ABSTRACT
Modified rice bran oil (RBO) and modified mustard oil (MO) were prepared by adding oleic acid and oxidized oil separately. The physical and chemical indices like dielectric constant, viscosity, peroxide value (PV), acid value (AV), free fatty acids (FFA), iodine value (IV), and saponification value (SV) of pure and modified RO and MO were determined and correlated. Dielectric constant and viscosity were found to decrease in a non-linear fashion with increase in temperature (30°C–75°C). The dependence of dielectric constant and viscosity with temperature was investigated using model empirical equations. The dielectric variation was studied using Akerlof and Oshry’s model equations, which exhibited high dependency of R2 ranges from 0.99 to 0.997. The behaviour of viscosity with temperature was studied with Wright’s equation and the correlation coefficient (R2) was found to be 0.998. Model equations were developed, which relates dielectric constant with IV, SV, and PV with the regression coefficients R2 = 0.955, 0.936, and 0.994, respectively. The developed equations can be used in processing, pipelining, and to predict the parameters at a desired temperature. On comparing the correlations between the physical and chemical properties of the oil samples, RBO and its modified forms exhibited more oxidative stability than modified MO.
Introduction
Vegetable oil plays an important role in preparing food items that provide energy, micronutrients (vitamins), and fatty acids for humans to lead a healthy life.[Citation1] Oil in the food industry is utilized for frying, baking, storing, and to add aroma, colour, and texture to the food items. Fats and oils are basically triesters of glycerol with long-chain fatty acids called triglycerides.[Citation2] On repeated heating near frying temperatures, non-volatile toxic compounds are formed.[Citation3] Peroxides are the primary oxidized products produced, which on further heating get degraded to aldehydes, ketones, esters, etc., which are secondary products. Other chemical reactions like hydrolysis and polymerization produce monomers, dimers, trimers, oligomers, free fatty acids (FFA), and water to make oil into a highly tarnished product.[Citation4] The different chemical compositions in oil undergo changes on degradation. Hence, oil quality analyses are being carried out in the quantification of degraded products using methods such as chromatography and spectroscopic methods like Fourier transform IR (FTIR), Nuclear Magnetic Resonance (NMR), Raman spectra, etc. To perform the analytical exploration, the oil has to be dissolved in organic solvents like benzene, acetone, etc. These solvents pollute the environment; moreover, the methods are time consuming and not economical.[Citation5]
Simple physical and chemical indices can be used for the quality assessment of oil and to quantify the toxic degraded products produced in the oil during successive heating.[Citation5,Citation6] Dielectric constant εr (permittivity) is a measure of polar compounds and water content in the oil. Although FFA was taken as an index of degradation since 1981, dielectric constant is being used as the primary index of oil quality.[Citation7] This is due to the fact that degraded products such as peroxides, FFA, monomers, and other non-volatile complex compounds add polar molecules to the oil. In the presence of an external electric field, these polar compounds change the dielectric nature and capacitive value of a parallel-plate capacitor. The dielectric constant of unheated refined oil ranges from 1.5 to 2.9 at 2 Mega Hertz (MHz).[Citation8] The dielectric constants of oils are generally measured using the capacitive method, which is by measuring the ratio of the capacitance in the oil to that of the air. The higher the level of degraded products in oil, the higher is the increase in the dielectric constant. For example, increased quantities of ester, the second degraded product, lead to an increase in permittivity to 3.2.[Citation9,Citation10,Citation11]
Resistance of flow between successive layers in a liquid is the measure of viscosity of the sample. Viscosity is a measure of polyunsaturated fatty acid (PUFA) and monounsaturated fatty acids (MUFA) in the oil. The higher the content of unsaturated fatty acids, the lower is the viscosity.[Citation12] The viscosity parameter also states the measure of saturated fatty acids, which is one of the degraded products. Hence, this parameter is highly related to the molecular structure of the liquid.[Citation13] The measures of carboxylic group acid value (AV), peroxide value (PV), FFA, and long-chain unsaturated fatty acids like iodine value (IV) and saponification value (SV) provide the basic chemical quality indices of the oil.[Citation5]
In the present work, variations of dielectric constant and viscosity with temperature were investigated. The non-linear variations of the parameters were modelled with standard empirical equations to estimate the accuracy of the measurement. Variation of dielectric constant and viscosity was correlated using a novel equation, so as to emphasize the dielectric constant as a non-invasive parameter to estimate the degradation level of oil. The chemical-quality indices (FFA, IV, SV, PV, and AV) of modified rice bran oil (RBO) and modified mustard oil (MO) were correlated independently with dielectric constant and viscosity. SV and IV indicate the level of unsaturation in oil and hence are inversely proportional with the dielectric property. Increase in PV indicates increases in the polar compounds in oil and hence vary directly with the dielectric constant of the oil. Thus, when the dielectric constant of the oil is less, the quality of the oil is good. This experiment helps us understand that an alternative measure in the form of dielectric property can be used to study the dilapidation level of the oil for reusability instead of performing chemical analysis, which is time consuming and costly. In this experiment, modified oil refers to either heating of oil or adding fresh oil with heated oil or adding 20 ml of oleic acid to heated oil.
Materials and methods
Oils: Refined rice bran (Oryza sativa) and mustard (Brassica juncea) were purchased in a local supermarket in Trichy, Tamil Nadu, India. The basic composites of the oil were as follows: RBO – MUFA = 41%, PUFA = 47%, and saturated fats = 12%. In MO – MUFA = 67%, PUFA = 21%, and saturated fats = 12%. The reagents used for titration for determining the chemical parameters and oleic acid were purchased from Sigma Aldrich Chemicals India Private Ltd. These samples were kept in dark brown bottles wrapped with a brown paper around them such that they are not influenced by external temperature or sunlight and to prevent from natural oxidation.
Sample preparation
One kilogram of cake flour was mixed with 500 ml of water, 100 g of butter, ½ table spoon of salt, one table spoon of baking powder, and 200 g of sugar. The mixture was made using whisker, then it was made into dough using an atta dough maker. The dough was flattened using the two flat circular plates and by exerting a pressure over it. The flattened dough was pieced in a diamond shape with the dimension of 3 cm x 3cm and a thickness of 1 cm. The biscuits were deep fried separately in the RBO and MO (to prepare heated samples) under a temperature-controlled induction stove (175±5°C). It took 2 h to fry the entire dough. To study the degree of degradation in oil exposed to frying, heated samples of rice bran oil (RBOH) and mustard oil (MOH) were taken and stored separately at ambient temperature. In most of the snacks industry, oil volume decreases on successive frying and hence fresh oil is added to heated (oxidized) oil. To study the characteristics of such types of oil, 50 ml of heated oil was mixed with 50 ml of unheated oil kept as a separate sample (RBO+H and MOH+H). Unheated oil of 80 ml RBO and MO was added with 20 ml of oleic acid to get another modified sample (RBO + O and MO + O), which has increased unsaturated fatty acids. In total, 24 oil samples were prepared to measure the physical and chemical properties in triplicate and average them.
Physical Parameters
Dielectric constant of the oil was measured by IEC60247 (International Electro-technical Commission) standards using the capacitive method using LCR (connecting inductance (L), capacitance (C) and resistance (R) in series) DCK-001 (Mittal Enterprises, New Delhi, India) at 2 MHz.[Citation14] Kinematic viscosities (η) of oil were measured using the ASTM D446 method.[Citation13]
Chemical parameters
The long-chain unsaturated fatty acids in sample, which are measured as SV, were found as per ASTM D5558 standard method.[Citation5] The unsaturated fatty acids in sample, which are measured as IV, were estimated using ASTM D5768 standard as in the previous study.[Citation5] Quantification of carboxylic acid in the sample as AV was estimated using standard ASTM D664 (5). The PVs of the oil samples were determined by the standard AOCS Cd 8-53 method.[Citation5]
Table 1. Akerlof and Oshry’s model equation relating dielectric constant and temperatureεr = A/T + B + C×T + D×T2.
Statistical analysis
The independent parameter temperature and dependent parameters dielectric constant and viscosity were fitted with different model equations and analysed using SPSS (version 12) as illustrated in , , , , and . These tables illustrate the value of specific constants A, B, and C along with the correlation coefficient (R2) and standard error estimate (SEE) using least square approximation. Each sample was prepared, stored in three bottles, and the measurements were performed for three trials and the standard deviation is illustrated in . The statistical analysis of the data, correlation, and regression were carried out with a graph pad prism and Microsoft Excel 10.0 software.
Table 2. Correlation equation relating dielectric constant and temperature εr = A log T + B.
Table 3. Andrade model equation relating viscosity and temperature Linear: η = A+BT.
Table 4. Andrade model equation relating viscosity and temperature non-linear: η = A+B/T+C/T2.
Table 5. Wright’s ASTM model equation relating viscosity and temperature ln ln fn (ƞ) = A + B×ln T.
Table 6. Constants and regression coefficient values for ln η = A/ln εr + B equation relating viscosity with dielectric constant.
Table 7. Basic chemical and physical quality factors of modified vegetable oil.
Results and discussion
Electrical property of oil and model oil
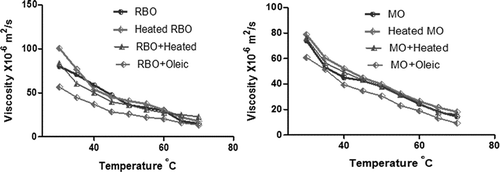
The insulation property of oil is due to the nonpolar (MUFA, PUFA, and a small amount of long-chain saturated fatty acid) compounds in it. When oil starts degrading, the chemicals present in it break down, which increases the polar compounds in the oil.[Citation14] The ratio of the capacitance of a liquid to that of air gives the dielectric constant (εr) of the liquid, which is a measure of polar compounds.[Citation7,Citation15] illustrates the variation in the dielectric constant of the oil measured from 30°C to 75°C with steps of 5°C. The permittivity of the medium is measured at 2 MHz as electronic and ionic polarization has a pronounced effect at frequency >1 MHz. It is observed that the dielectric constant of oil decreases with increase in temperature as the mobility of charge carriers increases with decrease in molecular interaction. The permittivity (εr) of heated oils is greater as the percentage of saturated fatty acid increases. The dielectric constant of modified oil decreases with the addition of oleic as the percentage of MUFA increases (unsaturated fatty acids). When a medium consists of a mixture of fatty acids, the polarization occurs based on the difference between the ratios of the electrical properties like conductivity and permittivity.
Figure 1. Dependence of dielectric constant with temperature of modified rice bran and mustard oils.
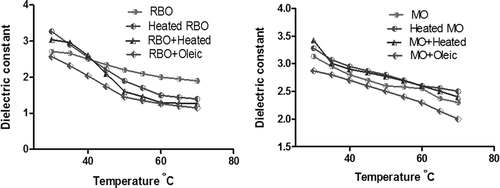
illustrates the variation of the dielectric constant of the samples, which ranges from 3.5 to 1.2. Comparing the dielectric constant of modified RBO, εr of heated oil increases by 17%, εr of the mixture of oxidized and unheated oil increases by 11%, and a mixture with monounsaturated oleic decreases by 5%. On the other hand, if the behaviour of the modified MO is analysed, εr of heated oil increases by 4.7%, the mixture of heated and unheated oil increases by 8.7%, and addition with oleic acid decreases by 8.3%. Relating RBO with MO, the increase in εr of heated oil is more for RBO as it has 47% of PUFA whereas MO has 21% of PUFA. The study also elucidates that the larger the number of unsaturated double bonds, the faster it gets oxidized to a single bond with the addition of hydrogen.[Citation8,Citation15] The mixture of oil with monounsaturated oleic acid decreases the dielectric constant value. Hence, the higher the percentage of unsaturated fatty acids, the smaller would be the dielectric constant value.[Citation16] These measurements support the factor that it could be used as an index in the quality analysis of the oil.
Viscosity of oil and modified oil
illustrates the dependency of viscosity of modified oil with temperature. It is observed that the disparity of viscosity decreases with increase in temperature due to the decrease in intermolecular force of attraction between the molecules.[Citation17,Citation18,Citation19] Kinematic viscosity (η) of oil was measured using redwood viscometer at temperatures ranging from 30 to 70°C. The concentric cylindrical system with oil in the inner copper vessel is used to measure the viscosity of oil at different temperature. Associating η of modified RBO with heated oil, viscosity upsurges by 4.3%, the mixture of heated and unheated oil increases by about 2.1%, and the mixture with unsaturated oleic fatty acid droops by 41%. Compared to MO, η of heated oil increases by 6.1%, the mixture of heated with unheated oil by 2.4%, and the addition with oleic acid decreases by 21%. Analysing the variation in viscosity of the modified oil, the value for the sample with 20% of oleic decreases compared to the other oil. The percentage of increase in viscosity is less in RBO compared to MO as it contains 47% of PUFA.
Effect of temperature on electrical property
The variation of dielectric constant with temperature is the same for all modified oils. shows the electrical property decreases non-linearly with temperature. Akerlof and Oshry’s Model equation is as follows:
The variation of dielectric constant with temperature is related using a combination of linear, quadratic, and non-linear Akerlof and Oshry’s Model as shown in Eq. (1). illustrates the correlation analysis for the modified oil samples. The regression coefficient R2 value is observed to exhibit the best fit of the equation as the value ranges between 0.990 and 0.997. The SEE values exemplify that the deviation between the computed and experimental values is less than 1.2%. From the values of scaling coefficient A, B, C, and D values of dielectric constant at any desired temperature can be predicted. Linear equation
Since dielectric constant endures a non-linear variation with temperature, the above linear equation is also analysed with the experimental data. illustrates the linear dependency of εr with the logarithmic value of T in Celsius of Eq. (2). The dependency coefficient R2 ranges from 0.922 to 0.985. This also states that the variation of dielectric constant with temperature was non-linear. The error estimated is of a very less value. From the specific constant values A and B, the εr value of the modified oil sample at any temperature can be estimated.
Effect of temperature on viscosity
The quality of the modified oil can be estimated from the change in the molecular structure, which in turn is categorized by the change in energy of intermolecular bonding. The unsaturated fatty acids exhibit cis isomerism; hence the bonding is not stronger. These molecules can be easily agitated using temperature. In the food industry, only viscosity can be used as an index to estimate the quality of oil. Viscosity is one of the testing methods used at factories in the nations of the Commonwealth of Independent States (CIS). Viscosity of modified oil like dielectric constant decreases with increase in temperature.[Citation18,Citation19,Citation20] The experimental variation of viscosity with temperature follows a simple Andrade equation represented by Eqs. (7) and (8). The Andrade equation is as follows:
where ƞ is the kinematic viscosity of the modified oil at different temperatures, A and B are the constants determined using linear estimation fitting, and T is the temperature in °C. The results of the regression analysis for the modified oil samples are presented in and . For Eq. (3), the R2 varies from 0.869 to 0.987 and the deviation of calculated viscosity from the measured viscosity varies by 1.3–15%. The correlation was also estimated for the modified order variation of temperature for the measured kinematic viscosity. The computed R2 for Eq. (4) ranges from 0.981 to 0.992 and the deviation between the calculated and experimental values ranges from 1.9% to 0.8%. Comparing the above two equations, the quadratic equation has a better fit than the linear one. Using the constants A, B, and C, the viscosity of oil at any temperature can also be predicted. The Wright equation (ASTM 341-93) is as follows:
Equation (5) is the modified Wright equation used to linearize the discrepancy of viscosity by varying the temperature in °C by including the natural logarithmic function of the quadratic equation relating viscosity. The R2 varies from 0.990 to 0.998. The performance of the equation is given in . The equation is highly correlated with the experimental data. The accuracy (≤1.5%) is observed to be more compared to all the above modelling, Eq. (5). A and B are the constants related to the fatty acids in the complex mixture in oils.[Citation12]
However, food oils in general exhibit significant variations in their composition; consequently, it is impossible to define unique values for the chemical and physical constants for any oil and it is usually necessary to combine several empirical constants to predict the chemical and physical properties of edible oils.[Citation21,Citation22] None of the data reported here represent a survey of the range of parameters for all varieties of a particular oil; hence, the degree of interspecies variability remains undefined.
Relating the electrical and rheological properties
The above-mentioned two non-invasive methods in determining the quality of oil highly depend on its total peroxide content (TPC).[Citation14] Both dielectric constant and viscosity decrease non-linearly with temperature. Viscosity of oil represents the flow property, which can be related to the activation energies of movement and the orientation of dipoles with respect to temperature. A combination of these two physical characteristics keeping temperature as the independent parameter is illustrated in . A novel equation using the logarithmic relation to relate the two dependent properties is as follows:
Since both the factors are inversely related with temperature, a linear equation is used to relate them. illustrates that the entire sample obeys the desired equation perfectly and the R2 has better fitness using least square (R2 ranges between 0.891 and 0.995). Good accuracy is observed for oil added with oleic acid and the lowest correlation is obtained for heated MO and RBO. A and B are specific constants and lower SEE values were computed. The existence of a dependency between the two-factor dielectric constant and viscosity is important in the food industry and processing.[Citation7,Citation8] Both properties of oil that have different combinations and types of fatty acids are related to the dissipation of energy on heating. As a fact-finding challenge based on this principle, the interrelationship between viscosity and dielectric constant has been resultant empirically. Both factor viscosity and dielectric constant depend on TPC in the oil as the factor increases with the saturated compound, monoglyceride, di- and triglyceride, etc.
Relating electrical property and chemical indices
indicates the relation between the electrical property dielectric constant (εr) with the chemical indices SV, IV, and PV. The experimental data were scattered and fitted with the least square fitting line. The deterioration of heated oil can be estimated using the dielectric constant of the lipids. The impact of the degree of oxidation in heated oil, oil mixed with heated oil, and added with oleic acid was investigated. During oxidation, the IV value of heated RBO is 99.8, which changes to 102.8 with the mixture of heated and unheated oil. The value still increases to 109 with the addition of 20% of oleic acid with the oil. The heated MO has an IV value of 95, which increases to 98 for the mixture of heated and unheated oil and still increases to 101 with the addition of MUFA (oleic acid). illustrates the variation of the chemical and physical indices of the modified oil. According to the literature survey, the dielectric constant of oil highly depends on the unsaturated fatty acids (IV value). The linear decrease in IV arises with an increase in the dielectric constant.[Citation8,Citation16] ) illustrates the variation by an equation using power law variation using excel software.
Figure 3. Dependency of dielectric constant with (a) IV (iodine value), (b) PV (peroxide value), and (c) SV (saponification value).
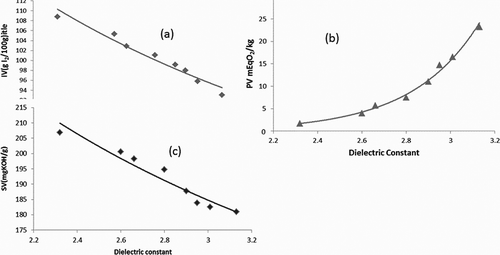
This equation can also be used to predict the IV of oil for the quality analysis independent of temperature. RBO and MO contain more than 75% of long-chain unsaturated fatty acids. When oil gets oxidized, the unsaturated fatty acids get saturated due to the addition of hydrogen in the double bond and increase the quantity of FFA. The quantification of SV is the index of available long-chain fatty acids and the possibility of using oil in successive frying. During frying, oil gets degraded due to successive heating and chemical breakdown. ) illustrates the variation of SV with dielectric constant. Similar to IV, it is also observed that as the dielectric constant increases, the SV decreases. On frying, rancidity in oil changes the SV value of the heated RBO from 200 to 188; in the mixture of heated and unheated oil, it rises to 198 as the unheated oil contributes more long-chain unsaturated FA. The value still increases to 206.8 with the addition of 20% of long-chain monounsaturated oleic acid with the oil. In MO on heating, the SV value decreases from 183 to 182. The mixture of oxidized and unheated oil 181 do not show increase as the behaviour of the oil changes with the addition of fresh oil. It is anticipated that addition of fresh oil with oxidized oil will upsurge the quantity of unsaturated fatty acids in the mixture. However, it is observed that there is no such increase in the FFA value. The addition of oleic acid with MO exhibits an 8% increase in the SV value. illustrates the variation of the chemical and physical indices of the modified oil. According to the literature survey, the dielectric constant of oil highly depends on the unsaturated fatty acids (SV value). The linear decrease in SV arises with the increase in the dielectric constant. ) illustrates the variation by the following equation:
This equation can also be used to predict the SV of oil in the quality analysis independent of the temperature. In oil-quality analysis there is no global standardization. In Europe and Asian countries TPC is used. If the oil contains more than 25% of TPC, the oil cannot be reused and should be discarded. Whereas in the United States and Japan, AV is used as the index.
RBO and MO contain more than 90% of short-chain, long-chain, saturated, and unsaturated fatty acids. When oil gets oxidized, there is a removal of hydrogen in the fatty acids and the formation of peroxides.[Citation23,Citation24] The polar chemical species are accelerated in their movements towards the relative potential gradients developed when the oil sample is kept in an external electric field. Dielectric constant is a measure of insulation, but when there is dielectric breakdown due to chemical and thermal energy, there is an increase in the dielectric constant value.[Citation14] The quantification of PV is a direct index to estimate the quality of oil. ) illustrates the variation of PV with dielectric constant. Dielectric constant has a positive variation with peroxide. Due to rancidity in oil, the PV value of heated RBO changes from 3.9 to 11.7. With the mixture of heated and unheated oil, it decreases to 7.5 as the unheated oil contributes to unsaturated FA. The value still decreases to 5.68 with the addition of 20% of oleic acid. In MO on heating the PV value increases from 14.7 to17. In the mixture of heated and unheated oil the value increases to 33.2, which provides more contribution to TPC and the oil should not be reused. It is anticipated that PV should decrease with the addition of fresh oil with oxidized oil as it increases the unsaturated fatty acids in oil. However, no such increase is observed in the value and yet the FFA value increases. Addition of oleic acid with MO exhibits a decrease in PV by 48% compared to the fresh oil. illustrates the variation of the chemical and physical indices of the modified oil at room temperature (30°C). According to an earlier research, the dielectric constant of oil increases with increase in PV. The variation of PV increases non-linearly with increase in dielectric constant. Using the Dakin–Arrhenius law relation Y = A e B/x, ) illustrates the exponential variation by the following equation:
All the above-mentioned factors state that the dielectric constant of oil is an eco-friendly, cost-effective, and better index in the real-time analysis of quality of oil. Oil exposed to several times of frying result in the production of FFA and total acid value (TAN), which counts the carboxylic acid in a gram of the modified RBO and MO. AVs are a classic method to determine the quality of edible oil. Inoue and co-workers[Citation5,Citation22,Citation25] correlated the increases in the dielectric constants of soybean oil as a result of heating to higher temperatures with AV, the analogue of the free fatty AV. exemplifies the variation of AV and FFA. During heating, hydrolysis of glycerides produces FFA; however, the changes in FFA and AV are not related to the dielectric constant as it is the measure of both saturated and unsaturated fatty acids.[Citation26] The product produced due to chemical reactions like polymerization, oxidation, and hydrolysis results in the variation of εr. epitomizes the increase in the FFA value, which is observed to be more with the addition of monounsaturated oleic acid. The developed Eqs. 7, 8, and 9 can be validated by substituting the value of the measured dielectric constant in the determination of IV, SV, and PV, respectively.
Conclusion
The current study elucidates that both permittivity and viscosity of oil are properties that depend on the measure of unsaturated fatty acids. These properties of modified oil were observed to decrease with increase in temperature. This non-linear variation was fitted with empirical equations to predict the data in the intermediate temperature. Akerlof and Oshry’s Model had prodigious R2 (R2 > 0.990) within the temperature range of 30–75°C. From the non-linear correlation equations that relate viscosity with temperature, Wright’s ASTM Model exhibited high R2 (R2 > 0.990) for all modified oils within the temperature range of 30–75°C. The chemical indices like SV, IV, FFA, AV, and PV were measured for every modified sample. To switch over from chemical analysis, the indices were correlated with the electrical property of the oil. The dependency factor R2 computed between dielectric constant with SV, IV, and PV ranges from 0.936 to 0.995. From the analysis, it was shown that chemical changes that take place in oil deterioration can be analysed using the electrical property. The study also indicates that RBO and its mixture exemplify more sustainability of unsaturated fatty acids compared to MO. The experiment elucidates that RBO has more oxidative stability compared to MO.
Acknowledgements
The authors gratefully acknowledge the Vice Chancellor of SASTRA University, for the support to carry out the research work in the university laboratory.
References
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oil. Journal of Food Science 2007, 72(5), 77–86.
- Fritsch, C.W. Measurements of Frying Fat Deterioration. Journal of American Oil Chemists’ Society 1981, 58(3), 272–274.
- Al-Kahtani, H.A. Survey of Quality of Used Frying Oil from Restaurants. Journal of American Oil Chemists’ Society 1991, 68(11), 857–862.
- Augustin, M.A.; Asap, T.; Heng, L.K. Relationship between Measurements of Fat Deterioration during Heating and Frying in RBD Olein. Journal of American Oil Chemists’ Society 1987, 64(12), 1670–1675.
- Rubalya Valantina, S.; Mukesh Kumar, V.; Devasena, T. Selected Rheological Characteristics and Physicochemical Properties on Vegetable Oil. International Journal of Food Properties 2015, 19(8), 1852–1862.
- Hampikyan, H.; Colak, H.; Akhan, M.; Turgay, I. Determination of Total Polar Compound (TPC) Levels in Frying Oil. Journal of Food, Agriculture and Environment 2011, 9(2), 142–144.
- Kumar, D.; Singh, A.; Tarsikka, P. S. Interrelationship between Viscosity and Electrical Properties for Edible Oil. Journal of Food Science and Technology 2013, 50(3), 549–554.
- Prevc, T.; Cigić, B.; Vidrih, R.; Ulrih, N. P.; Šegatin, N. Correlation of Basic Oil Quality Indices and Electrical Properties of Model Vegetable Oil Systems. Journal of Agriculture and Food Chemistry 2013, 61, 11355−11362.
- Pace, W.E.; Westphal, W.B.; Goldblith, S.A. Dielectric Properties of Commercial Cooking oil. Journal of Food Science 1968, 33, 30–36.
- Risman, P.O.; Bengtsson, N.E. Dielectric Properties of Foods at 3 GHz Determined by Cavity Perturbation Technique. Journal of Microwave Power & Electromagnetic Energy 1971, 6, 101–106.
- El-Shami, S.M.; Zaki Selim, I.; El-Anwar, I.M.; El-Malah, M.H. Dielectric Properties for Monitoring the Quality of Heated oil. Journal of American Oil Chemists’ Society 1992, 69(9), 872–875.
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of Fatty Acid Composition of Vegetable Oil with Rheological Behaviour and Oil Uptake. Food Chemistry 2010, 118, 398–402.
- Rubalya Valantina, S.; Chandiramouli, R.; Neelamegam, P. Detection of Adulteration in Olive Oil using Rheological and Ultrasonic Parameters. International Food Research Journal 2013, 20(6), 3197–3202.
- Rubalya Valantina, S.; Susan, D.; Bavasri, S.; Priyadarshini, V.; Ramya Saraswathi, R.; Suriya, M. Experimental Investigation of Electro-Rheological Properties of Modeled Vegetable Oils. Journal of Food Science and Technology 2015, 53(2), 1328–1337.
- Agrawal, S.; Batnagar, D. Dielectric Study of Binary Mixtures of Edible Unsaturated Oils. Indian Journal of Pure and Applied Physics 2005, 43, 624–629.
- Rudan-Tasič, D.; Klofutar, C. Characteristics of Vegetable Oils of Some Slovene Manufacturers. Acta Chimica Slovenica 1999, 46(4), 511–521.
- Erhan, S.Z.; Asadauskas, S.; Adhvaryu, A. Correlation of Viscosities of Vegetable Oil Blends with Selected Esters and Hydraca Rice Bran Oils. Journal of American Oil Chemists’ Society 2002, 79, 1157–1161.
- Fasina, O.O.; Hallman, H.; Craig Schmidt, M.; and Clements, C. Predicting Temperature Dependence Viscosity of Vegetable Oils from Fatty Acid Composition. Journal of American Oil Chemists’ Society 2006, 83, 899.
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Effect of heating and cooling on rheological parameters of edible vegetable oils. Journal of Food Engineering 2005, 67, 401–405.
- Hosahalli Ramaswamy, S.; Cuiren Chen, R.; Navneet Rattan, S. Comparison of Viscoelastic Properties of Set and Stirred Yoghurts Made from High Pressure and Thermally Treated Milks. International Journal of Food Properties 2015, 18(7), 1513–1523.
- Soriguer, F.; Rojo-Martinez, G.; Dobarganes, M.C.; Garcia Almeida, J.M.; Esteva, I.; Beltran, M. Hypertension is Related to the Degradation of Dietary Frying Oil. The American Journal of Clinical Nutrition 2003, 78(6), 1092–1097.
- Wang, T.; Jenni, L. Briggs, Rheological and Thermal Properties of Soybean Oils with Modified FA Compositions. Journal of American Oil Chemists’ Society 2002, 79(8), 831–836.
- Rubalya Valantina, S.; Neelamegam, P. Antioxidant Potential in Vegetable Oil. Research Journal of Chemistry and Environment 2012, 16(2), 87–94
- Wanasundara, P.K.J.P.D.; Shahidi, F. Report: Antioxidants: Science, Technology and Applications. Bailey’s Industrial Oil and Fat Products 2005, 6(6), 431–489.
- Velasco, J.; Marmesat, S.; Bordeaux, O.; Marquez-Ruiz, G.; Dobarganes, C. Formation and Evolution of Monoepoxy Fatty Acids in Thermoxidized Olive and Sunflower Oil and Quantitation in used fRying Oil from Restaurants and Fried Food Outlets. Journal of Agriculture and Food Chemistry 2004, 52(14), 4438–4443.
- Hui, Y.H. Bailey’s Industrial Oil and Fat Products Edible Oil and Fat Products; Wiley- Interscience Publication: New York, 1999; 2, 603–675.