ABSTRACT
Blends of fatty acid-balanced oil that was prepared by the aqueous enzymatic extraction, and with fully hydrogenated soybean oil in different weight ratios from 30:70 to 80:20 (wt%) were interesterified using Lipozyme RM IM in a supercritical CO2 system. The optimal immobilized enzyme dosage, pressure, substrate ratio, temperature, and time were 6% (w/w) of initial substrates, 8 MPa, blend ratio with 60:40 (wt%) of fatty acid-balanced oil and fully hydrogenated soybean oil, a temperature of 70°C, and reaction time of 2 h, respectively. It was observed that at the optimal conditions, under supercritical CO2 conditions, the reaction time of the interesterification was shorter than that of conventional enzymatic interesterification. The slip melting point, solid fat content, fatty acid composition, differential scanning calorimetry, polymorphic form and crystal morphology of the enzymatically interesterified fats were evaluated. The results indicated that the interesterified fats showed desirable physical properties with lower slip melting point and solid fat content, suitable crystal form (β′ polymorph), and without trans-fatty acid for possible use as a shortening and margarine stock.
Introduction
Margarine and shortening are important and very much needed ingredients used for bakery products in order to make the perfect baking formation. Their applications are also extending in the food industry and global market. The partial hydrogenation in margarine processing results in a substantial formation of trans-fatty acids. Recently, it has been known that potential adverse health effects, such as increased risk for coronary heart disease, inflammation,[Citation1,Citation2] have been associated with the consumption of semi-solid fats containing trans-fatty acids from partially hydrogenated oils.[Citation3,Citation4]
Many health organizations have recommended to reducing consumption of foods containing trans-fatty acids.[Citation5–Citation7] Therefore, semi-solid fat with low or no trans-fatty acid needs to be developed in the food industry. A number of investigators have attempted modification of oils and fats by physical, chemical, or biochemical methods.[Citation8,Citation9] Interesterification to produce specialty fats has received attention mainly due to the production of zero-trans fats. To produce low- or no-trans plastic fats, interesterification is used as an alternative process to partial hydrogenation in which physical and chemical properties and crystallization behavior are modified. In the interesterification, the fatty acids are not altered but are redistributed in the triacylglycerol (TG) molecules. The interesterification modifies the TG composition of the oil or fat and its physical properties, such as crystallization and melting behavior, solid fat content (SFC), textural and crystal habit. What’s more, it contributes to a greater availability of oil fractions for various food applications.[Citation10,Citation11]
Two major challenges facing the food and ingredient industry today are providing health-conscious products to the public and developing environmentally responsible production technology. Among many alternative processing methods, enzymatic interesterification is reported as an effective method to produce zero or low trans-fats such as stick margarine fat and shortening.[Citation12] There are also many studies about immobilization of lipase used in transesterification reaction, and the immobilized lipase can be easily separated from the reaction mixture at the end of the reaction process and it can be reused.[Citation13–Citation17] In combination with an environmentally benign and safe medium, such as supercritical CO2, enzymatic catalysis makes supercritical fluids (SFs) extremely attractive to the food industry. The main reason for the frequent use of lipase in SFs is the increased solubility of hydrophobic lipid substrates in nonpolar SF, and the reversal of hydrolysis reactions in favor of synthesis, such as esterification and transesterification. Nonpolar substrates such as lipids are more soluble in dense gases than in aqueous solution. Moreover, the advantages of carbon dioxide as a SF include its low toxicity, low cost, lack of flammability, lack of reactivity, and a wide range of solvent properties at different pressures and temperatures and improved properties of separated components in certain cases.[Citation18] Therefore, enzyme interesterification in supercritical CO2 system is efficient and less time consuming. It increases processing flexibility and also provides numerous functional characteristics including a sharper melting profile and a low or zero trans alternative that is fully functional.
Recently, the concept that oils with balanced fatty acids promote good health has been widely recognized by nutritionists and medical scientists. The United States Recommended Dietary Allowances, the World Health Organization, and the American Heart Association recommend an ideal dietary fatty acid ratio of 1:1:1 (SFA, saturated fatty acid(s); MUFA, monounsaturated fatty acid(s); PUFA, polyunsaturated fatty acid(s)).[Citation6,Citation19] In addition, a report from the Chinese Nutrition Society regarding dietary reference intakes suggested a fatty acid ratio of 0.27:1:1 (SFA:MUFA:PUFA), after subtracting the daily consumption of fatty acids derived from animal fat.[Citation20] Therefore, fatty acid-balanced oil (FABO) was prepared from a mixture of soybeans, peanuts, linseeds, and tea seeds using an aqueous enzymatic method. The FABO has an ideal dietary fatty acid ratio of 0.27:1:1 (SFA:MUFA:PUFA) and no trans-fatty acids.
Looking for interesterified fats with desirable physical properties, our study aims to assess enzymatically interesterified of FABO and fully hydrogenated soybean oil (FHSO) blends. The enzymatic-interesterified fats by using supercritical CO2 process could be used as a replacement for partially hydrogenated fats and oils for the production of margarine and shortening. It’s considered as more efficient and less time-consuming process.
In the present study, the FABO was prepared by the enzyme-assisted aqueous extraction processing that was chosen as the materials due to its nutritional qualities, such as trans-free fatty acid and the ideal dietary fatty acid ratio of 0.27:1:1 (SFA:MUFA:PUFA). Varying proportions of FABO and FHSO blends were interesterified and their physical properties, such as slip melting point (SMP), SFC, melting thermograms, crystal morphology, and polymorphic forms, were examined. The generated results can be used to provide information for industrial scale-up of enzymatic interesterification application.
Materials and methods
Materials
FABO was prepared using the method described by Li et al.[Citation21] Lipozyme RM IM immobilized on ion-exchange resin (Novozymes A/S, Bagsvaerd, Denmark), from Rhizomucor miehei [EC number 3.1.1.3] with sn-1,3-specific selectivity, was used as the biocatalyst. CO2 (>99.9%) was supplied by the Harbin Liming Gas Co., Ltd. (Harbin, China). The fatty acid methyl ester standards were obtained from the Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals were analytical grade.
Fully hydrogenated oils
FHSO was prepared from the FABO in a high-pressure reaction kettle under supercritical CO2 system. Reactions were carried out for 95 min at 120°C, reaction pressure of 14 MPa, and palladium on carbon (Pd/C) catalyst of 0.5%, under magnetic agitation. After the reaction, the oil samples were collected for further analyses.
Interesterification
Interesterified oils were prepared from the blends of FABO and FHSO in different ratios from 30:70 to 80:20 (wt%) using Lipozyme RM IM in a high pressure reaction kettle under a supercritical CO2 system. Reactions were carried out for 2 h at 70°C, reaction pressure of 8 MPa, and lipase dosage of 6%, under magnetic agitation. After the interesterification reaction, the oil samples were collected and stored at 4°C for further analysis.
SFC
SFC values of samples were determined according to the AOCS Official Method Cd 16b-93 on a Bruker Minispec pulse Nuclear Magnetic Resonance spectrometer (Karlsruhe, Germany).[Citation22] The samples in the NMR tube were first melted at 70°C for 30 min, followed by chilling at 0°C for 90 min, and then held at each measuring temperature for 30 min prior to measurement. SFC was measured at intervals of 5°C from 5 up to 60°C.
Fatty acid composition
The fatty acid compositions of samples were determined according to AOCS Official Method Ca 5a-40.[Citation22]
Differential scanning calorimetry
Melting and crystallization profiles of lipid samples were determined with PE Pyris 6 model differential scanning calorimetry (DSC) (Netzsch, Germany) following AOCS Official Method Cj 1-94.[Citation22] The 8–12-mg samples were weighed into aluminum pans and sealed. Normal standardization was performed with n-decane and indium. The samples were initially heated rapidly from room temperature to 80°C and held for 10 min to erase the crystal memory; then, the samples cooled to −60°C at 10°C/min and held for 30 min, after that, the samples were heated to 80°C at 5°C/min to determine the crystallization and melting properties. Currently, 80°C was considered as the temperature to determine the melting properties.
SMP
SMP values of samples were determined using an open capillary tube according to AOCS Official Method Cc 3-25.[Citation22] Capillary tubes filled with a 1-cm high column of fat were chilled at 10 ± 1°C for 16 h before being immersed in a beaker of cold distilled water. The water was stirred and heated, and the temperature was recorded when the column of fat rose in the tube.
X-ray diffraction spectroscopy
The crystal polymorphism of samples was determined by X-ray diffraction, using a XRD-6000 (Rigaku Int. Corp., Tokyo, Japan) with a fine copper X-ray tube, operating at 40 kV and 35 mA. The short spacing was observed with ranging from 12° to 32° (2θ, scale), at a scan rate of 2°/min. The samples were melted (80°C) and poured into a rectangular plastic mold and tempered at 24°C for 16 h. The α form shows a single strong spacing at ca. 4.15 Å, while β′ form exhibits two strong spacings at ca. 3.8 and 4.2 Å, or three spacing at ca. 3.71, 3.97, and 4.27, whereas β form was identified with a strong spacing at ca. 4.6 Å.
Experimental statistical analysis
All tests were carried out in triplicate and the data were reported as means ± standard deviations. ANOVA SPSS 14 statistical software was used for analysis of variance (SPSS Inc., Chicago, IL). Differences were considered statistically significant at p < 0.05. An analysis of variance was used to compare results.
Results and discussion
Effect of different ratios (FABO/FHSO) on SMP values of interesterified products
Effects of different ratios (FABO/FHSO) from 30:70, 40:60, 50:50, 60:40, 70:30, and 80:20 (wt%) on the SMP values of interesterified products were investigated using Lipozyme RM IM as a biocatalyst in a high-pressure reaction kettle equipped in supercritical CO2 system. The reactions were carried out for 2 h at a temperature of 70°C, reaction pressure of 8 MPa, and lipase dosage of 6%, under magnetic agitation of 400 r/min.
SMP is a significant parameter for characterizing and developing interesterified fats. shows that the SMP of the interesterified products decreased from 59.3 to 27.4°C with an increase of the blend ratios of FABO and FHSO from 30:70 to 80:20 significantly (p < 0.05). It caused considerable rearrangement of TG species because of interesterification reaction. Increasing FABO proportions in the blended oil mixture would reduce trisaturated TG content and increase in monounsaturated and diunsaturated TGs, resulting in lowering of respective melting points. Other researchers have also reported similar results.[Citation23,Citation24] The results indicated that the interesterified oil products of the blend ratio at 60:40 (FABO/FHSO) with lower SMP value of 40.5°C are most desired for shortenings and margarines preparation. Based on these results, the samples with optimal blend ratio of 60:40 (FABO/FHSO) were selected for the next set of reactions to assess the optimization of enzymatic interesterification under supercritical CO2 condition.
Table 1. SMP of interesterified products.
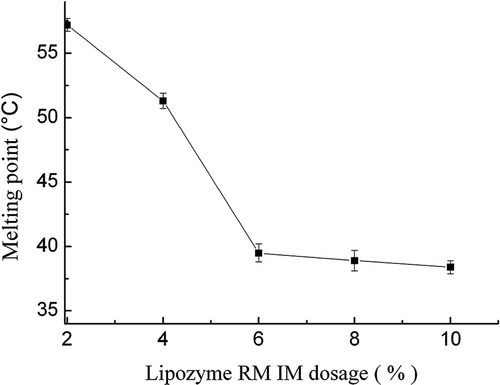
Effect of the lipase dosage on the SMP values of interesterified products
Effect of the lipase dosage on the SMP values of interesterified products was evaluated at the range from 2% to 10% of dosage under supercritical CO2 condition. The reactions were carried out for 2 h at a temperature of 70°C, a reaction pressure of 8 MPa, and blend ratio of 60:40 (FABO/FHSO), under magnetic agitation of 400 r/min. As shown in , the SMP values of interesterified products decreased sharply with increasing lipase dosage up to 6%, but it did not significantly decrease the SMP values after above that amount. Obviously, a great amount of lipase provides abundant active sites and sufficient mass contact, consequently leads to a higher conversion rate; therefore, SMP values of interesterified products decreased sharply. However, when the lipase dosage exceeded 6%, the SMP values remained unchanged. It may be due to the lack of substrates corresponding to the active sites of the enzyme, and the difficulties in maintaining a uniform suspension of the biocatalysts. Yadav et al.[Citation25] reported that the higher viscosity of the reaction medium with the addition of higher amounts of enzyme results in a less effective transfer of the substrates to the active sites of the enzyme. Therefore, the optimal lipase dosage of 6% in the samples was used for further optimization of enzymatic interesterification with supercritical CO2 system.
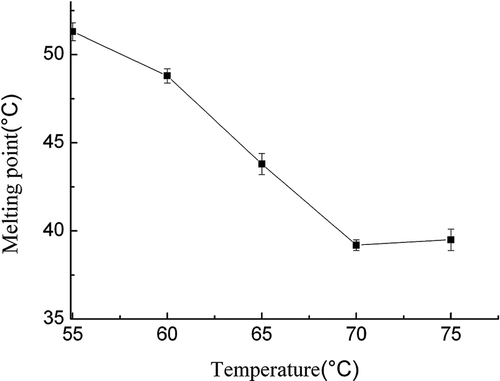
Effect of the reaction temperature on SMP values of interesterified products
The enzymatic transesterification was carried out under the following conditions: the reaction temperature in the range of 55–75°C, the reaction time of 2 h, the bend ratio of 60:40 (FABO/FHSO), the reaction pressure of 8 MPa, lipase dosage of 6%, and magnetic agitation of 400 r/min. presents the effect of reaction temperatures on the SMP values of interesterified products. The results indicate that temperature had significant effects on the SMP values. The SMP values decreased sharply from 51 to 39.2°C by increasing reaction temperature from 55 to 70°C, significantly (p < 0.05). In fact, an increase in temperature would reduce the mixture viscosity of substrates and thus reduces mass transfer limitations which favor the interactions between enzyme particles and substrates. Furthermore, the SMP values slightly increased with the further increase of temperature at temperature ranges higher than 70°C, which could be due to partial denaturation of the enzyme and loss of its activity at a higher temperature. Therefore, the results indicated that the highest suitable reaction temperature was observed at a temperature of 70°C. Pande et al.[Citation26] studied the enzymatic synthesis of trans-free structured margarine fat analogs with high-stearate soybean oil; they obtained the optimum reaction temperature of 50–65°C.
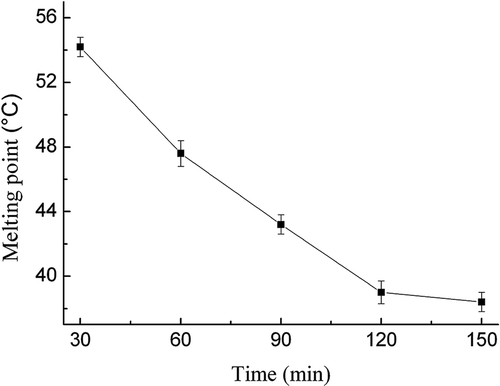
Effect of the reaction time on SMP values of interesterified products
illustrates the effects of reaction time on the SMP values of interesterified products. The effects of reaction time were evaluated under the following conditions: reaction time in the range of 30–150 min, blend ratio of 60:40 (FABO/FHSO), reaction temperature of 70°C, reaction pressure of 8 MPa, lipase dosage of 6%, and magnetic agitation of 400 r/min. As shown in , the SMP values of interesterified products decreased rapidly from 51 to 39.2°C with increasing reaction times from 30 to 120 min significantly (p < 0.05). After that, the SMP values of interesterified products did not significantly decreased with further increase of reaction time from 120 to 150 min. Therefore, the optimum reaction time was determined at 2 h. Adhikari et al.[Citation23] obtained the optimum reaction time of 24 h in the study of the scaled-up production of zero-trans margarine fat using pine nut oil and palm stearin in normal pressure. In contrast, the mass transfer resistance decreases under the supercritical CO2 system; therefore, the reaction time of enzymatic transesterification could be shortened significantly. Jenab et al.[Citation27] also observed similar results in the research of lipase-catalyzed interesterification between canola oil and fully hydrogenated canola oil in contact with supercritical carbon dioxide.
Physicochemical properties of interesterified products
Fatty acid composition: Fatty acid composition of the FABO, FHSO, and interesterified products is shown in . In FABO, the main fatty acids were oleic (32.32%), linoleic (33.36%), and palmitic acids (12.24%) which has an ideal dietary fatty acid ratio of 0.27:1:1 (SFA:MUFA:PUFA), and no trans-fatty acids, while SFA of palmitic acids (12.06%) and stearic acids (84.32%) was found in FHSO, respectively. The results showed that the fatty acid composition of FABO and FHSO blends had not been changed before and after enzymatic interesterification. Trans-fatty acids were not detected in FABO and FHSO blends before and after enzymatic interesterification. The fatty acids in TAG were largely rearranged during the interesterification, and then the physicochemical properties of interesterified product were significantly modified by interesterification. Rozendaal et al.[Citation28] reported that in the interesterification, the fatty acids are not altered but are redistributed in the TG molecules. The process thus consists in simultaneously breaking the existing ester bonds and forming new bonds in the glycerol molecules.
Table 2. Fatty acid composition (area %) of FABO, FHSO, and interesterified products.
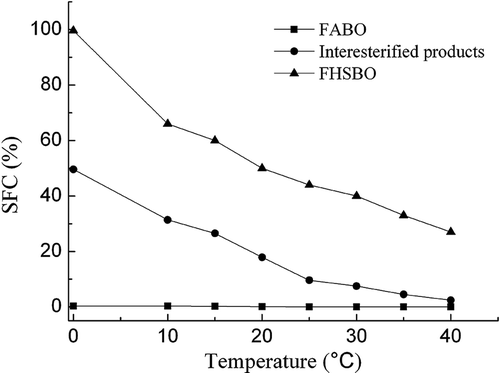
SFC: SFC is the percentage of lipid in solid form at a given temperature, and these values are responsible for many characteristics of margarines, including their appearance, ease of packaging, spreadability, oiling-out, and organoleptic properties. shows the SFC curves of the FABO, FHSO, and interesterified product as a function of temperature, respectively. FHSO and interesterified products exhibited a similar trend in the SFC curves with a marked decline between 0 and 40°C. The FHSO showed highest SFC values than the interesterified products, it may attribute that FHSO contains a higher amount of high-melting tristearin. The SFC curve of the FABO showed a plateau at all measured temperatures because of a high level of USFA content (80.0%, ). After interesterification, the SFC curve of interesterified product showed 16.0% at 25°C and 20.0% at 20°C, and thereafter, SFC curve decreased to 2.4% at 40°C. SFC curves at room temperature (25°C) should be 15–35% for desirable spreadability as plastic fats.[Citation29] Therefore, the interesterified product had an ideal SFC curve that might be suitable for trans-free margarine and shortenings stock.
Melting and crystallization properties
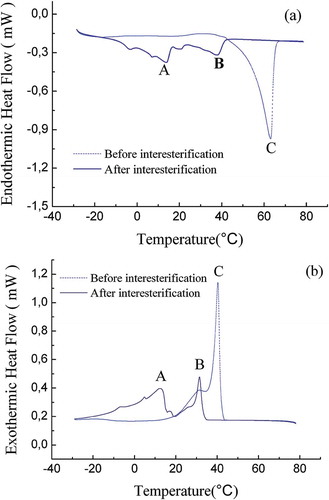
DSC provides information about the amount of energy exchanged for melting and crystallization of fats and oils. Fat melting is an endothermic process in which the energy is absorbed, whereas crystallization is an exothermic process in which the energy is released.[Citation30] DSC crystallization and melting thermograms of FABO and FHSO blends before and after enzymatic interesterification are presented in and . The results indicated that the interesterification changed largely the melting profile of the FABO and FHSO blends as shown in . The melting thermograms of the non-interesterified FABO and FHSO blends showed a sharp peak C at 42°C which represented highest melting TGs. After interesterification, changes in the shape of the melting curve were observed since peak C in the blends disappeared, while the highest melting peaks of the interesterified products were observed at 16 (peak A) and 32°C (peak B), respectively, which indicating the formation of a softer fat as a result of the randomization of the fatty acids present in the glycerides of both oils. DSC cooling curve shows one distinct crystallization peak C at 62°C in the non-interesterified FABO and FHSO blends as shown in . This crystallization behavior of the FABO and FHSO blends was changed by the interesterification resulting in two crystallization peaks (A and B) observed at 17 and 38°C in the interesterified product. These results showed that the harder product being formed with an increase in the stearin content. Before interesterification, in the melting profile (), peaks C of the non-interesterified blend disappeared. However, after interesterification, new peaks A and B were formed which indicating a softer product being formed as a result of the randomization of the fatty acids present in the glycerides of both oils.
Crystal Polymorphism by X-ray diffraction spectroscopy
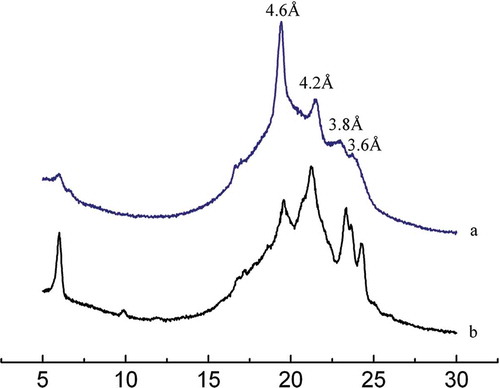
Polymorphic forms and crystal morphology are the most important criteria for determining the functional properties of margarines and shortenings. The main polymorphs of fat crystals are α, β′, and β polymorphic forms. Each polymorph has different characteristics with α form characterized as unstable with the lowest melting point and short spacing at 4.15 Å; β′ form, metastable, intermediate melting point and two strong short spacings at 3.80 and 4.20 Å, and three minor short spacings at 4.27, 3.97, and 3.71 Å, respectively. The β form is very stable, highest melting point, and short spacing at 4.60 Å.[Citation31] The β′ form having small crystal size is a desirable polymorphic form for margarine and shortening stock.
The polymorphic forms of the FABO and FHSO blends before and after enzymatic interesterification are presented in . X-ray diffraction spectra of FABO and FHSO blends before and after enzymatic interesterification indicated that the FABO and FHSO blends displayed stronger intensities at 3.8, 4.2, or 4.3 Å than 4.6 Å, whereas the interesterified product showed only two stronger intensities at 4.2 and 3.8 Å, which indicates the β′ crystal form. After interesterification, the spacing at 4.6 Å had disappeared. The results indicated that the predominant crystal form had changed from β to β′. A similar result was also presented by Ref. [Citation24].
Conclusion
The study demonstrated that blends of FABO and FHSO in different ratios from 30:70 to 80:20 (wt%) were effectively interesterified using Lipozyme RM IM in the supercritical CO2 system. In this work, the FABO samples were obtained by using the enzyme-assisted aqueous extraction processing due to its nutritional qualities, such as trans-free fatty acid and the ideal dietary fatty acid ratio of 0.27:1:1 (SFA:MUFA:PUFA), which could confer health benefits. Furthermore, in the supercritical CO2 system, the reaction time of the interesterification was shorter than that of conventional enzymatic interesterification. The evaluation of the interesterified product indicated that the fatty acid composition, melting, and crystallization behavior, and SFC of initial blends of FABO and FHSO, were considerably modified with the rearrangement of fatty acids within or between TAGs after enzymatic interesterification. The produced interesterified fats showed desirable physical properties (lower SMP and SFC), suitable crystal form (β′ polymorph), and without trans-fatty acid. Therefore, the enzymatic-interesterified fats by using supercritical CO2 process could be used as a replacement for partially hydrogenated fats and oils for the production of margarine and shortening. The method can save the reaction time and have guiding significance for the development of healthy functional foods and further study.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (NSFC): Study on the method of controlling TFAs in oil by orientated hydrogenation and mechanism of molecular reaction under CO2 supercritical state (grant number 31271886) General program. This work was also supported by a grant from the Project of Fundamental Research Funds of Heilongjiang Provence: Pilot study on the phytosteryl esters of soybean oil. (grant number DADOU2014-2). This work was also supported by a grant from the Key Laboratory of Soybean Biology in Chinese Ministry of Education Northeast Agricultural University, Harbin, China, 150030.
Additional information
Funding
References
- Astorg, P. Dietary Fatty Acids and Colorectal and Prostate Cancers: Epidemiological Studies. Bulletin du Cancer 2005, 92(7), 670–684.
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary Intake of Trans Fatty Acids and Systemic Inflammation in Women. The American Journal of Clinical Nutrition 2004, 79(4), 606–612.
- Gurr, M.I. Trans Fatty Acids and Health-an Update. Lipid Technology 1990, 2, 105–107.
- Hunter, J.E. Dietary Levels of Trans-Fatty Acids: Basis for Health Concerns and Industry Efforts to Limit Use. Nutrition Research 2005, 25(5), 499–513.
- Food, Drug Administration, H.H.S; others. Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims, Final Rule. Federal Register 2003, 68, 41433.
- Organization, W.H.; Organization, W.Hothers. Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids, Genebra Jt, FAOWHO Expert Consult, Fats Fat, Acids Hum, Nutr.
- Stender, S.; Dyerberg, J.; Hølmer, G.; Ovesen, L.; Sandström, B. The Influence of Trans Fatty Acids on Health: A Report from the Danish Nutrition Council. Clinical science, London, England, 1995, 88(4), 375–392.
- De, B.K.; Patel, J.D. Modification of Palm Oil by Chemical and Enzyme Catalyzed Interesterification. Journal of Oleo Science 2010, 59, 293–298.
- Pacheco, C.; Palla, C.; Crapiste, G.H; Carrín, M. E. Optimization of Reaction Conditions in the Enzymatic Interesterification of Soybean Oil and Fully Hydrogenated Soybean Oil to Produce Plastic Fats. Journal of the American Oil Chemists Society 2013, 90(3), 391–400.
- Ribeiro, A.P.B.; Grimaldi, R.; Gioielli, L.A; Gonçalves, L.A. Zero Trans, Fats from Soybean Oil and Fully Hydrogenated Soybean Oil: Physico-Chemical Properties and Food Applications. Food Research International 2009, 42(3), 401–410.
- Makeri, M.U.; Karim, R.; Abdulkarim, M.S.; Ghazali, H.M.; Miskandar, M.S.; Muhammad, K. Comparative Analysis of the Physico-Chemical, Thermal and Oxidative Properties of Winged Bean and Soybean Oils. International Journal of Food Properties 2016, 19(12), 2769–2787.
- Adhikari, P.; Zhu, X.M.; Gautam, A.; Shin, J.A.; Hu, J.N.; Lee, J.H.; Akoh, C.C.; Lee, K.T. Scaled-up Production of Zero- Trans, Margarine Fat using Pine Nut Oil and Palm Stearin. Food Chemistry 2010, 119(4), 1332–1338.
- Xie, W.; Chi, Z. Propylsulfonic and Arenesulfonic Functionalized SBA-15 Silica as an Efficient and Reusable Catalyst for the Acidolysis of Soybean Oil with Medium-Chain Fatty Acids. Food Chemistry 2016, 211, 74–82.
- Xie, W.; Hu, L. Biguanide-Functionalized Mesoporous SBA-15 Silica as an Efficient Solid Catalyst for Interesterification of Vegetable Oils. Food Chemistry 2016, 197, 92–99.
- Xie, W.; Chen, J. Heterogeneous Interesterification of Triacylglycerols Catalyzed by Using Potassium-Doped Alumina as a Solid Catalyst. Journal of Agricultural and Food Chemistry 2014, 62, 10414–10421.
- Xie, W.; Zang, X. Immobilized Lipase on Core–Shell Structured Fe3O4–MCM-41 Nanocomposites as a Magnetically Recyclable Biocatalyst for Interesterification of Soybean Oil and Lard. Food Chemistry 2016, 194, 1283–1292.
- Li, C.; Zhang, G.; Liu, N.; Liu, L. Preparation and Properties of Rhizopus oryzae Lipase Immobilized using an Adsorption-Crosslinking Method. International Journal of Food Properties 2016, 19(8), 1776–1785.
- Jackson, M.A.; King, J.W.; List, G.R.; Neff, W.E. Lipase-Catalyzed Randomization of Fats and Oils in Flowing Supercritical Carbon Dioxide. Journal of the American Oil Chemists Society 1997, 74, 635–639.
- Allowances, R.D. Committee on Dietary Allowances. Food Nutr. Board Comm. Life Sci. Natl. Res. Counc; 9th Ed.; Natl, Acad, Press: Wash, DC. 1980, 5.
- Society, C.N. Chinese Dietary Reference Intakes; Zhongguo qing gong ye chu ban she: Beijing, 2000.
- Li, Y.; Zhang, Y.; Wang, M.; Jiang, L.; Sui, X. Simplex-Centroid Mixture Design Applied to the Aqueous Enzymatic Extraction of Fatty Acid-Balanced Oil from Mixed Seeds. Journal of the American Oil Chemists Society 2013, 90(3), 349–357.
- Society, A.O.C.; Firestone, D. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Champaign: IL, USA, 1989.
- Adhikari, P.; Shin, J.A.; Lee, J.H.; Hu, J.N.; Zhu, X.M.; Akoh, C.C.; Lee, K.T. Production of Trans -Free Margarine Stock by Enzymatic Interesterification of Rice Bran Oil, Palm Stearin and Coconut Oil. Journal of the Science of Food & Agriculture 2010, 90(4), 703–711.
- Jeunghee, L.; Casimirc, A.; Kiteak, L. Physical Properties of trans-Free Bakery Shortening Produced by Lipase-Catalyzed Interesterification. Journal of the American Oil Chemists Society 2008, 85, 1–11.
- Yadav, G.D.; Trivedi, A.H. Kinetic Modeling of Immobilized-Lipase Catalyzed Transesterification of N -Octanol with Vinyl Acetate in Non-Aqueous Media. Enzyme & Microbial Technology 2003, 32, 783–789.
- Pande, G.; Akoh, C.C. Enzymatic Synthesis of Trans -Free Structured Margarine Fat Analogs with High Stearate Soybean Oil and Palm Stearin and their Characterization. LWT - Food Science and Technology 2013, 50, 232–239.
- Jenab, E.; Temelli, F.; Curtis, J.M. Lipase-Catalysed Interesterification between Canola Oil and Fully Hydrogenated Canola Oil in Contact with Supercritical Carbon Dioxide. Food Chemistry 2013, 141, 2220–2228.
- Rozendaal, A.; Macrae, A.R. Interesterification of Oils and Fats. In Lipid Technol. Appl.; Gunstone, F.D.; Padley, F.B.; Eds.; Marcel Dekker, Inc.: New York, USA, 1997; pp. 223–263.
- Rao, R.; Sankar, K.U.; Sambaiah, K.; Lokesh, B.R. Differential Scanning Calorimetric Studies on Structured Lipids from Coconut Oil Triglycerides Containing Stearic Acid. European Food Research & Technology 2001, 212(3):334–343.
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; dos Santos, A.O.; Cardoso, L.P.; Gonçalves, L.A.G. Influence of Chemical Interesterification on Thermal Behavior, Microstructure, Polymorphism and Crystallization Properties of Canola Oil and Fully Hydrogenated Cottonseed Oil Blends. Food Research International 2009, 42, 1153–1162.
- Solís-Fuentes, J.A.; Carmendurán-De-Bazúa, M.D. Determination of the Predominant Polymorphic Form of Mango (Mangifera indica) Almond Fat by Differential Scanning Calorimetry and X-ray Diffraction. European Journal of Lipid Science & Technology 2005, 107, 395–401.