ABSTRACT
Four drying methods, viz hot air drying (HD), vacuum drying (VD), microwave-vacuum drying (MVD), and vacuum freeze-drying (FD), were used to dry white Hypsizygus marmoreus (WHM). The volatile and taste components of the dried WHM were comparatively analysed by electronic nose technology (E-nose) and head-space solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GS-MS). Principal component analysis (PCA) showed that E-nose could distinguish fresh WHM and four dried products clearly. Dominant volatile components in fresh WHM were found to be 1-octen-3-ol, 3-octanone, and (E)-2-nonenal. However, it was found that the drying methods caused damage to the 8-carbon compounds (C8), especially to 8-carbon alcohol greatly. For instance, HD produced more ester materials, MVD yielded more aldehyde compounds, FD brought more hydrocarbon products, and VD produced more ketone substances.
Introduction
Hypsizygus marmoreus (Peck) Bigelow has gained popularity in the past decade, with studies focusing on its bioactivity[Citation1,Citation2]. The white Hypsizygus marmoreus (WHM), also named “seafood mushroom” because its taste is very similar to that of seafood, is a white variety of H. marmoreus. Due to its delicious flavour and crunchy texture, WHM has been used as a popular food or a food-flavouring material in regions of East Asia, including China, Japan, and Taiwan.
The typical aver substances of mushrooms can be classified into volatile compounds and non-volatile components. Non-volatile components are responsible for the taste of stored or processed mushrooms[Citation3]. Non-volatile components of WHM mainly include soluble sugar, free amino acid, organic acid, and flavour nucleotides[Citation4,Citation5]. Volatile compounds are more complicated and liable to be changed. They originate mainly from chemical or enzymatic oxidation of unsaturated fatty acids and further interactions with proteins, peptides, or free amino acids. In addition, some of them may be produced by Maillard reactions or Strecker degradation[Citation6]. Of all the food processing methods, drying, which is a traditional process that has been used for centuries to preserve agricultural products by reducing the moisture content, plays a major role in food manufacturing. However, drying and dewatering influence the quality of food products, especially flavour [Citation7–Citation9].
Electronic nose technology (E-nose) and headspace solid-phase micro-extraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) have popularly been used to analyse food flavour [Citation10,Citation11]. E-nose represent a promising method for the general, rapid, objective evaluation of aroma profiles of various foods[Citation12–Citation14]. Compared with E-nose, HS-SPME-GC-MS has advantages of less sample, high-sensitivity rapid analysis, and identifying of the characteristic flavour of components[Citation15–Citation17].
The influence of four drying methods on the non-volatile taste components of WHM was reported in our previous study. The results showed that the four drying methods had different influence on the soluble sugar, free amino acids, organic acids, and flavour nucleotides. Considering the maximum retention of non-volatile taste components, vacuum freeze-drying (FD) is the most optimal drying method[Citation5]. In the present study, to further discuss the effect of drying on the flavour of white H. marmoreus, four drying methods were used to dehydrate fresh WHM using E-nose technology and HS-SPME-GC-MS to evaluate the volatile taste components changes after drying. This study helps clarify the volatile flavour components formation mechanism in WHM during drying and provides a reference for further development of WHM processing products.
Material and methods
Materials
Fresh WHM were purchased from a local supermarket (Suguo Supermarket, Nanjing, China). Samples were dried by four different methods and ground into powder by an FW-177 herbal medicine smashing machine (Tianjin Taisite Instrument Co. Ltd, Tianjin, China). The powder was sieved to pass through 60-mesh sieves and stored at 4°C until used.
Preparation of dried samples
HD:Pretreated samples were spread uniformly as a single layer on the tray bed of an electric blast drying oven (DHG-9030A, Shanghai Yiheng Science and technology Co., Ltd., Shanghai, China). The air temperature was maintained at 60°C. VD:Pretreated samples were evenly distributed on a vacuum oven tray and dried by a vacuum dryer (Shanghai Jinghong Equipment Co., Ltd., Shanghai, China) at 60°C and 0.1 MPa. In MVD, the pretreated samples were evenly distributed on the tray of a microwave vacuum oven (WZD1S, Nanjing Sanle Electronic Information Industry Group Co., Ltd, Nanjing, China). Samples were dried at 0.1 MPa and 1000 W. In FD, pretreated materials were spread as a thin layer in the plates and frozen to a temperature around –24°C for 24 h and the cavity was placed in the freeze dryer (DHG-9030A, Shanghai Yiheng Science and technology Co., Ltd., Shanghai, China) at 10 Pa for 9 h. The condenser temperature was set at –45°C. A 10% moisture content in the samples was attained under these conditions.
E-nose analysis
An e-nose system (FOX 3000, Alpha MOS, Toulouse, France) equipped with 12 metal oxide gas sensors (MOS sensors) based on different sensing materials was used to analyse the odours of fresh and dried WHM. With the minor modification of the method of Du[Citation18], samples were placed in sealed vials and heated at 50°C for 10 min. The headspace gas was pumped into the sensor chamber at a constant rate of 150 ml/min via Teflon-tubing connected to a needle. Each sample was then analysed by e-nose for 2 min with data collected every second. The maximum value for each sensor during the 120 s sampling period was used to plot data on a radar graph and for Principal Component Analysis (PCA) and Radar fingerprint chart using AlphaSoft V9.1.
HS-SPME-GC-MS analysis
HS-SPME-GC-MS analysis was conducted according to Huang with some modification[Citation19]. Fresh WHM (2.5 g) or 0.2 g dry samples were immediately introduced into a 15-ml headspace vial. A fused-silica fibre coated with a 75-lm carboxen/polydimethylsiloxane (CAR/PDMS) was used for the absorption of the volatile compounds. The sample vials were equilibrated at 60°C followed by fibre exposure to the headspace for 40 min to collect the analytes. Then the fibre was removed from the vial and introduced directly into the injector of the 7890A/5975C GC-MS apparatus for analysis of volatile compounds. GC-MS condition settings are described next.
GC conditions
Analyte removal from the fibre was carried out by holding the injector temperature at 250°C. The analytes were separated on an HP-5 MS capillary column (30 m × 250 μm, 0.25 μm) and carried out using helium as carrier at 0.8 ml/min. The column oven temperature was initially set at 40°C for 1 min, increased at a rate of 4°C /min to 120°C (held for 2 min), and then 5°C /min to 135°C (held for 2 min), then 10°C /min to 180°C (held for 3 min), finally ramped up to 230°C at a rate of 10°C /min, which was held for 8 min. Helium (99.999%) was used as a carrier gas at a flow rate of 1 ml/min.
MS conditions
Electron ionization source temperature was 230°C and the ionization electron energy was 70 eV. The temperatures of interface and quadrupole were 250°C and 150°C, respectively. The mass range scanned was 25–450 amu in a full-scan acquisition mode.
Statistical analyses
The measured data were analysed by PCA and Radar fingerprint chart using AlphaSoft V9.1. The volatile compounds were tentatively identified by matching the mass spectra with the spectra of reference compounds in both the Wiley mass spectra library (6th edition) and the NIST/EPA/NIH mass spectra library (NIST08, version 1.5a). Every experiment was performed in triplicate, and statistical analysis was performed using IBM SPSS Statistics 19 software. Analysis of variance (ANOVA) and least significant difference (LSD, defined when p < 0.05) were used to analyse the significant difference between samples from different drying treatments. All the results were expressed as the mean value ± standard deviation (SD).
Results and discussion
E-nose analysis to volatile compounds of WHM
E-nose is sensitive to obtain smell information of samples, and slight changes in the odour, composition, and concentration of volatile compounds could result in a different sensor response. In this study, E-nose equipped with 12 different metal oxide gas sensors was used to monitor shifts in the aroma composition of WHM after drying. The responses from each of the 12 sensors during the 120 s sampling period and maximum values for each sensor were recorded and used for data analysis.
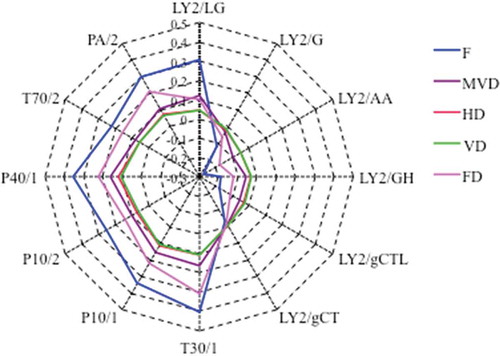
Radar fingerprint chart of the volatile compounds from fresh and dried WHM samples is shown in . Different sensors had different responses to WHM volatiles. The relative values of T30/1, P10/1, P10/2, P40/1, T70/2, PA/2, and LY2/LG of fresh samples were stronger than those of the other sensors, and the dried samples took on the same curve drawing. Sensors LY2/G, LY2/AA, LY2/GH, LY2/gCTL, and LY2/gCT provided negative response values to fresh and dried samples, and the relative values of fresh samples were significantly lower (p<0.05) than those of dried samples. In the four kinds of dried samples, the response value of FD was more similar to that of fresh WHM. In addition, the VD and FD samples took on an almost same response from 12 sensors, which indicated VD and FD had a similar effect on the volatile components of WHM.
Figure 1. Ladar map of five white Hypsizygus marmoreus samples (F: Fresh sample; HD: Hot-air drying; VD: Vacuum drying; MVD: Microwave-vacuum drying; FD: Vacuum-freeze drying).
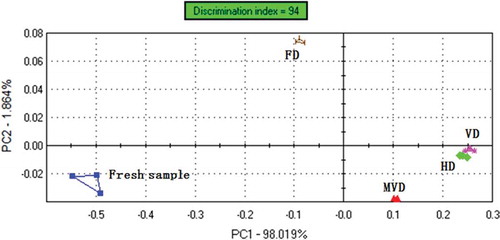
PCA is a statistical procedure that uses an orthogonal transformation to convert a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables[Citation20]. Higher contribution rate represents the principal components reflect the original multi-index information better. When the total contribution rate is over 85%, it can generally be considered feasibility of the methods[Citation21]. shows the PCA score plots of e-nose sensor responses to the headspace volatiles from WHM. The variance contribution rates of the first and second PCs were 98.019% and 1.864%, respectively. The accumulative variance contribution rate of the first two PCs was 99.883% (more than 85%), indicating that the two principal components include the most information of the volatile compounds. The value of PC1 increased in the following order of Fresh < FD < MVD < HD < VD; HD and VD almost located in the same area. Discrimination index was 94%, and the PCA plots can distinguish fresh WHM, HD, VD, MVD, and FD samples and five kinds of samples located in different areas.
Identification of the volatile compounds by HS-SPME-GC-MS
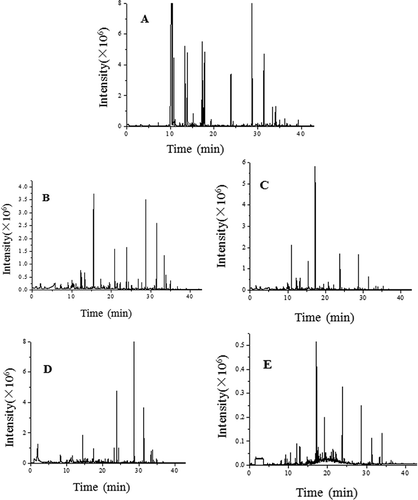
HS-SPME is a relatively simple, fast, and sensitive method to analyse the volatile components. To evaluate the effect of different drying methods on the flavour of WHM, volatile compounds were monitored using SPME-GC-MS. GC-MS total ion chromatograms of the volatile compounds of fresh samples and various dried samples are shown in . As shown in , about 45, 49, 42, 72, and 49 classes of volatile compounds were identified in fresh WHM, HD, VD, MVD, and FD samples, respectively. Flavour compounds were classified into alcohols, hydrocarbons, acids, esters, ketones, aldehydes, sulphides, pyrazines, furans, and phenols (). Content and types of flavour components significantly changed after the WHM was dried. Moreover, HD, VD, MVD, and FD had different influence on the volatile components.
Table 1. Relative content of volatile compounds in samples of H. marmoreus.
Table 2. Relative content of different kinds of volatile compounds in samples of H. marmoreus.
Influence of drying methods on the volatile compounds of WHM
Volatile compounds in fresh WHM
In this study, 45 classes of volatile compounds were determined (). Alcohols, aldehydes, and ketones were the dominant volatile compounds of the fresh white H. marmoreus, and their relative contents were 54.73%, 14.24%, and 12.05%, respectively. Among all the aroma compounds, 1-octen-3-ol(32.97%), 3-octanone (11.86%), and (E)-2-octenal (6.18%) contributed over 50%. The main odorants of mushroom aroma are eight carbon atom (C8) compounds, and 1-octen-3-ol, described as “mushroom alcohol”, is a characteristic aroma compound of mushrooms[Citation22]. 3-Octanone had fruit flavours. (E)-2-Octenal had the flavour of roasted meat, and it took on the flavour of cucumber when diluted to 1 mg/kg. Sulphide compounds were not detected in the fresh WHM.
Influence of HD on the volatile compounds of WHM
As shown in and , 49 classes of volatile compounds were determined in the HD samples. Acids (6.26%), sulphides (0.37%), and pyran (0.45%) were determined, which were not detected in fresh samples. These new aroma compounds are related to the activation of some enzymes in the early period of HD. Maillard reaction has been shown to promote the formation of hydrocarbons, heterocyclic, and aromatic compounds[Citation23]. HD samples had a higher alcohol content (27.08%), but the alcohol content fell by almost half compared to fresh WHM. The most abundant aroma compounds were henylethyl alcohol (16.99%), 2-phenylethyl ester (5.3%), 2,4,7,9-tetramethyl-5-decyn-4,7-diol (4.95%), butanoic acid, 2-methyl- (3.36%), (E)-2-Octenal (2.36%), 1-hexanol (2.14%), 1-octen-3-ol (1.83%), and 3-octen-2-one (1.58%).
Influence of VD on volatile compounds of WHM
In the VD samples, 42 classes of volatile compounds were determined (). Similar to HD, new aroma compounds, such as acids (0.08%), sulphides (0.35%), and pyran (0.28%), were detected, but different from HD and fresh samples, phenols were not detected. Alcohols, acids, and esters were less than in HD and the contents of hydrocarbons and ketones were higher. The contents of henylethyl alcohol (8.40%), 2-dodecenal (3.02%), hexanal (2.50%), 3-octen-2-one (2.45%), 1-octen-3-ol (2.22%) and 3-octanone, 2-methyl-(2.03%) were relatively higher among all the volatile compounds.
Influence of MVD on the volatile compounds of WHM
After drying by MVD, 72 classes of volatile compounds were detected (). Similar to HD and VD, new aroma compounds, such as acids (3.27%), sulphides (0.28%), and pyran (2.28%), were determined. Among all the four kinds of drying methods, pyrazines were detected only in the MVD samples, and the content of pyrazines detected was 23.64%. With strong flavour, pyrazines are intermediate products of Maillard reaction, which play a significant role in the flavour formation of MVD white H. marmoreus[Citation24].
MVD took much less time than the other drying methods and Maillard reaction stopped at the intermediate stage, causing more accumulation of pyrazines. Other drying methods lasted longer, which promoted further reaction and the production of other flavour compounds. The contents of pyrazine, 3-ethyl-2,5-dimethyl-(5.71%), pyrazine, 2,5-dimethyl-(3.81%), and pyrazine, 2,5-dimethyl-3-(3-methylbutyl)-(3.66%) were relatively higher among all pyrazine compounds.
Influence of FD on the volatile compounds of WHM
A total of 49 classes of volatile compounds were determined in FD samples(). Theoretically, flavour substances are stable under the low temperature of FD. However, the present study showed that FD significantly promoted the reduction of alcohols (54.73% to 4.70%), aldehydes (14.24% to 2.28%), and the generation of hydrocarbons (1.11% to 32.41%) and esters (0.52% to 2.63); this is probably because vacuum and low temperature are beneficial to the formation of esters by chemical reactions of alcohols, then esters further converted into hydrocarbons[Citation25]. Compared to the fresh WHM, new aroma compounds such as sulphides (2.41%) were detected in the FD product, but original furans, pyrazines, and phenols disappeared.
Conclusion
PCA and LDA showed that e-nose could distinguish fresh WHM and four dried products (HD, VD, MVD, and FD) clearly. Results showed that fresh WHM mainly contained alcohols, aldehydes, and ketones with 1-octene-3-alcohol, trans-2-nonene aldehyde, and 3-ketone being the dominant volatile compounds; HD samples mainly contained alcohols and esters; benzene ethanol and HD samples had a higher content of 2-phenylethylester. VD samples mainly contained alcohols and ketones, with 2-3-octene-ketonea and 2-methyl-3-symplectic ketone being higher than the other volatile compounds. MVD samples mainly contained pyrazines, and had a higher content of 3-ethyl-2,5-dimethyl pyrazine and 2,5-dimethyl pyrazine. VD samples mainly had hydrocarbon compounds and the contents of 2,6-dimethyl undecane and 2-methyl undecane were higher. The four different drying methods had great influence on the volatile flavour components, which promoted the gradual reduction of alcohols, ketones, aldehydes, and the generation of hydrocarbons, esters, acids, and sulphides. The methods showed varying extents of damage (MVD > FD > VD > HD) to C8, especially to 8-carbon alcohol. HD produced more ester materials while MVD produced more aldehyde compounds. VD yielded more ketone substances and no acids were detected. In conclusion, the overall purpose of this study was to compare the composition profiles of WHM aroma during the different drying processes. Accordingly, in combination with our previous works, the results would provide a reference for the further development of WHM processing products.
Funding
This work was financially supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-24) and Special Fund for Agro-scientific Research in the Public Interest (No. 201303080).
Additional information
Funding
References
- Tsai, P.F.; Ma, C.Y.; Wu, J.S.B. A Novel Glycoprotein from Mushroom Hypsizygus marmoreus (Peck) Bigelow with Growth Inhibitory Effect Against Human Leukaemic U937 Cells. Food Chemistry 2013, 141, 1252–1258.
- Oka, K.; Ishihara, A.; Sakaguchi, N.; Nishino, S.; Parada, R.Y.; Nakagiri, A.; Otani, H. Antifungal Activity of Volatile Compounds Produced by an Edible Mushroom Hypsizygus marmoreus Against Phytopathogenic Fungi. Journal of Phytopathology 2015, 163, 987–996.
- Phat, C.; Moon, B.; Lee, C. Evaluation of Umami Taste in Mushroom Extracts by Chemical Analysis, Sensory Evaluation, and an Electronic Tongue System. Food Chemistry 2016, 192, 1068–1077.
- Lee, Y. L.; Jian, S. Y.; Mau, J. L. Composition and Non-volatile Taste Components of Hypsizigus marmoreus. LWT - Food Science and Technology 2009, 42, 594–598.
- Wu, F.; Tang, J.; Pei, F.; Wang, S.; Chen, G.; Hu, Q.; Zhao, L. The Influence of Four Drying Methods on Nonvolatile Taste Components of White Hypsizygus marmoreus. European Food Research and Technology 2015, 240, 823–830.
- Siegmund, B. 7 – Biogenesis of Aroma Compounds: Flavour Formation in Fruits and Vegetables. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K.; Elmore, J.S.; Methven L.; Eds.; Woodhead Publishing: Cambridge, England, 2015; 127–149.
- Arumuganathan, T.; Manikantan, M.; Indurani, C.; Rai, R.; Kamal, S. Texture and Quality Parameters of Oyster Mushroom as Influenced by Drying Methods. International Agrophysics 2010, 24, 339–342.
- Mittal, T.C.; Sharma, S.R.; Muker, J.S.; Gupta, S. K. Drying Behaviour and Change in Colour and Textural Properties of Mushroom During Drying. International Journal of Food Engineering 2012, 8(1), 1–11.
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of Different Drying Methods on the Product Quality and Volatile Compounds of Whole Shiitake Mushrooms. Food Chemistry 2016, 197, 714–722.
- Papotti, G.; Bertelli, D.; Plessi, M. Use of HS-SPME-GC-MS for the Classification of Italian Lemon, Orange and Citrus spp. Honeys. International Journal of Food Science and Technology 2012, 47, 2352–2358.
- Torri, L.; Rinaldi, M.; Chiavaro, E. Electronic Nose Evaluation of Volatile Emission of Chinese Teas: From Leaves to Infusions. International Journal of Food Science and Technology 2014, 49, 1315–1323.
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic Noses and Tongues: Applications for the Food and Pharmaceutical Industries. Sensors (Basel) 2011, 11(5), 4744–4766.
- Berna, A. Metal Oxide Sensors for Electronic Noses and their Application to Food Analysis. Sensors (Basel) 2011, 10(4), 3882–3910.
- Papadopoulou, O.S.; Panagou, E.Z.; Mohareb, F.R.; Nychas, G.J.E. Sensory and Microbiological Quality Assessment of Beef Fillets using a Portable Electronic Nose in Tandem with Support Vector Machine Analysis. Food Research International 2013, 50(1), 241–249.
- Dong, L.; Piao, Y.; Zhang, X.; Zhao, C.; Hou, Y.; Shi, Z. Analysis of Volatile Compounds from a Malting Process using Headspace Solid-phase Micro-extraction and GC-MS. Food Research International 2013, 51, 783–789.
- Luis C.G.; Elizabeth O.V.; Jorge A.P.; Enrique S.D. Floral Classification of Yucatan Peninsula Honeys by PCA & HS-SPME/GC–MS of Volatile Compounds. International Journal of Food Science and Technology 2012, 47, 1378–1383.
- Eggink, P.M.; Maliepaard, C.; Tikunov, Y.; Haanstra, J.P. W.; Bovy, A.G.; Visser, R.G.F. A Taste of Sweet Pepper: Volatile and Non-volatile Chemical Composition of Fresh Sweet Pepper (Capsicum annuum) in Relation to Sensory Evaluation of Taste. Food Chemistry 2012, 132, 301–310.
- Du, X.; Bai, J.; Plotto, A.; Baldwin, E.; Whitaker, V.; Rouseff, R. Electronic Nose for Detecting Strawberry Fruit Maturity. Proceedings of the Florida. State Horticultural Society 2010, 123, 259–263.
- Huang, B.; Lei, Y.; Tang, Y.; Zhang, J.; Qin, L.; Liu, J. Comparison of HS-SPME with Hydrodistillation and SFE for the Analysis of the Volatile Compounds of Zisu and Baisu, two Varietal Species of Perilla frutescens of Chinese Origin. Food Chemistry 2011, 125, 268–275.
- Rattray, N.J.W.; Hamrang, Z.; Trivedi, D.K.; Goodacre, R.; Fowler S.J. Taking your Breath Away: Metabolomics Breathes Life in to Personalized Medicine. Trends in Biotechnology 2014, 32(10), 538–548.
- Liu, M.; Wang, J.; Li, D.; Wang, M. Electronic Tongue Coupled with Physicochemical Analysis for the Recognition of Orange Beverages. Journal of Food Quality 2012, 35(6), 429–441.
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The Smell and Odorous Components of Dried Shiitake Mushroom, Lentinula Edodes I: Relationship Between Sensory Evaluations and Amounts of Odorous Components. Journal of Wood Science 2004, 50(4), 358–364.
- Su, G.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.; Zhao, M. Characterization of Antioxidant Activity and Volatile Compounds of Maillard Reaction Products Derived from Different Peptide Fractions of Peanut Hydrolysate. Food Research International 2011, 44(10): 3250–3258.
- Malheiro, R.; Pinho, P.G.; Soares, S.; Ferreira, A.C.S.; Baptista, P. Volatile Biomarkers for Wild Mushrooms Species Discrimination. Food Research International 2013, 54(1), 186–194.
- Kjeldsen, F.; Christensenn, L.P.; Edelenbos, M. Changes in Volatile Compounds of Carrots (Daucus carota L.) During Refriferated and Frozen Storage. Journal of Agricultural and Food Chemistry 2003, 51(18): 5400–5407.