ABSTRACT
Changes in quality indices [total volatile base nitrogen (TVB-N), salt extractable protein (SEP), hypoxanthine (Hx), K-value, sensory assessment (SA), and electrical conductivity (EC)] for shrimp (Solenocera melantho) stored at −28, −20, and −12°C for 112 days were investigated in this study. The Arrhenius model and the radial basis function neural network (RBFNN) model were established to predict changes in the quality of shrimp during storage. Quality of shrimp stored at −12°C changed more quickly during 56–112 days, but those stored at −28°C deteriorated slowly during the entire storage period. Additionally, the indicators SEP, EC, and SA all fitted to the Arrhenius model well (relative errors within ±10%), but this model did not perform well in the prediction of K-value, Hx, and TVB-N on some days. However, the RBFNN model showed excellent accuracy for all indicators (relative errors within ±0.5%). The RBFNN model performed better than the Arrhenius model in predicting the quality of shrimp stored at −28°C to −12°C.
Introduction
Shrimp play an important role in the seafood market worldwide, which comprises about 20% of the total value in international seafood trade.[Citation1] Shrimp are popular because they are nutritious and delicious. Because of globalization, people can consume shrimp from all over the world. However, shrimp die quite quickly after being caught. Similar to other seafood, shrimp are perishable because they are rich in water, protein, non-protein nitrogenous compounds, and other nutrients, which can be used rapidly by microorganisms.[Citation2]The degree of loss in the quality of frozen seafood is mainly influenced by storage temperature.[Citation3]The rapid spoilage and the close relationship between temperature and loss of quality make freezing preservation particularly important for maintaining good quality.
The Arrhenius model has been used widely for predicting the quality of food. In research on aquatic products, the Arrhenius model proved worthwhile and effective for predicting changes in the quality of crucian carps (Carassius auratus gibelio) and silver carp (Hypophthalmichthys molitrix) during storage.[Citation4,Citation5] However, Wu et al.[Citation3] applied the Arrhenius model for predicting the quality of bighead carp fillets, and found that several indicators could not be predicted accurately. Yao et al.[Citation6] also reported that the Arrhenius model did not perform well in the late stage of storage for predicting the quality of crucian carp (Carassius carassius). Thus, more general and precise models need to be constructed.
The RBFNN model, a type of new model for predicting quality, has been applied rarely in food science. Nevertheless, the advantages of the RBFNN model in prediction are obvious from some reports. For example, RBFNN models have shown great accuracy for predicting the quality of water.[Citation7,Citation8] The RBFNN model with a learning feature can train datasets automatically and obtain accurate predictive results.[Citation9] However, there are only a few reports on the application of the RBFNN model for predicting changes in the quality of shrimp during storage. Ahmad et al.[Citation10] reported that the RBF model can predict some quality parameter values of shrimp from −23 ± 0.5 to −10 ± 0.5 °C satisfactorily with high reliability.[Citation10]
The objectives of this work were to explore changes in key quality indicators [total volatile base nitrogen (TVB-N), salt extractable protein (SEP), K-value, hypoxanthine (Hx), electrical conductivity (EC), and sensory assessment (SA)] of shrimp stored at different frozen temperatures (−28, −20, and −12°C), and to establish and compare the RBFNN and Arrhenius models for predicting the quality of stored shrimp. The average storage temperatures in about 30% of retail freezers were −18 to −28°C, while 20% were held at −10 to −12°C.[Citation11] Thus, −28°C, −20°C, and −12°C were chosen as the experimental temperatures to address the practical application of this study.
Materials and methods
Materials
Raw fresh shrimp (Solenocera melantho; size: about 8 g of per shrimp) were harvested in Zhejiang Province, China, in December 2015. On the same day as capture, the shrimp were quick-frozen (refrigerator, Qingdao Haier Joint Stock Co., Ltd., Qingdao, China) to −28°C, and transported to the laboratory in Beijing, China. They were packaged and sealed in plastic bags. All shrimp were divided into three groups, and then stored in a refrigerator at −28°C, −20°C, and −12°C, respectively. Three replicate samples were taken randomly for analysis at specified intervals from samples at each storage temperature, and they were thawed at 4°C for 12 h before experiments.
TVB-N
TVB-N was determined by the semi-micro steam distillation method of Zhang et al.[Citation12] with some modification. Five grams of homogenized shrimp sample was stirred with 50 mL distilled water for 30 min, and filtered at medium speed. The mixture of 5 mL filtrate and 5 mL MgO (10g L−1) was distilled for 5 min with a Kjeldahl Apparatus (KDY-9820, Beijing, China), with 5 mL distilled water as a control. An acid receiver contained boric acid and a mixed indicator absorbed volatile base components, and the result was determined by titration.
SEP
SEP was measured by the method of Wu et al.[Citation3] with some modifications. One gram of shrimp sample was homogenized with 15 mL of cold distilled water for 30 s. After 20 min of extraction at 4°C, the homogenate was centrifuged at 10000 rpm for 20 min, and the precipitate was collected. Afterward, 15 mL pH 7.0 Tris-maleate buffer (0.6 M NaCl-20 Mm Trismaleate) was added to the precipitate and homogenized for 30 s. After 60 min of extraction at 4°C, the homogenate was centrifuged at 10000 rpm for 20 min again. The supernatant was diluted to 25 mL using the Tris-maleate buffer. Then 1 mL of dilutant was mixed with 4 mL Biuret reagent, and allowed to react for 20 min at 20°C. The absorption value at 540 nm of this sample was detected by a spectrophotometer (UNICO Instruments Co., Ltd, Shanghai, China) and SEP was calculated using the absorption value.
K-value and Hx
K-value and Hx determinations were carried out by the modified method of Liu et al.[Citation13] One gram of shrimp sample was ground using 2 mL of perchloric acid (10% (v/v)), and centrifuged for 5 min at 6000 rpm with the supernatant collected. The sediment was washed twice by 2 mL 5% (v/v) perchloric acid and centrifuged (6000 rpm, 5 min) to collect the supernatant. The collected supernatants were all neutralized to pH 6.40±0.05 with 10 mol L−1 KOH, 1 mol L−1 KOH, and 5% (v/v) perchloric acid, and then centrifuged (6000 rpm, 5 min) to obtain the supernatant. The supernatant was diluted to 10 mL with pH 6.40 perchloric acid for analysis. After being filtered through a 0.22 μm membrane filter, the dilutant was analysed for ATP and its related compounds by a high-performance liquid chromatography (HPLC, (Shimadzu, LC-10ATseries, Japan), equipped with an SPD-10A (V) detector (254 nm) and a COSMOSIL 5C18-PAQ column (4.6ID 250 mm) as the stationary phase. A 50 μL sample was injected at the flow rate of 1 mL/min with pH 6.8 phosphate buffer as the mobile phase. The amount of ATP and its related compounds were identified and quantified by comparing the retention time and spectra based on their Sigma external standards. The equation for the K-value is as follows:
where ATP is triphosphate, ADP is adenosine diphosphate, AMP is adenosine monophosphate, IMP is inosine monophosphate, HxR is hypoxanthine riboside, and Hx is hypoxanthine.
Electrical conductivity
Five grams of the homogenized shrimp sample was stirred with 50 mL distilled water for 30 min, and filtered at medium speed. EC of the filtrate was determined using a digital EC meter (Mettler Toledo FE20/EL20, Shanghai, China).
Sensory assessment
A panel of five trained members from the laboratory carried out the sensory evaluation experiment based on a six-point scale, in which six was the best and one was the worst for each aspect. Each panellist evaluated one whole shrimp from a random pack of shrimp. The SA of the shrimp sample was evaluated by five aspects [colour (6, no discoloration; 1, extreme discoloration), morphological integrity (6, tight and complete; 1, extremely loose and incomplete), smell (6, extremely desirable; 1, extremely unacceptable), musculature (6, elastic; 1, extremely inelastic), and soap turbidity of boiled shrimp (6, clear; 1, extremely turbid)]. Scores of different aspects were all added up to obtain the overall sensory score. The total score below 15 was considered unacceptable.
Establishment of the arrhenius model
First, kinetic analysis of the experimental data was carried out by the method of Liu et al.[Citation13] The rate of changes in quality can be indicated by the general kinetic reaction:[Citation14]
where B is the value of quality indices (TVB-N, SEP, K-value, Hx, EC, and SA) for samples stored for t days, k is the rate constant, and n is the kinetic order. Different orders of reaction equations can be obtained when substituting n =0, 1, and 2 into Eqn (1) with some transformations:
where B0 is the initial value. The data were fitted to zero-order, first-order, and second-order reaction equations and analysed using the linear regression based on the plot of quality indicators (B, ln B, or 1/B) versus time. The coefficient of determination (r2) was used to evaluate the appropriateness of each reaction.
Arrhenius equation:[Citation15]
where k0 is the pre-exponential constant, Ea is the activation energy (J mol–1), T is the absolute temperature (K), and R is the universal gas constant (8.3144 J mol–1K–1). When substituting Eqn (5) into Eqns. (2), (3), and (4), the Arrhenius predictive equations can be obtained:
Establishment of the RBFNN model
The RBFNN model is a promising feed-forward model that can predict nonlinear data effectively.[Citation16] The model is built by adding new samples to the hidden layer and cutting the disused hidden nodes; information granulation and a genetical algorithm were used to optimize its parameters.[Citation17,Citation18] The RBFNN can be trained to respond to specific inputs with target outputs.
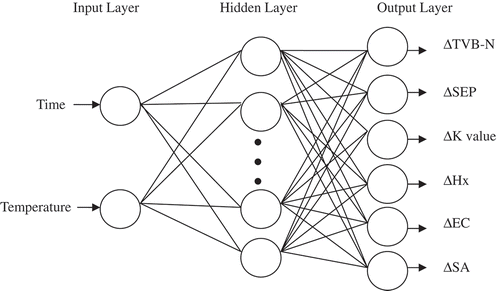
The RBFNN is composed of an input layer, a hidden layer, and an output layer. The input layer is the link between the external environment and the network. Many nodes in the hidden layer use a radial basis function to apply a nonlinear transformation to the input data.[Citation19] A Gaussian function is commonly used as the radial basis function in RBFNN, which is shown in equation (9).[Citation20] The outputs were generated based on linear combinations of activations of basis functions figured by hidden nodes.[Citation15] The formula for calculating outputs is shown in Eq. (10).[Citation20,Citation21]
where X(i) is the input vector (the ith input node); Sj is the centre of the jth hidden node; σ is spread (σ=0.5 is specified in this study).
where Zm(x) is the mth component of the output vector; Wjm is the weight from the jth hidden layer neuron to the mth output neuron. The input layer is composed of two neurons, which received input signals for storage temperature (K) and time (day) and the output layer consists of six neurons, which are ΔTVB-N, ΔSEP, ΔK-value, ΔHx, ΔEC, and ΔSA (ΔB represents the quantity variance between indicator at time t and the initial value) in this study ().
Sample data (experimental values of TVB-N, SEP, K-value, Hx, EC, and SA at −28, −20, and −12°C) were normalized to the range of −1 to 1 and then they were used to train the RBFNN until the training error reached an acceptable range.[Citation22] The number of neurons in the hidden layer was decided by the mean square error (MSE) value.[Citation8] A well-trained RBF network model can calculate the results of the predicted values correctly.[Citation20]
Validation of the predictive models
The predicted values of the two models were compared with the experimental value at −28°C. Relative error (%), MSE, and r2 were adopted to evaluate the capability of the models, which are as follows:
where Bpre is the predicted data, Bexp is the experimental data, and is the mean of experimental data.
Statistical analysis
Analysis of linear regression was conducted by Microsoft Office, Excel 2007. MatlabR2013b was used to establish the RBFNN model. Analysis of variance (ANOVA) and least significant differences (LSD) (testing difference between means at the level of P < 0.5 = were calculated using SPSS Statistics 21.0.
Results and discussion
TVB-N
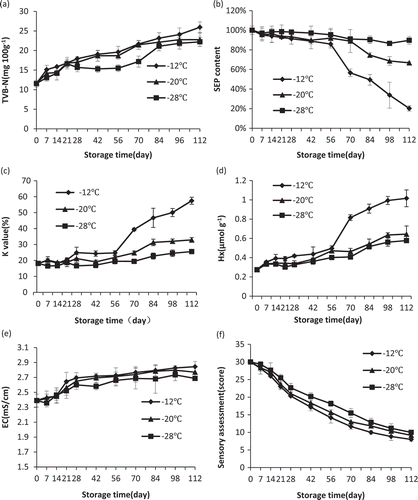
TVB-N increased significantly (P < 0.05) during storage () and the increasing rates of TVB-N increased with temperatures. The initial value was 11.6 mg/100g, which was higher than 9.94±0.86 mg/100g for Pacific white shrimp (Litopenaeus vannamei)[Citation23] and lower than 13.3 mg N/100 g for deep-water pink shrimp (Parapenaeus longirostris).[Citation24] On the 112th day, TVB-N of samples frozen at −28 and −20°C reached 22.2 and 22.8 mg/100g, respectively, which were significantly (P < 0.05) lower than 26.0 mg/100g of shrimp stored at −12°C. In addition, Gonçalves and Gindri Junior[Citation25] reported that 20 mg /100 g was considered fresh for TVB-N. According to this level, samples at −28, −20, and −12°C could keep fresh for 84, 70, and 70 days, respectively. The lower the temperature, the slower the TVB-N increased. Similar results were obtained by Tsironi et al.[Citation11] This may be because low temperatures can inhibit the growth and reproduction of bacteria as well as slow down the process of oxidative deamination for non-protein nitrogen compounds.[Citation12]
SEP
SEP is an important indicator for frozen shrimp, which can reflect the degree of protein denaturation.[Citation26] SEP decreased slightly during the first 56 days, while the rate of decrease became faster during the last 56 days, especially for samples stored at −20 and −12°C (). On the 56th day, SEP of shrimp frozen at −28, −20, and −12°C decreased to 95.42, 91.95, and 85.94% of the initial value, respectively, while it declined to 89.73, 66.76, and 20.25% at those same temperatures, respectively, on the 112th day. The results also showed that the higher the temperature was, the faster the SEP decreased, which was similar to the study of Wu et al.[Citation3] SEP decreased significantly (P < 0.05 = for shrimp at −20°C and −12°C, especially after storage for 56 days. Nevertheless, the decrease for shrimp at −28°C was not significant (P < 0.05), which means that the storage temperature of −28°C can inhibit the decrease of SEP effectively. The formation of hydrophobic bonds, hydrogen bonds, disulphide bonds, and ionic interactions may contribute to protein denaturation and a decrease in SEP.[Citation26,Citation27]
K-value
The initial K-value was 18.15% (Fig. 2c), which was similar to the result for white shrimp (18.97%).[Citation28] The K-values of shrimp frozen at “−28,−20, and −12°C increased to 25.54%, 32.89%, and 57.47% on the 112th day, respectively. With extended storage time, the K-value increased significantly (P < 0.05), but it increased faster after 56 days. Moreover, the K-values of shrimp stored at higher temperatures increased more quickly, which was consistent with the conclusion of Xu et al.[Citation21] That is, lower temperatures can slow down the autolysis rate of shrimp and inhibit the activity of 5’-nucleotidase, and the slower speed in ATP degradation can lead to a lower increasing rate of K-value.[Citation29]
Hx
The process of ATP degradation in shrimp is as follows: ATP → ADP → AMP → IMP → HxR → Hx.[Citation28] ADP, IMP, AMP, HxR, and Hx are all produced in the process of ATP degradation, in which IMP and AMP represent freshness, and HxR and Hx are variables associated with a loss of freshness.[Citation34] Thus Hx is an effective indicator to evaluate the degree of spoilage for shrimp. Hx values increased gradually during the storage period (Fig. 2d). The increase in Hx at the low temperatures was slower than that at high temperatures, which was more obvious during the late period of storage. On the 56th day, the Hx values of shrimp frozen at “−28,−20, and −12°C were 0.401, 0.471, and 0.496 μmol/g, respectively, while on the 112th day, they reached 0.577, 0.644, and 1.016 μmol/g for those same temperatures, respectively. High temperatures may raise the activity of 5’-nucleotidase and expedite the degradation of ATP, which would contribute to the accumulation of Hx.[Citation34]
EC
EC is a convenient index, which can affect the ultimate tenderness, water-holding capacity, and other qualities of seafood.[Citation30] EC increased significantly (P < 0.05) with the extension of storage time at all experimental temperatures (). The higher the temperature was, the faster the EC increased. Shi et al.[Citation5] reported similar findings. This may be due to ionic substances produced by bacteria, which can result in an increase in EC.[Citation30] Additionally, decomposed muscle tissues may influence cell sap and surrounding extracellular tissues, which could also lead to increase in EC.[Citation4] Low temperatures can partly inhibit the microbial population, and decomposition of muscle tissues, so the increase in EC was slow.
Sensory assessment
SA scores of shrimp stored at −28, −20, and −12°C decreased regularly during the storage period (P < 0.05) (). An SA score of 30 equals shrimp that is absolutely fresh, and 15 equals shrimp that has decayed. According to this standard, shrimp at −28, −20, and −12°C spoiled after storage at 84, 70, and 56 days, respectively, which was partly correlated with chemical indicators. Imran[Citation31] also reported that the spoilage point in SA was reached faster at higher storage temperatures for chilled shrimp (Litopenaeus vannamei). Ketones, volatile aldehydes, sulphides, and esters produced by microbes, as well as fat oxidation, may result in rotten, fruity, and sulphydryl odours, which is equivalent to poor quality in SA.[Citation31] The growth of microbes and biochemical reaction was more quick at high temperatures, so quality indicators (colour, morphological integrity, smell, musculature, and soap turbidity of boiled shrimp) declined soon.[Citation32]
Establishment of the arrhenius model
Reaction orders were determined by fitting the experimental values of TVB-N, SEP, K-value, Hx, EC, and SA with different kinetic equations (). Reaction orders with higher Σr2 values represent better correlation, so 0, 0, 1, 1, 0, and 1 were chosen as the reaction orders for TVB-N, SEP, K-value, Hx, EC, and SA, respectively. Ea of TVB-N, SEP, K-value, Hx, EC, and SA were 8.24, 64.43, 39.93, 20.03, 24.58, and 6.06 kJ/mol, respectively, while k0 was 4.82, −4.92 × 1010, 9.37×105, 106.27, 708.39, and −0.20 for these same indicators, respectively. The Arrhenius models for TVB-N, SEP, K-value, Hx, EC, and SA in frozen shrimp are shown as follows:
Table 1. Estimation of the reaction orders in Arrhenius models of quality indicators by r2.
where BTVB-N, BSEP, BK, BHx, BEC, and BSA are predictive values of TVB-N, SEP, K-value, Hx, EC, and SA, respectively, for shrimp stored at temperature T (K) on time t (day), while BTVB-N 0, BSEP 0, BK0, BHx0, BEC0, and BSA0 are the initial values.
Establishment of the RBFNN model
The experimental values of TVB-N, SEP, K-value, Hx, EC, and SA at −28, −20, and −12°C (594 data points) were used to establish, train, and test the RBFNN model. Only 70% of the data were selected randomly to establish the model, and 15% of the data were used to train the model, while the rest of the data were applied to verify the results in the case of overfitting. The number of neurons in the hidden layer was decided by the testing MSE (). In this study, 29 neurons in the hidden layer contributed to the smallest MSE. Thus, the RBF network with 29 hidden neurons was constructed based on the training data.
Table 2. MSE of RBFNN with varying quantities of hidden neurons.
Evaluation of the arrhenius model and the RBFNN model
To evaluate the effectiveness of the Arrhenius model and the RBFNN model based on shrimp frozen at different temperatures, predictive values were compared with measured values at −28°C (). As Kaymak-Ertekin and Gedik[Citation33] reported, models with relative errors below 10% can be considered acceptable. For the Arrhenius model, relative errors for SEP, EC, and SA were all within ±10%, while relative errors for TVB-N exceeded 10% on the 14th, 21st, 28th, and 84th days; relative errors for K-values exceeded 10% on the 7th, 14th, 28th, 42nd and 70th days; and relative errors for Hx exceeded 10% on the 7th, 14th, 84th, and 98th days. However, relative errors of all the indicators were below 0.5% for the RBFNN model. Moreover, all relative errors for the RBFNN model were smaller than those for the Arrhenius model. Thus, the RBFNN model was more accurate than the Arrhenius model in predicting the quality of frozen shrimp.
Table 3. Measured values and predictive values of the Arrhenius model and the RBFNN model for shrimp stored at –28°C.
To evaluate the overall performance of each index, we compared the two models by MSE and r2 calculated using the predicted values and the measured values (). The MSE for each indicator calculated by the Arrhenius model was larger than that calculated by the RBFNN model, while the r2 calculated by the Arrhenius model was smaller than that calculated by the RBFNN model. Therefore, the RBFNN model outperformed the Arrhenius model in overall performance.
Table 4. r2 and MSE of the Arrhenius model and the RBFNN model between the predicted values and the measured values.
Conclusion
Changes in quality of shrimp stored at −28°C occurred slowly during the entire storage period, while shrimp stored at −12°C deteriorated more quickly, especially in the last 8 weeks of storage. Reaction orders in the Arrhenius model of TVB-N, SEP, K-value, Hx, EC, and SA were 0, 0, 1, 1, 0, and 1, respectively. SEP, EC, and SA were well-fitted by the Arrhenius model with low relative errors (< 10%= between the measured values and predictive values, while the relative errors of TVB-N, K-value, and Hx surpassed 10% on some days. However, lower relative errors (within ± 0.5%)were found using the RBFNN model, which represented excellent accuracy in prediction. Overall, the RBFNN model is a promising tool for predicting the changes in quality of shrimp frozen at −28 to −12°C during the entire storage period, and the RBFNN model performed better than the Arrhenius model. The models provide a convenient way to predict changes in quality during storage, which is of great practical value to the shrimp industry. Further studies can be carried out to apply the RBFNN and Arrhenius models to predict changes in the quality of shrimp stored under different conditions. Finally, more work should be encouraged to establish and verify new types of models for predicting the quality of stored shrimp.
Funding
This study was financially supported by the National Science and Technology Ministry of China [award number2015BAD17B03].
Additional information
Funding
References
- Oosterveer, P. Globalization and Sustainable Consumption of Shrimp: Consumers And Governance in the Global Space of Flows. International Journal of Consumer Studies 2006, 30, 465–476.
- Broekaert, K.; Heyndrickx, M., Herman, L.; Devlieghere, F.; Vlaemynck, G. Molecular Identification of the Microbiota of Peeled and Unpeeled Brown Shrimp (Crangon crangon) During Storage on Ice and at 7.5 DEGREES C. Food microbiology 2013, 36, 123–134.
- Wu, H.; Wang, Z.; Luo, Y.; Hong, H.; Shen, H. Quality Changes and Establishment of Predictive Models for Bighead Carp (Aristichthys nobilis) Fillets During Frozen Storage. Food and Bioprocess Technology 2014, 7, 3381–3389.
- Zhu, S.; Luo, Y.; Feng, L.; Bao, Y. Establishment of Kinetic Models Based on Electrical Conductivity and Global Stability Index for Predicting the Quality of Allogynogenetic Crucian Carps (Carassius auratus gibelio) during Chilling Storage. Journal of Food Processing and Preservation 2015, 39, 167–174.
- Shi, C.; Lu, H.; Cui, J.; Shen, H.; Luo, Y. Study on the Predictive Models of the Quality of Silver Carp (Hypophthalmichthys molitrix) Fillets Stored under Variable Temperature Conditions. Journal of Food Processing and Preservation 2014, 38, 356–363.
- Yao, L.; Luo, Y.; Sun, Y.; Shen, H. Establishment of Kinetic Models Based on Electrical Conductivity and Freshness Indictors for the Forecasting of Crucian Carp (Carassius carassius) Freshness. Journal of Food Engineering 2011, 107, 147–151.
- Deng, W.; Wang, G.; Zhang, X.; Guo, Y. Water Quality Prediction Based on a Novel Hybrid Model of ARIMA and RBF Neural Network. IEEE International Conference on Cloud Computing and Intelligence Systems 2014, 33–40.
- Han, H.G.; Chen, Q.L.; Qiao, J.F. An Efficient Self-Organizing RBF Neural Network for Water Quality Prediction. Neural Networks, 2011, 24, 717–725.
- Tudu, B.; Jana, A.; Metla, A.; Ghosh, D.; Bhattacharyya, N.; Bandyopadhyay, R. Electronic Nose for Black Tea Quality Evaluation by an Incremental RBF Network. Sensors and Actuators B: Chemical 2009, 138, 90–95.
- Ahmad, I.; Jeenanunta, C. Application of Support Vector Classification Algorithms for the Prediction of Quality Level of Frozen Shrimps (Litopenaeus vannamei) Suitable for Sensor-Based Time-Temperature Monitoring. Food and Bioprocess Technology 2015, 8, 134–147.
- Tsironi, T.; Dermesonlouoglou, E.; Giannakourou, M.; Taoukis, P. Shelf life Modelling of Frozen Shrimp at Variable Temperature Conditions. LWT - Food Science and Technology 2009, 42,664–671.
- Zhang, L.; Li, X.; Lu, W.; Shen, H.; Luo, Y. Quality Predictive Models of Grass Carp (Ctenopharyngodon idellus) at Different Temperatures During Storage. Food Control 2011, 22, 1197–1202.
- Liu, X.; Jiang, Y.; Shen, S.; Luo, Y.; Gao, L. Comparison of Arrhenius Model and Artificial Neuronal Network for the Quality Prediction of Rainbow Trout (Oncorhynchus mykiss) Fillets During Storage at Different Temperatures. LWT - Food Science and Technology 2015, 60, 142–147.
- Boekel, M. Statistical Aspects Of Kinetic Modeling for Food Science Problems. Journal of Food Science 1996, 61, 477–486.
- Ratkowsky, D.; Olley, J.; McMeekin, T.; Ball, A. Relationship Between Temperature and Growth Rate of Bacterial Cultures. Journal of Bacteriology 1982, 149, 1–5.
- Chaudhuri, A. Forecasting Financial Time Series Using Multiple Regression, Multi Layer Perception, Radial Basis Function and Adaptive Neuro Fuzzy Inference System Models: A Comparative Analysis. Computer and Information Science 2012, 5, 13–24.
- Park, H.S.; Chung, Y.D.;Oh, S.K.; Pedrycz, W.; Kim, H.K. Design of Information Granule-Oriented RBF Neural Networks and its Application to Power Supply for High-Field Magnet. Engineering Applications of Artificial Intelligence 2011, 24, 543–554.
- Yin, J.-C.; Zou, Z.-J.; Xu, F. On-Line Prediction of Ship Roll Motion During Maneuvering Using Sequential Learning RBF Neuralnetworks. Ocean Engineering 2013, 61, 139–147.
- Mohammadi, R.; Fatemi Ghomi, S.M.T.; Zeinali, F. A New Hybrid Evolutionary Based RBF Networks Method for Forecasting Time Series: A Case Study of Forecasting Emergency Supply Demand Time Series. Engineering Applications of Artificial Intelligence 2014, 36, 204–214.
- Yao, Y.; Lian, Z.; Hou, Z.; Liu, W. An Innovative Air-Conditioning Load Forecasting Model Based on RBF Neural Network And Combined Residual Error Correction. International Journal of Refrigeration 2006, 29, 528–538.
- Xu, Z.; Liu, X.; Wang, H.; Hong, H.; Yu, X.; Luo, Y. Establishment of the Arrhenius Model and the Radial Basis Function Neural Network (RBFNN) Model to Predict Quality of Thawed Shrimp (Solenocera melantho) Stored at Different Temperatures. Journal of Food Processing and Preservation 2016, 40, 882–892.
- Lu, W.Z.; Wang, W.J.; Wang, X.K.; Yan, S.H.; Lam, J.C. Potential Assessment of a Neural Network Model with PCA/RBF Approach for Forecasting Pollutant Trends in Mong Kok Urban Air, Hong Kong. Environmental Research, 2004, 96,79–87.
- Okpala, C.O.R. Investigation of Quality Attributes of Ice-Stored Pacific White Shrimp (Litopenaeus vannamei) as Affected by Sequential Minimal Ozone Treatment. LWT - Food Science and Technology 2014, 57, 538–547.
- Cadun, A.; Kışla, D.; Çaklı, Ş. Marination of Deep-Water Pink Shrimp with Rosemary Extract and the Determination of its Shelf-Life. Food Chemistry 2008, 109, 81–87.
- Gonçalves, A.A.; Gindri Junior, C.S.G. The Effect of Glaze Uptake on Storage Quality of Frozen Shrimp. Journal of Food Engineering 2009, 90, 285–290.
- Auh, J.; Lee, H.; Kim, J.; Kim, J.; Yoon, H.; Park, K. Highly Concentrated Branched Oligosaccharides as Cryoprotectant for Surimi. Journal of Food Science 1999, 64, 418–422.
- Xiong, G.; Cheng, W.; Ye, L.; Du, X.; Zhou, M.; Lin, R.; Cai, Y.-Z. Effects of Konjac Glucomannan on Physicochemical Properties of Myofibrillar Protein and Surimi Gels from Grass Carp (Ctenopharyngodon idella). Food Chemistry 2009, 116, 413–418.
- Kalleda, R.K.; Han, I.Y.; Toler, J.E.; Chen, F.; Kim, H.J.; Dawson, P.L. Shelf Life Extension of Shrimp (White) Using Modified Atmosphere Packaging. Polish Journal of Food and Nutrition Sciences 2013, 63, 87–94.
- Abu Bakar, F.; Salleh, A.B.; Razak, C.N.A.; Basri, M.; Ching, M.K.; Son, R. Biochemical Changes of Fresh and Preserved Freshwater Prawns (Macrobrachium rosenbergii) During Storage. International Food Research Journal 2008, 15, 181–191.
- Ekanem, E.O.; Achinewhu, S.C. Mortality and Quality Indices of Live West African Hard-Shell Clams (Galatea paradoxa Born) During Wet and Dry Postharvest Storage. Journal of Food Processing and Preservation 2006, 30, 247–257.
- Imran, A.; Chawalit, J.; Somrote, K. Characterization of Quality Degradation During Chilled Shrimp (Litopenaeus vannamei) Supply Chain. International Food Research Journal, 2013, 20, 1833–1842.
- Hong, H.; Luo, Y.; Zhu, S.; Shen, H. Establishment of Quality Predictive Models for Bighead Carp (Aristichthys nobilis) Fillets During Storage at Different Temperatures. International Journal of Food Science and Technology, 2012, 47, 488–494.
- Kaymak-Ertekin, F.; Gedik, A. Kinetic Modelling of Quality Deterioration in Onions During Drying and Storage. Journal of Food Engineering, 2005, 68, 443–453.
- Howgate, P. A Review of the Kinetics of Degradation of Inosine Monophosphate in Some Species of Fish During Chilled Storage. International Journal of Food Science and Technology 2006, 41, 341–353.