutralize the acid, and possess high potential to dissolve the minerals of enamel and dentin[Citation24].
Fruit juices contained high level of calcium compared with other beverages. On the other hand, phosphate levels were high in Fanta and black cola drinks, and negligible in other drinks. Presence of minerals can induce resistance to dissolution, which can somewhat alter the erosive potential[Citation9,Citation24]. Because of the low mineral and phosphate contents, the degree of saturation was very low for few of the soft drinks and most of the bottled drinking waters, and moderate for fruit juices. Therefore, these drinks have the potential to stimulate leaching minerals from the enamel and dentin. The supplementation of beverages with calcium and phosphate seems to be a viable alternative to reduce the erosive potential of the drinks.
SEM images demonstrated that prolonged exposure of dental sample to beverage could cause the tooth surface to erode and crack. The experimental results showed that erosive beverages could cause the dissolution of phosphate and calcium contents of dental samples when exposed to these beverages. Dental erosion is one of the major concerns of detrimental dental health[Citation36]. Dental erosion caused by beverages and bottled water is a complex phenomenon that depends on the key parameters and conditions. Along with the physicochemical parameters, it is also important to analyze biological and behavioral factors[Citation37]. and to perform in vitro analysis of beverages and bottled waters using real dental sample. Considering a single key parameter may not represent the true dental erosion potential of a beverage or bottled drinking water sample. As an example, a rather neutral or low TA drink is less likely to dissolve the hard tissues of dental samples. However, if the drink possesses low degree of saturation, then it may stimulate leaching of minerals of a dental sample with fractured or dissolved enamel layer.
Worldwide dental erosion has been increasingly prevalent among the young and adult population; however, teenage males and teenage females are at higher risks of dental erosion because of the consumption of large amounts of acidic beverages[Citation38–Citation40]. In Bangladesh, about 47.8% of the population is within the age group of 0–24 years[Citation41]. Therefore, the dental health of this large population is a major concern for Bangladesh (and for other countries in similar situation). Awareness and early detection of dental erosion will allow to take preventive measures. To reduce dental erosion, different preventive actions may be suggested, such as to consume modified drinks, to improve dental hygiene, and to improve drink pattern of acidic beverages. Drink modification has been developing in recent years with varying success[Citation42–Citation45]. Addition of calcium has shown to reduce the erosive potential of beverages[Citation42–Citation46].
The frequency and duration of acid (i.e., acidic beverages) contact are important variables for the development of dental erosion[Citation47,Citation48]. Special drinking habits such as holding or moving the liquid in the mouth prior to swallowing, drawing from a straw, or nipping from a bottle lead to prolonged duration of an acidic-pH liquid in the oral cavity[Citation49]. Therefore, it is advisable to abstain from these drinking habits to reduce the duration of erosive attack. In addition, saliva plays an important role in reducing enamel and dentin erosion due to its buffering and remineralization capacities, and ability to form a protective pellicle layer on dental hard tissues[Citation50,Citation51]. Stimulation of salivary flow can yield an increase in salivary mineral content, which can re-deposit calcium and phosphate onto the enamel and dentin surface, and minimize dental tissue loss[Citation52,Citation53]. Chewing sugar-free gums may stimulate saliva production, and hence, promote remineralization[Citation54]. Furthermore, rinsing with milk, or eating cheese may increase remineralization, as they contain higher levels of calcium and phosphate[Citation55,Citation56].
This study aimed to conduct systematic scientific research to characterize dental erosion potential of beverages and bottled drinking water in Bangladesh, and to perform in vitro analysis using dental samples. This article reports the dental erosion potential of soft drinks, energy drinks, fruit juices, and bottled drinking water available locally in terms of their pH, TA, PO43−, Ca2+, and degree of saturation. The in vitro analysis of dental samples using selected beverages and bottled drinking water will be addressed in a separate article. In Bangladesh, there are very limited primary data (or no data) available to analyze the dental erosion potential of the locally available soft drinks, fruit juices, energy drinks, and bottled drinking waters. Therefore, the results presented in this article will serve as the baseline data to the health professionals, researchers, regulatory authorities, and government policy-makers to conduct future studies, and to manage and monitor the dental erosion caused by beverages and bottled drinking waters available in Bangladesh.
Conclusion
This research work showed that beverages commonly available in Bangladesh have dental erosion potential based on the values of different physicochemical properties affecting enamel dissolution, and may cause enamel erosion on long-term consumption. Low pH and high TA, which indicate high potential to dissolve enamel and root dentin, were found to be the common criteria of the locally available soft drinks, energy drinks, and fruit juices. However, the moderate degree of saturation of fruit juices, and high degree of saturation of few soft drinks including black cola and Fanta, indicates possible inhibition of demineralization of enamel and dentin. Bottled drinking water appears to have lower TA and neutral pH, and therefore, less likely to dissolve enamel or dentin. However, most of the bottled drinking water samples showed very low degree of saturation, which indicates regular consumption may stimulate mineral removal from enamel and dentin by leaching. Raising the degree of saturation of certain drinks may inhibit dissolution to a certain extent. Hence, fortification of beverages with calcium and phosphate can reduce the impact of the acidity of the drinks. Evaluation of dental erosion potential of beverages and drinking water reported in this study will help conducting in vitro analysis to model the dynamics of dental erosion, and will serve as the basis for determining the potential erosion risk toward the population of Bangladesh. Early intervention can help create awareness among general public, health workers, and nutritionists about the potential risks.
Acknowledgments
Authors acknowledge the Department of Chemical Engineering and the Department of Glass and Ceramic Engineering at BUET for providing laboratory facilities. A part of the results of this paper were first presented in the Second International Conference on Food Properties (iCFP 2016) held in Bangkok, Thailand, between 31 May 2016 and 2 June 2016, and it received one of the best papers recognition.
Funding
This research was supported by BCEF Academic Research Fund, and BUET CASR Research Grant.
Additional information
Funding
References
- Lussi, A. Dental Erosion: From Diagnosis to Therapy, in Dental Erosion. Monogr Oral Sci. Basel, A. Lussi, Editor. 2006, Karger. pp. 66–76.
- Lussi, A.; Schaffner, M.; Jaeggi, T. Dental Erosion - Diagnosis and Prevention in Children and Adults*. International Dental Journal 2007, 57(S6), 385–398.
- Dawes, A.S. A three-dimensional contact algorithm for sliding surfaces. International Journal for Numerical Methods in Fluids 2003, 42(11), 1189–1210.
- Imfeld, T. Dental erosion. Definition, classification and links. European Journal of Oral Sciences 1996, 104(2), 151–155.
- Zero, D.T. Etiology of dental erosion–extrinsic factors. European Journal of Oral Sciences 1996, 104(2), 162–177.
- Jensdottir, T., et al. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among Icelanders. Clinical Oral Investigations 2004, 8(2), 91–96.
- Ren, Y.-F. Dental Erosion: Etiology, Diagnosis and Prevention. The Academy of Dental Therapuetics and Stomatology 2011, 31(8), 75–84.
- Brown, C.J., et al. The Erosive Potential of Flavoured Sparkling Water Drinks. International Journal of Paediatric Dentistry 2007, 17(2), 86–91.
- Cochrane, N.J., et al. Erosive potential of sports beverages. Australian Dental Journal 2012, 57(3), 359–364.
- Kitchens, M.; Owens, B. Effect of Carbonated Beverages, Coffee, Sports and High Energy Drinks, and Bottled Water on the in vitro Erosion Characteristics of Dental Enamel. Journal of Clinical Pediatric Dentistry 2007, 31(3), 153–159.
- Hasnaoui, N., et al. Organic Acids, Sugars, and Anthocyanins Contents in Juices of Tunisian Pomegranate Fruits. International Journal of Food Properties 2011, 14(4), 741–757.
- Featherstone, J.; Lussi, A. Understanding the chemistry of dental erosion. 2006.
- Dawes, C. What is the Critical pH and Why Does a Tooth Dissolve in Acid? Journal-Canadian Dental Association 2003, 69(11), 722–725.
- Larsen, M.; Nyvad, B. Enamel Erosion by Some Soft Drinks and Orange Juices Relative to Their pH, Buffering Effect and Contents of Calcium Phosphate. Caries Research 1999, 33(1), 81–87.
- Pinto, S.C., et al. Erosive Potential of Energy Drinks on the Dentine Surface. BMC Research Notes 2013, 6(67), 1–6.
- Amaechi, B.T.; Higham, S.M. Dental Erosion: Possible Approaches to Prevention and Control. Journal of Dentistry 2005, 33(3), 243–252.
- Amaechi, B.; Higham, S.; Edgar, W. Factors Influencing the Development of Dental Erosion in Vitro: Enamel Type, Temperature and Exposure Time. Journal of Oral Rehabilitation 1999, 26(8), 624–630.
- Johansson, A.-K., et al. Dental Erosion and Its Growing Importance in Clinical Practice: From Past to Present. International Journal of Dentistry 2012, 2012.
- Mahmud, S. Z.; Begum, F.; Uddin, M.M. Assessment of Common Oral and Dental Diseases Among Pregnant Women at Dhaka City in Bangladesh. South American Journal of Medicine 2014, 2(2), 165–177.
- Sarwar, A.F.M., et al. Oral Hygiene Practice Among the Primary School Children in Selected Rural Areas of Bangladesh. Journal of Dhaka National Medical College & Hospital 2012, 18(1), 43–48.
- Khan, M.H.A., et al. Evaluation of School Oral Health Education Program-A Review. Bangladesh Journal of Dental Research & Education 2013, 3(2), 45–50.
- Silvi, A.S., et al. Awareness Among Caregivers on Oral Health of Children. American Research Thoughts 2014, 1(2), 960–972.
- Kitchens, M.; Owens, B. Effect of Carbonated Beverages, Coffee, Sports and High Energy Drinks, and Bottled Water on the in vitro Erosion Characteristics of Dental Enamel. Journal of Clinical Pediatric Dentistry 2007, 31(3), 153–159.
- Featherstone, J.D.; Lussi, A. Understanding the Chemistry of Dental Erosion. In Dental Erosion. Monographs in Oral Science; Lussi, A.; Ed.; Karger, 2006; 66–76.
- Pinto, S., et al. Erosive Potential of Energy Drinks on the Dentine Surface. BMC Research Notes 2013, 6(1), 1–6.
- Hughes, J.A., et al. Development and Evaluation of a Low Erosive Blackcurrant Juice Drink in Vitro and in Situ1. Comparison with Orange Juice. Journal of Dentistry 1999, 27(4), 285–289.
- West, N.X., et al. Development of Low Erosive Carbonated Fruit Drinks 2. Evaluation of an Experimental Carbonated Blackcurrant Drink Compared to a Conventional Carbonated Drink. Journal of Dentistry 2003, 31(5), 361–365.
- McDonald, J.L.; Stookey, G.K. Influence of Whole Grain Products, Phosphates, and Tin Upon Dental Caries in the Rat. The Journal of Nutrition 1973, 103(11), 1528–1532.
- Attin, T., et al. Effect of Mineral Supplements to Citric Acid on Enamel Erosion. Archives of Oral Biology 2003, 48(11), 753–759.
- Barbour, M.E.; Lussi, A.; Shellis, R.P. Screening and Prediction of Erosive Potential. Caries Research 2011, 45(Suppl. 1), 24–32.
- Zero, D.T.; Lussi, A. Erosion — Chemical and Biological Factors of Importance to the Dental Practitioner. International Dental Journal 2005, 55(S4), 285–290.
- Featherstone, J.D.B., Lussi, A. Understanding the Chemistry of Dental Erosion. In Dental Erosion from Diagnosis to Theory. Monographs in oral science. 2006; 66–76.
- Amaechi, B.T.; Higham, S.M.; Edgar, W.M. Factors Influencing the Development of Dental Erosion in Vitro: Enamel Type, Temperature and Exposure Time. Journal of Oral Rehabilitation 1999, 26(8), 624–630.
- Dawes, C. What is the Critical pH and Why Does a Tooth Dissolve in Acid? Journal of the Canadian Dental Association 2003, 69(11), 722–724.
- von Fraunhofer, J.A.; Rogers, M.M. Dissolution of Dental Enamel in Soft Drinks. General Dentistry 2004, 52(4), 308–312.
- Lussi, A.; Schaffner, M.; Jaeggi, T. Dental Erosion‐diagnosis and Prevention in Children and Adults*. International Dental Journal 2007, 57(S6), 385–398.
- Lussi, A.; Jaeggi, T. Erosion—Diagnosis and Risk Factors. Clinical Oral Investigations 2008, 12(1), 5–13.
- Enam, F., et al. Characterizing dental Erosion potential of beverages and bottled drinking water in Bangladesh.
- Árnadóttir, I.B.; Sæmundsson, S.R.; Holbrook, W.P. Dental Erosion in Icelandic Teenagers in Relation to Dietary and Lifestyle Factors. Acta Odontologica Scandinavica 2003, 61(1), 25–28.
- Isaksson, H., et al. Prevalence of Dental Erosion and Association with Lifestyle Factors in Swedish 20-year olds. Acta Odontologica Scandinavica 2014, 72(6), 448–457.
- CIA. The World Factbook: Bangladesh. The World Factbook 2016 [cited 2016 September 24]; Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/bg.html.
- Grenby, T.H. Lessening dental erosive potential by product modification. European Journal of Oral Sciences 1996, 104(2), 221–228.
- Hughes, J., et al. Development and Evaluation of a Low Erosive Blackcurrant Juice Drink in Vitro and in situ1. Comparison with Orange Juice. Journal of Dentistry 1999, 27(4), 285–289.
- Hughes, J., et al. Development and Evaluation of a Low Erosive Blackcurrant Juice Drink 3. Final Drink and Concentrate, Formulae Comparisons in Situ and Overview of the Concept. Journal of Dentistry 1999, 27(5), 345–350.
- West, N., et al. Development and Evaluation of a Low Erosive Blackcurrant Juice Drink 2. Comparison with a Conventional Blackcurrant Juice Drink and Orange Juice. Journal of Dentistry, 1999, 27(5), 341–344.
- Beiraghi, S., et al. Effect of Calcium Lactate in Erosion and S. Mutans in Rats when Added to Coca-Cola. Pediatr Dent 1989, 11(4), 312–315.
- Eisenburger, M.; Addy, M. Evaluation of pH and Erosion Time on Demineralisation. Clinical Oral Investigations 2001, 5(2), 108–111.
- West, N.; Hughes, J.; Addy, M. Erosion of Dentine and Enamel in vitro by Dietary Acids: The Effect of Temperature, Acid Character, Concentration and Exposure Time. Journal of Oral Rehabilitation, 2000, 27(10), 875–880.
- Bassiouny, M.; Yang, J. Influence of Drinking Patterns of Carbonated Beverages on Dental Erosion. General Dentistry 2004, 53(3), 205–210.
- Hall, A., et al. The Effect of Saliva on Enamel and Dentine Erosion. Journal of Dentistry, 1999, 27(5), 333–339.
- Meurman, J.; Frank, R. Scanning Electron Microscopic Study of the Effect of Salivary Pellicle on Enamel Erosion. Caries Research 1991, 25(1), 1–6.
- Dawes, C. The Effects of Flow Rate and Duration of Stimulation on the Concentrations of Protein and the Main Electrolytes in Human Parotid Saliva. Archives of Oral Biology 1969, 14(3), 277–294.
- Bardow, A., et al. Saliva Composition in Three Selected Groups with Normal Stimulated Salivary Flow Rates, but Yet Major Differences in Caries Experience and Dental Erosion. Acta Odontologica Scandinavica 2014, 72(6), 466–473.
- Rios, D., et al. Effect of Salivary Stimulation on Erosion of Human and Bovine Enamel Subjected or not to Subsequent Abrasion: An in situ/ex Vivo Study. Caries Research 2006, 40(3), 218–223.
- Gedalia, I., et al. Enamel Softening with Coca-Cola and Rehardening with Milk or Saliva. American Journal of Dentistry 1991, 4(3), 120–122.
- Desobry‐Banon, S.; Vetier, N.; Hardy, J. Health Benefits of Yogurt Consumption. A Review. International Journal of Food Properties 1999, 2(1), 1–12.