ABSTRACT
Thermal mortalities of adult red flour beetles Tribolium castaneum (Herbst) infesting canola seeds at various moisture contents and volumes were determined after radio frequency (RF) heating (i.e. temperature between 30 and 80°C). The mortality of 92% was achieved at the end temperature of 343 K for small-volume (1.96 × 10−4 m3, 0.250 kg) seeds, and the mortality of 99% at 333 K for large-volume (1.77 × 10−3 m3, 2.26 kg) seeds. Regardless of sample volume, the thermal mortalities of the test insects increased significantly after the seed temperature reached 333 K (60°C). The kinetic parameters of the thermal death of the adult T. castaneum were estimated using inverse simulation. The ordinary differential equation-based kinetic model with the Arrhenius temperature-dependent reaction rate constant was solved using the fourth-order Runge–Kutta method. The kinetics followed first-order reaction with the activation energy of 100 kJ/mol. Good agreements were observed between the mortalities predicted using the kinetic model and the experiments (R2 = 0.972–0.987) except for the small-volume seeds at 11% MC (11 g/100 g raw materials) (R2 = 0.741). The predicted lethal times (s) to achieve 95 and 99% mortalities using the kinetic model agreed well with those determined from the experiments.
Introduction
The world’s total production of canola seeds in 2015–2016 was 67.7 million metric tons.[Citation1] Major canola-producing countries are Canada, Austria, China, European Union, India, and the United States. Over 90% of the canola produced in Canada is exported to markets around the world. The canola industries had injected $19.3 billion to the Canadian economy, including 249,000 jobs and $12.5 billion in wages.[Citation2]
In Canada, the losses of oilseed production average out 8–10% in annual crop yield due to pest insects, causing hundreds of millions of dollars loss in the Canadian economy.[Citation3] Insect pest infestations that make barriers to export are a major concern in the production, storage, and process of all food products.[Citation4] The trade regulations of domestic and international markets have required post-harvest treatments of all food products to ensure quarantine security from insect pests.[Citation5,Citation6] One of the most common insect pests in the stored grains and oilseeds around the world is T. castaneum.[Citation7]
The fumigation with methyl bromide has been widely used and is regarded as one of the practical methods to disinfest various insect pests found in grains and oilseeds.[Citation7] However, it was prohibited since the Montreal Protocol because of the health hazards caused by excessive insecticide residues and the destructive effect to the ozone layer.[Citation5,Citation8,Citation9] The other non-chemical treatments to control insect pests such as ionizing radiation, hot air, and cold storage have certain disadvantages. These techniques require a long treatment time and a substantial capital investment, and may leave live insect pests after the treatments.[Citation9,Citation10] RF heating based on electromagnetic radiation is volumetric and selective heating, and this technique may avoid the problems posed by the aforementioned non-chemical treatments.[Citation11] There is a possibility of destroying the insect pests using RF heating without significant thermal degradation of the grain. Lagunas-Solar et al.[Citation12] reported the mortalities of Angoumois grain moths, Sitotroga cerealella (Olivier), and lesser grain borers, Rhyzopertha dominica (Fabricius), in rice using RF heating. A mortality of 99% of S. cerealella was achieved at 55–60°C for 5 min of heating, and 100% mortality of R. dominica was attained at 60°C for 1 h of heating. There were no significant changes in moisture level and milling quality of the rice. Shrestha et al.[Citation13] achieved 100% mortality for all the life stages of rusty grain beetles, Cryptolestes ferrugineus (Stephens), in stored wheat at 60°C with RF heating (1.5 kW, 27.12 MHz) without significant degradation of the wheat qualities.
Several kinetic models for the mortalities of insect pests have been studied for thermal processing. Tang et al.[Citation11] demonstrated a possibility of developing high-temperature, short-time thermal treatments to control the larvae of codling moth, Cydia pomenella (Linnaeus), with a minimal thermal impact on fruit quality using the thermal death kinetics of the insect. Wang et al.[Citation14] reported the thermal death kinetic parameters for the fifth-instar C. pomenella, which were heated to four different temperatures (46, 48, 50, and 52°C) using a heating block system. The thermal death kinetics of the insects followed a 0.5th-order reaction with an activation energy of 472 kJ/mol at a heating rate of 18°C/min. Johnson et al.[Citation15] estimated the lethal exposure times for the fifth-instar larvae of Indian meal moth, Plodia interpunctella (Hubner), at 44–52°C during RF heating using a 0.5th-order kinetics model. They obtained the RF heating time of less than 5 min to achieve 95% mortalities at 50°C and 52°C. A thermal death kinetic model of T. castaneum at larvae stage (the most heat-tolerant life stage of the insect) with 0.5th-order reaction was developed to estimate lethal exposure times at 48, 50, and 52°C during RF heating.[Citation16] They reported the lethal times (LTs) to achieve 99% mortalities were 1.6 min, 9.1 min, and 76.8 min at the constant temperatures of 52°C, 50°C, and 48°C, respectively. The thermal death kinetics were determined based on experiments at constant system temperatures, and only applied for the larvae stage of the insect. These kinetic models could not be used in this study. The target life stage of the insect in this study was adult, the heating process was transient (the temperature of the infested sample was continuously increased), and the immediate mortality (the mortality determined immediately after the RF heating) was considered.
Thermal death kinetics of the adult T. castaneum based on the immediately determined mortalities and temperature histories of the host material (canola seeds) will be helpful. The determined kinetic parameters and model can predict mortalities at specific RF heating times without knowing the insect body temperatures. They can also be used to determine the proper RF heating conditions for disinfestation of the adult T. castaneum. Therefore, in this study, the thermal death kinetics of the adult T. castaneum infesting canola seeds at various moisture contents and volumes of seeds were characterized based on dynamic temperature changes of the canola seeds and the experimental data of insect mortalities during RF heating.
Materials and methods
RF heating system
The RF heating system used in this work is shown in . A 1.5 kW, 27.12 MHz RF system (Strayfield Fastran, Berkshire, England) with two parallel electrode plates (250 mm × 250 mm) was used to heat the samples. The upper electrode can be adjusted vertically up or down by turning the crank attached to the plate, and the bottom plate was fixed and grounded. The electric arcing was observed when the air gap between the upper electrode and the top surface of the samples was less than 1 mm and 15 mm for the small- and large-volume samples due to evaporated moisture from the sample during RF heating. To prevent the electric arcing, air gaps of 1 mm and 15 mm, respectively, for the small- and large-volume samples were set. The larger air gap for the large-volume samples was determined due to intensified electric arcing by more evaporated moisture from the seeds compared to that of the small-volume samples. Those air gap sizes were determined from our previous work.[Citation17]
Figure 1. The side and rear view of the RF heating system (a) and the interior view of the RF heating system (b) showing I: reflex signal conditioner; II: temperature controller; III: data acquisition device; IV: data acquisition software; V: electrodes; VI: canola seeds; VII: fibre optic temperature sensors.[Citation20]
![Figure 1. The side and rear view of the RF heating system (a) and the interior view of the RF heating system (b) showing I: reflex signal conditioner; II: temperature controller; III: data acquisition device; IV: data acquisition software; V: electrodes; VI: canola seeds; VII: fibre optic temperature sensors.[Citation20]](/cms/asset/0d1306ae-719b-40cd-b11e-8afdcb661940/ljfp_a_1272609_f0001_oc.jpg)
Canola seed samples
Top-quality canola seeds (B. napus L.) at the initial moisture content (MC) of 7% wet basis (w.b.) were provided by our industrial partner, Viterra Inc., Regina, SK, Canada. The standard method[Citation18,Citation19] was used to determine the MC of the canola seeds. Four different MCs (5%, 7%, 9%, and 11%) of the bulk canola seeds were prepared. The seed samples at 5% MC were prepared by drying the original canola seeds in a hot-air oven. The seed samples at 9% and 11% MCs were prepared by adding pre-calculated amounts of distilled water to the seeds at the initial MC. A sprayer was used to do that. The prepared canola seed samples were stored in a cold storage at 4°C before using them. The details on preparing the seed samples at the required MCs can be found in Yu et al.[Citation17]
RF exposure time of the canola seeds
Small-volume (1.96 × 10−Citation4 mCitation3, 0.250 kg) seed samples were contained in an RF transparent polycarbonate sample holder that can minimize the effect of the holder on RF heating, measuring 50 mm and 100 mm in diameter and height, respectively, and larger-volume (1.77 × 10−Citation3 mCitation3, 2.26 kg) seed samples were contained in a sample holder made of the identical material measuring 150 mm and 100 mm in diameter and height, respectively. The former and the latter were referred to as small-volume sample and large-volume sample, respectively, in this work.
A ReflexTM signal conditioner and the fibre optic temperature sensors with an accuracy of ± 0.8°C (Neoptix, Québec City, Québec, Canada) were used to measure and monitor the temperatures of samples. The fibre optic temperature sensors were located at the hottest spot of the seed samples. The hottest spot for the small-volume samples was at the geometric centre regardless of MC of the seeds. However, for the large-volume samples, the hottest spot was shifted from the geometric centre to near the inner wall of the sample holder as the MCs of the samples were changed from 5% and 7% to 9% and 11%, respectively, as illustrated in . Those hottest spots were obtained from our previous research.[Citation17] A dedicated Labview (2010v.10) program was developed to interface with the data acquisition device connected to the temperature sensors. The temperatures of the sample were displayed and recorded every 2 s. RF exposure times (s) of the canola seeds were determined when the temperature of the hottest spot of the bulk seeds reached up to each desired temperature (303 K, 313 K, 323 K, 333 K, 343 K, and 353 K) from 297 K (initial temperature) for each MC (5%, 7%, 9%, and 11%) of the seeds. Three different batches of canola seeds in total were used for each MC. The same amount of seeds was used for each batch with the same operating condition of the RF unit. The RF exposure times (s) were obtained from our previous research.[Citation20] The average of triplicate measurements was used for the RF exposure time (s) of the seeds. Then regression models were developed for the temperature (K) of the seeds as a function of RF exposure time (s) for input data in the kinetic model used in this study.
Figure 2. The location of the hottest spot of the small -volume samples (a), and the large-volume samples at MCs of 5% and 7% (b), and 9% and 11% (c).[Citation20]
![Figure 2. The location of the hottest spot of the small -volume samples (a), and the large-volume samples at MCs of 5% and 7% (b), and 9% and 11% (c).[Citation20]](/cms/asset/42203d3d-58bf-471a-8ce2-62d4e35eea54/ljfp_a_1272609_f0002_b.gif)
Test insect culture
The adult T. castaneum were collected from the Agriculture and Agri - Food Canada /University of Manitoba, Canada. The insects were cultured following the procedure adopted by Shrestha et al.[Citation13]. In brief, a total of 500 adult insects were reared in a jar (2 L) containing 1.5 kg of a mixture of wheat at MC of 14% and wheat germs in a proportion of 7:3 by weight. The jar was covered with a fabric for better ventilation and placed in a temperature and humidity chamber (30°C and 70% RH, respectively) for 70 days. The insects with the rearing mixture were separated using the Canadian standard sieve # 40 and collected using a suction assembly. The insects were exposed to carbon dioxide gas at various stages of the experiment when it was required to stop them from moving around. The details on the procedures of rearing and handling the test insects can be found in Yu et al.[Citation20]
Insects mortality
The insect mortalities were tested by introducing the adult insects in small and large seed samples. A total of 30 adult insects (70–72 days old from the time of hatching from eggs) were introduced at the hottest spot (ball shape) of the small-volume samples and its vicinity, and 90 adult insects at the hottest spot (doughnut shape) of the large-volume samples as shown in . The hottest spot was chosen to show how effectively insects can be killed with optimally designed RF heating systems. The vicinity of the hottest spot was also taken by the adult insects for the small-volume samples as the hottest spot was too tiny to hold 30 adult insects at the exact hottest spot. The infested canola seeds were heated until the hottest spot of the bulk seeds reached up to each desired temperature (303 K, 313 K, 323 K, 333 K, 343 K, and 353 K) from 297 K (initial temperature) for each MC (5%, 7%, 9%, and 11%) of the seeds. The dead and live insects were counted to calculate mortalities. The immediate mortalities of the adult T. castaneum at each desired temperature were tested in triplicate and then averaged for each MC of the seeds. The more detailed procedures of the insect mortality are available in Yu et al.[Citation20]
Kinetic modelling
The thermal death rate of the adult T. castaneum can be modelled using the following kinetic model:
where t is the heating time (s), N and N0 are, respectively, the number of live insects at time and the initial number of the insects, respectively, k is the reaction rate constant (1/s), and n is the order of the reaction.[Citation21] The temperature-dependent reaction rate constant can be expressed using the Arrhenius relationship as follows:
where k0 is the frequency factor, Ea is the activation energy (J/mol), R is the universal gas constant (8.314 J/mol·K), and T is the temperature of the bulk canola seeds (K).[Citation21] Temperature histories of the canola seeds during RF heating were used instead of those of the insects as measuring the insect-body temperatures was extremely difficult due to their tiny size (3–4 mm) and mobility. This makes the activation energy and frequency factor as apparent or effective values as the temperature of the insects is not the same as that of the canola seeds during RF heating. It is, however, assumed that the canola seeds had similar heating patterns as those of the insects despite their heating speed being slower. Using the temperature of the canola seeds instead of the insect-body temperature was the limitation of this study; however, at the same time, this approach would be more practical for real-world applications. Only a limited mobility of the insects in the seed sample during RF heating was observed due to the natural compactness of the tiny seeds (the diameter of the seed is approximately 1.8 mm).
Substitution of the value of k from Eq. (2) into Eq. (1) results in the thermal death rate of the adult T. castaneum as shown in Eq. (3).
The ordinary differential equation, Eq. (3), was solved using the ODE 45 solver based on the fourth-order Runge–Kutta method in MATLAB R2013a (The MathWorks Inc., Natick, MA, USA). The optimum values of k0, Ea, and n were determined by minimizing the root mean square error (RMSE) between the values predicted from the kinetic model and the experimental data as depicted in Eq. (4).
where m is the number of data, xi is the measured value, and is the value predicted using the kinetic model, Eq. (3). The unknown parameters k0, Ea, and n were determined by trial and error from eight different sets of time–temperature data (six points per set) depending on RF heating rates. As n was found to be 1 ± 0.01 in our preliminary parameter estimation, we set the order of kinetics (n) as 1 for all of the estimation. Then, only the other two unknown parameters k0 and Ea were estimated. The kinetic model was also used to estimate LTs to achieve 95% (LT95) and 99% (LT99) mortalities of the insects infesting the canola seeds at different MCs and volumes.
Statistical analysis
The stepwise regression and bivariate correlation analysis in SPSS (SPSS Inc., Chicago, IL, USA) were used to derive the regression models for temperature of the canola seeds versus RF exposure time, and to investigate the correlation between the mortalities of the insects from the kinetic model and the experiments.
Results and discussion
Temperature histories of canola seed samples
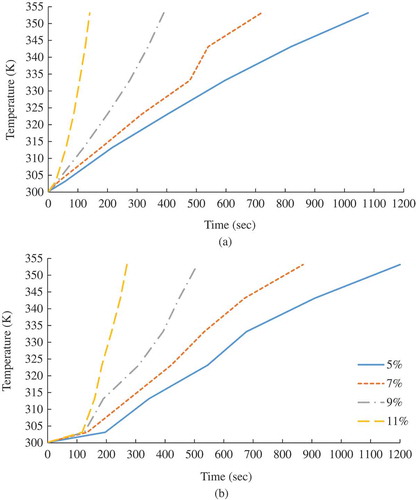
shows the temperature histories of the canola seed samples at various MCs during RF heating. The rate of the temperature increment of the seeds was apparently proportional to the MC of the seeds. It was attributed to the higher dielectric loss factor of the seeds due to the enhanced conductivity of dipoles and ions in the material, with higher MC causing higher dissipation of electromagnetic energy.[Citation13] In case of the large-volume samples, the temperatures were increased significantly faster after 303 K. The RF exposure time ranged from 25 to 1200 s. The differences between the RF exposure times to attain the same temperature for the small- and large-volume samples ranged from 0.024 to 186 s. The small- and large-volume samples at 5% MC took approximately eight and four times longer RF exposure times than those at 11% MC to reach 353 K, respectively. The regression models for the temperatures (K) of the canola seeds as a function of the RF exposure time (s) at the interested MCs for both the small- and large-volume samples are listed in . The developed regression models were used as input data in Eq. (3) to determine the thermal death kinetics of the adult T. castaneum.
Table 1. The regression models for the temperature of the canola seeds as a function of the RF exposure time (s) at different seed MCs and volumes during the RF heating.
Thermal mortality of the insects
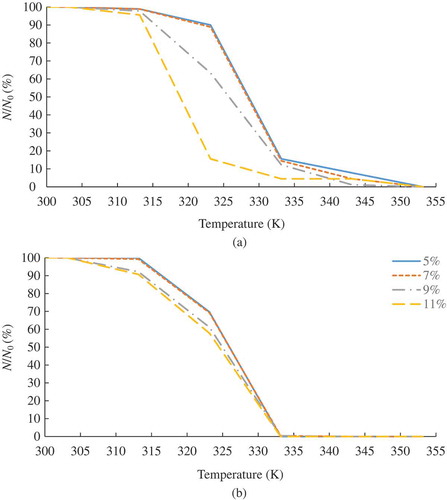
shows the measured survival rates (100% mortality %) of the adult T. castaneum in the small- and large-volume samples when the samples at MCs of 5%, 7%, 9%, and 11% were RF heated from 303 K to 353 K by RF energy. In general, the survival rate of the insect decreased with increasing temperature. At any end temperatures of the seeds, the survival rate of the insect decreased with increasing MC of the seeds. The insects thoroughly surrounded by the seeds are exposed to the evaporated hot steam from the wet seeds by RF heating. The survival rate of the insects in the seed samples at high MC (11%) was lower than that at low MC (5%) at the same end RF heating temperature due to the more evaporated moisture from the seeds of high MC (11%). The survival rates of the insect in the large-volume samples had significantly dropped over the temperature compared to those in the small-volume samples due to the more evaporated moisture from the large-volume samples. Placement locations of the adult T. castaneum in the small- and large-volume samples (mentioned in section 2.4) could be one of the reasons for the difference in the survival rates between the small- and large-volume samples. Over 84% and 99% mortalities of the insects were achieved at 333 K for the small- and large-volume samples, respectively. Those mortalities required RF exposure times of 106.0–597.0 s and 198.5–765.0 s for the small- and large-volume samples at 5–11% MC. The thermal effect on insect mortality was attributed to the thermal degradation of carbohydrates, proteins, DNA, RNA, and lipids of the insects.[Citation22] Yu et al.[Citation20] reported that 100% mortality of T. castaneum infesting the canola seeds at MCs of 5%, 7%, 9%, and 11% could be achieved without significant degradation of the seed quality at 333 K (60°C) with proper design of RF heating applicators.
Thermal death kinetics
The best fitted kinetic model of thermal death of the adult T. castaneum infesting the canola seeds during RF heating was as follows:
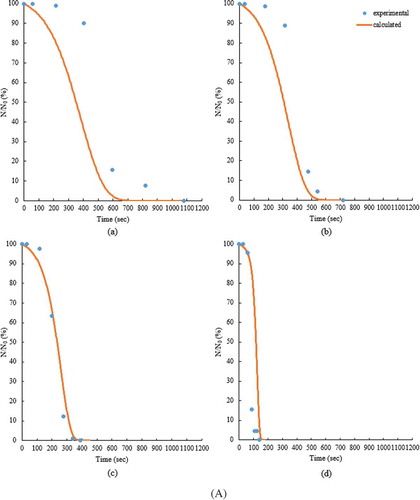
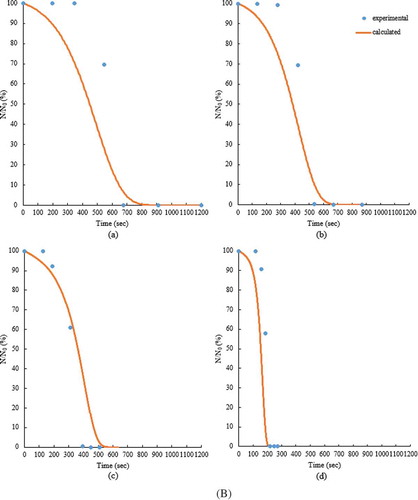
The performance of the model in predicting the mortalities for both the small- and large-volume samples is summarized in . The mortalities determined from the kinetic model agreed reasonably well with the experimental values, resulting in RCitation2 values of 0.958, 0.978, 0.972, and 0.741, and RMSE values of 16.20, 11.55, 12.84, and 43.32 for 5%, 7%, 9%, and 11% MCs of the small-volume samples, respectively. In case of the large-volumes samples, the RCitation2 values were 0.975, 0.987, 0.979, and 0.908, and the RMSE values were 12.30, 8.745, 11.02, and 25.73 for 5%, 7%, 9%, and 11% MCs, respectively. The kinetic parameters should be independent of MC and volume of samples if the effect of steam release is not reflected in the thermal death kinetics. Relatively high RMSE (43.32) and low RCitation2 (0.741) for the small-volume samples at 11% MC were caused by the sharply dropped survival rate of insects. compares the survival rates of insects determined from the kinetic model with those from the experiments for the small- and large-volume samples at 5%, 7%, 9%, and 11% MCs during RF heating. In , the scatter data present the experimental values, and the continuous lines represent the calculated values from the kinetic model. The first-order kinetic model was much more suitable to describe the thermal death kinetics of the insect than 0.5th-order, which was used by Wang et al.[Citation14] and Johnson et al.[Citation15,Citation16] for the thermal death kinetics of other insects. Shimoda et al.[Citation23] reported the inactivation of Saccharomyces cerevisiae by combinations of dissolved CO2 and heating (30–38°C) followed by first-order kinetics. First-order reaction was the most suitable to describe the thermal death kinetics of eggs of Mediterranean flour moth, Ephestia kuehniella (Zeller), which were heated in hot water at constant temperatures (46–75°C) for 5–1200 s.[Citation24] Our determined activation energy of 100 kJ/mol for the adult T. castaneum infesting the canola seeds was similar to that of eggs of E. kuehniella (102 kJ/mol)[Citation24] and lower than that for the fifth instar of C. pomonella (472 kJ/mol),[Citation14] the fifth instar of P. interpunctella (506.3 kJ/mol),[Citation15] and the fifth-instar navel orangeworm, Amyelois transitella (Walker) (510 kJ/mol to 520 kJ/mol).[Citation25] The lower activation energy indicated that the adult T. castaneum was more susceptible to thermal death by RF heating than the other insects infesting the food commodities. It should be, however, noted that in our study for kinetic parameter estimation, the temperatures of the canola seeds were used instead of the adult insect body temperatures.
Table 2. The performance of the kinetic model (Eq. 5) in predicting the mortalities of the adult T. castaneum during the RF heating.
Lethal time
shows the experimental LT required to kill 100% of the insects as well as the LT95 and the LT99 determined from the experiments and the kinetics model. The predicted LT95 and LT99 from the kinetic model were in good agreement with those from the experiments except for the small-volume seeds at 5% MC. This exception was caused by the slow heating rate of the seeds at the lower moisture level. Complete destruction of the adult insect in the canola seeds was achieved with the RF exposure time ranging 140–1080 s and 199–762 s for the small- and large-volume samples at 5–% MC, respectively. The differences between LT95 and LT99 determined from the experiments and the kinetic model ranged from 17.40 to 287.1 s, and 19.49 to 348.6 s, respectively. Similar discrepancies of LT95 and LT99 of mortalities of fifth instar of A. trasitella were observed by Wang et al. (2002b). The LT95 and LT99 from the experiments for the small-volume samples at 5% MC were 7.41 times and 7.66 times longer than those at 11% MC. This means disinfestation of the insect by RF could be done more effectively with higher MC seeds due to the shorter RF heating time and steam release effect to achieve the specific mortalities compared to seeds at lower MC.
Table 3. The RF exposure times (s) to achieve 100% mortality and the LTs (s) determined from the experimental and simulated data for the adult T. castaneum at the indicated seed MCs and volumes.
Conclusion
The survival rate of the adult T. castaneum infesting the canola seeds at different seed MCs (5%, 7%, 9%, and 11%) and volumes (small and large) decreased with temperature (303–353K) during RF heating. The kinetic parameters of the thermal death of the adult T. castaneum were estimated using an inverse simulation. The kinetics followed first-order reaction with the activation energy of 100 kJ /mol and it produced insect mortalities that were comparable to the experiments. The determined LT95 and LT99 from the experiments and the kinetic model were in good agreement. The thermal death kinetic model developed in this research would be useful in designing post-harvest RF thermal processes to control T. castaneum and similar insects in stored canola seeds and other commodities.
Acknowledgements
We would also like to express our thanks to Mr Ian Armer (Manager, Grain quality control division, Viterra Inc.) for providing us with canola seeds and valuable technical supports.
Funding
We gratefully acknowledge the Saskatchewan Ministry of Agriculture and the Western Grains Research Foundation, Saskatchewan, Canada for their financial supports through the Agriculture Development Fund program (ADF#20130219).
Additional information
Funding
References
- USDA Foreign Agricultural Service. Production, Supply and Distribution Online Database, query for Commodity: “Oilseed, Rapeseed”; Data Type: “Production”; Country: “All countries”; Year: “2015”, ( accessed July 25, 2016).
- Canola Council of Canada. http://www.canolacouncil.org/markets-stats/industry-overview/( accessed July 25, 2016).
- Canola Watch. http://www.canolawatch.org/2011/05/09/estimating-flea-beetle-damage-in- canola/, ( accessed July 25, 2016).
- Gao, M.; Tang, J.; Wang, Y.; Powers, J.; Wang, S. Almond Quality as Influenced by Radio Frequency Heat Treatments for Disinfestation. Postharvest Biology and Technology 2010, 58, 225–231.
- Birla, S.L.; Wang, S.; Tang, J.; Tiwari, G. Characterization of Radio Frequency Heating of Fresh Fruits Influenced by Dielectric Properties. Journal of Food Engineering 2008, 89 (4), 390–398.
- Jiao, S.; Johnson, J.A.; Tang, J.; Tiwari, G.; Wang, S. Dielectric Properties of Cowpea Weevil, Black-eyed Peas and Mung Beans with Respect to the Development of Radio Frequency Heat Treatments. Biosystems Engineering 2011, 108 (3), 280–291.
- Sinha, R.N.; Watters, F.L. Insect Pests of Flour Mills, Grain Elevators, and Feed Mills and their Control; Agriculture Canada: Ottawa, Canada, 1985.
- Benedick, R.E. Montreal Protocol On Substances that Deplete the Ozone Layer. International Negotiation 1996, 1(2), 231–246.
- Wang, S.; Tang, J. Radio Frequency Heating: A Potential Method for Post-harvest Pest Control in Nuts and Dry Products. Journal of Zhejiang University Science 2004, 5 (10), 1169–1174.
- Heather, N.W.; Hallman, G.J. Pest Management and Phytosanitary Trade Barriers; CABI: Oxfordshire, UK, 2008.
- Tang, J.; Ikediala, J.N.; Wang, S.; Hansen, J.D.; Cavalieri, R.P. High-temperature-short-time Thermal Quarantine Methods. Postharvest Biology and Technology 2000, 21 (1), 129–145.
- Lagunas-Solar, M.C.; Pan, Z.; Zeng, N.X.; Truong, T.D.; Khir, E.; Amaratunga, K.S.P. Application of Radio Frequency Power for Non-chemical Disinfestations of Rough Rice with Full Retention of Quality Attributes. Applied Engineering in Agriculture 2007, 23 (5), 647–654.
- Shrestha, B.; Yu, D.; Baik, O. D. Elimination of Cruptolestes Ferrungineus S. in Wheat by Radio Frequency Dielectric Heating at Different Moisture Contents. Progress in Electromagnetics Research 2013, 139, 517–538.
- Wang, S.; Ikediala, J.N.; Tang, J.; Hansen, J.D. Thermal Death Kinetics and Heating Rate Effects for Fifth-instar Cydia pomonella (L.) (Lepidoptera: Tortricidae). Journal of Stored Products Research 2002a, 38 (5), 441–453.
- Johnson, J.A.; Wang, S.; Tang, J. Thermal Death Kinetics of Fifth-instar Plodia interpunctella (Lepidoptera: Pyralidae). Journal of Economic Entomology 2003, 96 (2), 519–524.
- Johnson, J.A.; Valero, K.A.; Wang, S.; Tang, J. Thermal Death Kinetics of Red Flour Beetle (Coleoptera: Tenebrionidae). Journal of Economic Entomology 2004, 97 (6), 1868–1873.
- Yu, D.; Shrestha, B.; Baik, O.D. Temperature Distribution in a Packed-bed of Canola Seeds with Various Moisture Contents and Bulk Volumes During Radio Frequency (RF) Heating. Biosystems Engineering 2016, 148, 55–67.
- ASAE. ASAE S352.2, Moisture Measurements-unground Grains and Seeds; In ASAE standards; St: Joseph, MI, USA, 2002.
- Brusewitz, G.H. Density of Rewetted High Moisture Grains. Transactions of the ASAE 1975, 18 (5), 935–938.
- Yu, D.; Shrestha, B.; Baik, O.D. Radio Frequency (RF) Control of Red Flour Beetle (Tribolium castaneum) in Stored Rapeseeds (Brassica napus L.). Biosystems Engineering 2015, 151, 248–260.
- Datta, A.K. Biological and Bioenvironmental Heat And Mass Transfer; Cornell University: Ithaca, New York, 2002, 189.
- Hallman, G.J.; Denlinger, D.L. Temperature Sensitivity in Insects and Application in Integrated Pest Management; Westview Press, Inc: Boulder, CO , 1998.
- Shimoda, M.; Cocunubo‐Castellanos, J.; Kago, H.; Miyake, M.; Osajima, Y.; Hayakawa, I. The Influence of Dissolved CO2 Concentration on the Death Kinetics of Saccharomyces cerevisiae. Journal of Applied Microbiology 2001, 91 (2), 306–311.
- Ben-Ialli, A.; Méot, J. M.; Bohuon, P.; Collignan, A. Survival Kinetics of Ephestia kuehniella Eggs during 46–75 C Heat Treatment. Journal of Stored Products Research 2009, 45 (3), 206–211.
- Wang, S.; Tang, J.; Johnson, J.A.; Hansen, J.D. Thermal-death Kinetics of Fifth-instar Amyelois transitella (Walker) (Lepidoptera: Pyralidae). Journal of Stored Products Research 2002b, 38 (5), 427–440.