ABSTRACT
Aroma-impact compounds of dry jujubes obtained from Z. jujuba Mill. cv. “tangzao” (Y1), Z. jujuba Mill. cv. “muzao” (Y2), Z. jujuba Mill. cv. “lizao” (Y3), and Z. jujuba Mill. cv. “qingrunhongzao” (Y4) were analyzed by gas chromatography-olfactometry (GC-O). The result revealed 35, 29, 34, and 33 aroma-impact volatiles in the Y1, Y2, Y3, and Y4 samples, respectively. The compounds bearing the highest FD factor in each sample were (E)-2-hexenal, (E)-2-heptenal (FD = 256) for Y1, 2,3-butanedione, (E)-2-hexenal, 2,5-dimethylpyrazine, (E)-2-heptenal and β-damascenone (FD = 64) for Y2, 2,3-butanedione and β-damascenone (FD = 256) for Y3, and 2,5-dimethylpyrazine (FD = 256) for Y4.
Introduction
Jujube (Zizyphus jujuba Mill.) is indigenous to China with a cultivation history over 4000 years, which is also commonly called as Chinese date or red date.[Citation1] The plant belongs to the Rhamnaceae family.[Citation2] Jujube is currently widely cultivated all over the world and mainly distributed in Europe, southern and eastern Asia, and Australia. So far, over 700 cultivars of jujube have been found in China.
Jujube is rich in carbohydrates, salts, minerals, vitamins, fatty acids, amino acids, and proteins. It is well known for its nutritional value and desirable aromas. It is often consumed in fresh or processed by food industries to make various food products, such as jams, dried fruit, or flavoring ingredient.[Citation3]
In recent years, some studies of jujubes have been reported in the mainstream food chemistry journals. Mourad et al. reported the chemical composition and antioxidant properties of three Tunisian jujube varieties.[Citation4] Hédi et al. investigated the polyphenol profiles, including flavanols, flavonols, flavones, and hydroxycinnamates, of two Tunisian varieties of jujubes.[Citation5] Manjeshwar et al. presented a comprehensive analysis of the phytochemistry and pharmacological properties of jujube fruits and their seeds.[Citation6] However, there was lack of literatures relevant to the systematic and effective evaluation of volatile compounds of jujubes.
Aroma profile is one of the major characteristics that define the quality differences among various fruits. It consists of a complex mixture of volatile compounds resulted from various biological conversions of sugars, amino acids, and other chemical metabolites.[Citation7] These metabolites, along with the intrinsic compounds in fruits, are responsible for characterization and differentiation of fruit aromas.
Aromas, particularly in fruits, are often constituted by small molecular volatile chemicals and organoleptically desirable alcohols, esters, organic acids, aldehydes, ketones, lactones, monoterpenes, etc. However, it is worthy of mention that aroma-impact chemicals are considered to play more critical roles in aromatic contribution of food. In other words, it is the aromatic intensity, rather than the quantity, of volatile components to determine the flavor characteristics of food. In most cases, gas chromatography-olfactometry (GC–O), combined with qualitative MS technique, is commonly used to identify the aroma-impact compounds because this technique can detect flavor chemicals in a food as low as at 10–19 mol. Thus, this method is frequently used, regardless of some dissents, to differentiate the important aroma-impact volatiles.[Citation8]
However, to the best of our knowledge, there were few attempts to identify aroma-impact compounds of jujubes by GC-O. Therefore, the aims of this research were (1) to identify the volatile chemicals, particularly the key aroma-impact compounds in jujubes by GC-MS and GC-O, and (2) to characterize the aroma profiles of jujubes with aid of sensory evaluation and correlate its relationship with the identified aroma-impact compounds by aforementioned objective.
Experimental
Chemicals
Butanal, 3-methylbutanal, 2-methylbutanal, pentanal, 2,3-btanedione, 2,3-pentandione, hexanal, (E)-2-pentenal, 1-penten-3-ol, 2-methypyrazine, (E)-2-hexenal, ethyl hexanoate, 2,5-dimethylpyrazine, octanal, (E)-2-heptenal, 3-hydroxy-2-butanone, 2,6-dimethylpyrazine, 2,3-octanedione, 2-ethylpyrazine, hexanol, nonanal, trimethylpyrazine, (E)-2-octenal, ethyl octanoate, acetic acid, tetramethylpyrazine, decanal, benzaldehyde, butanoic acid, β-damascenone, hexanoic acid, geraniol, benzyl alcohol, γ-octalactone, phenylethyl alcohol, and octanoic acid were purchased from Sigma-Aldrich (Saint Louis, EUA). All of them were ARG quality.
Materials
Ripen samples, including four varieties of jujubes (i.e., Z. jujuba Mill. cv. “tangzao,” Y1, Z. jujuba Mill. cv. “muzao,” Y2, Z. jujuba Mill. cv. “lizao,” Y3, and Z. jujuba Mill. cv. “qingrunhongzao,” Y4), were collected in a local farm during the 2014 harvest season. The amounts of those four jujubes were 12.1, 12.5, 11.8, and 13.1 kg, respectively. The voucher specimens were SXU SD00011504, PE 01637044, SXU SD00012786, and IBSC 0405566, which were deposited at the Chinese Virtual Herbarium (CVH). The samples were carefully identified by Xishuangbanna Tropical Botanical Garden of Chinese Academy of Sciences, China. The ripen fruits were in full of pulp with bright red color. Moreover, only the jujube fruits that had neither any physical damage nor fungal infections were selected and used for all the following experiments.
The jujubes were processed with microwave drying, which was under an output power of 4000 W for 150 s. Then, the treated samples were stored in a dry environment for a year. Then, one kilogram of jujubes was crushed and manually deseeded: Thereafter, the deseeded samples and Milli-Q deionised water (400 g) were blended together by a kitchen blender. The mixtures were obtained. Then, the mixtures were filtered. Then, the mixture was filtered to get the supernatant. All filtered jujube juices were kept in a refrigerator (4°C) until chemical analysis.
Extraction of the volatile compounds
The simultaneous distillation extraction (SDE) technique was used to obtain the aromatic extracts of jujubes. One hundred fifty grams of the filtered jujube supernatant, two internal chemical standards (i.e., 40 μL of 230 mg/L methoxy-d3-phenol and 40 μL of 200 mg/L 2-octanol), and 500 mL pure water were poured into a 1000 mL distillation flask which was placed in a heating mantle at 100°C. High purity dichloromethane (50 mL) in HPLC grade was poured into a 100 mL distilled flask, which was placed in a water bath at 62°C. SDE was performed for 3 h. The extract was dehydrated by anhydrous sodium sulfate and then condensed to 0.5 mL under a gentle stream of pure nitrogen.
E-nose
An E-nose system (FOX 3000, Alpha MOS, Toulouse, France) equipped with 18 metal oxide sensors (LY2/LG, LY2/G, LY2/AA, LY2/GH, LY2/gCTL, LY2/gCT, T30/1, P10/1, P10/2, P40/1, T70/2, PA/2, P30/1, P40/2, P30/2, T40/2, T40/1, TA/2) based on different sensing materials was used in the experiment. The system was fitted with a headspace auto sampler HS100. The electronic nose was employed to compare the difference between jujubes extracts and the filtered juices by radar plot. In the experiment, 1 g of those samples were prepared in a 10 mL glass vial and capped with a Teflon rubber cap. Headspace (2500 mL) carried by air (150 mL/min) was injected into the E-nose. Sensor resistance was measured during 120 s at the rate of one acquisition in every 1 s. All samples were run in triplicates.
GC-MS of volatile compounds in jujubes
A Hewlett-Packard 7890 gas chromatograph with a 5975C mass selective detector (MSD) (Agilent Technologies, USA) and a HP-INNOWAX analytical fused silica capillary column (60 m × 0.25 mm × 0.25 μm, Agilent, Santa Clara, CA, USA) were used for analyzing of the volatile compounds. The injection port was set in a splitless mode for 3 min at 250°C. The carrier gas was the ultra-high-purity (UHP) helium that was set at a constant flow rate of 1 mL/min. The MSD was used for chemical identification. Its electron impact energy was 70 eV. The ion source temperature was set at 230°C. The quadrupole mass filter was operated at 150°C. The transfer line temperature was at 250°C. The chromatograms were recorded by monitoring the total ion currents in 30–450 m/z. The MS turned off during the detection time of 6–9 min. The oven temperature was held at 40°C for 6 min, then ramped to 100°C at the rate of 3°C/min, and ramped at the rate of 5°C/min to 230°C for the last 20 min. The volatile compounds were identified by comparing retention indices (RIs), retention times with those of the corresponding authentic standards, or with mass spectra in the Wiley7n.l Database (Hewlett-Packard, Palo Alto, CA, USA). The RIs of unknown compounds were determined via sample injection with a homologous series of alkanes (C6-C30) (Sigma-Aldrich, Saint Louis, MO, USA). To quantify the volatiles, the samples were run in triplicate, and the integrated areas based on the total ion chromatograms were normalized to the areas of the internal standard and averaged. In this experiment, 2-octanol was used as an internal standard to quantify the compounds with retention time less than 30 min. Otherwise methoxy-d3-phenol was used as an internal standard.
GC-O-AEDA of compounds in jujubes
The GC-FID-O system was consisted of an Agilent 7890A chromatograph instrument equipped with a flame ionization detector (FID) and an ODP-2 Olfactory Detector Port (Gerstel, Mulheim an der Ruhr, Germany). This system allowed us to simultaneously obtain a FID signal for the quantification and the odor characteristics of each compound detected by the sniffing port. The GC effluent was split into a ratio of 1:1 between the FID and sniffing port. The volatile chemicals in the samples were separated on the DB-INNOWAX (60 m length × 0.25 mm i.d. × 0.25 μm thickness; J&W Scientific, Folsom, CA, USA) column. Conditions for GC-O analysis were as follows: The flow rate of carrier gas (hydrogen) was 2 mL/min; the oven temperature was first increased from 40°C (6 min), ramped at 3°C/min to 100°C, and then ramped at 5°C/min to 230°C (20 min). The temperatures of the injector and FID detector temperatures were set at 250°C and 280°C, respectively. Moist air was pumped into the sniffing port at 50 mL/min to quickly remove the odorant eluted from sniffing port. Injection volume of the sample was 2 μL that was injected in a splitless mode. A group of three sensory panelists, including two females and one male, ages were 28, 25 and 31 years, respectively, were trained to evaluate the effluents.
For the volatile effluents, each panelist was asked to independently record the chemical retention time and gave an odor description. The sniffing time for each assessor was 72 min. The concentrated aromatic extract (0.5 mL) of the jujubes was stepwise diluted by dichloromethane as the solvent to obtain a series of dilutions to 1:4, 1:16, 1:64, and up to 1:256 of the original extracts. Sniffing of dilutions was continued until no odorant could be detected under the highest dilution by the sniffers in the sniffing port. Each odor was thus assigned a flavor dilution factor (FD factor) representing its greatest dilution at which that compound could be smelled for the last time. The higher FD factor of an aroma compound indicates the higher intensity of its flavor activity.
Sensory analysis
The jujubes were evaluated by a well-trained panel of 20 members (12 males and 8 females). Before the quantitative descriptive analysis, the panelists had profoundly discussed the aroma compositions of the jujubes through three preliminary sessions (each spent 2 h), until all of them had agreed to the degree of aromatic flavor. Subsequently, the organoleptic characteristic descriptors were quantified using six sensory attributes (roast, sweet, green, sour, fruity, and mellow) to evaluate the aroma defects and positive features. The complete blocks were estimated for each sample in triplicate for each treatment at random. Finally, 10 g of jujube juice was placed in a 100 mL of plastic cup covered with a Teflon cap, which was subjected to a panelist in a sensory laboratory without any peculiar smell at 25°C. The mean value of each sample was presented by the triplicate means score based on ten-point scales.
Statistical analysis
The quantitative descriptive sensory analysis was submitted to variance analysis (ANOVA). Duncan’s multiple comparison tests were applied to determine significant differences between the samples and sensory attributes using XLSTAT ver.7.5 (Addinsoft, New York, NY, USA).
Results and discussion
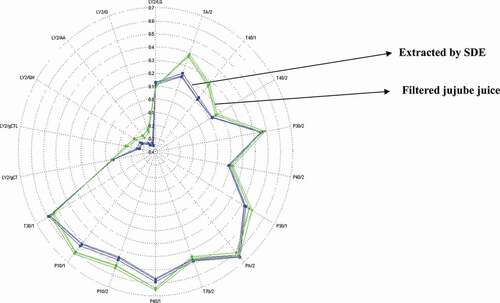
The comparison of aroma profiles between jujubes extracts and filtered juices by E-nose
Compared with sensory evaluation, the electronic nose method was more precise and objective in a certain degree from analytical point of view. Thus, the electronic nose was employed to compare the difference between the jujube extracts and its filtered juices by a radar plot. In this context, the sample Y1 was employed to evaluate the similarity of the aroma profiles of the jujube extract and its juice, which is displayed in that exhibited significant overlaps in most sensory indices. Specifically, these two samples presented similar response values in sensors P30/2, P30/1, P40/2, PA/2, T70/2, T30/1, LY2/LG, and LY2/gCT, although the response values of jujube juices to the sensors TA/2, T40/1, P10/1, and P10/2 were a little larger than those of the extracts. This indicated that the aroma profiles of these two samples were similar, which ensured the validity of the extraction method of simultaneous distillation extraction.
GC-O analysis
Results of the olfactometric analyses are listed in . Application of the aroma extract dilution analysis (AEDA) on the jujubes revealed 35, 29, 34, and 33 aroma-impact compounds (FD ≥ 4) in the Y1, Y2, Y3, and Y4 samples, respectively. Besides, the results showed that those volatile compounds had different aroma intensities in the four samples, in which the FD values of those aroma-impact compounds ranged from 4 to 256. The compound bearing the highest FD factor was (E)-2-hexenal, (E)-2-heptenal (FD = 256) for Y1, 2,3-butanedione, (E)-2-hexenal, 2,5-dimethylpyrazine, (E)-2-heptenal and β-damascenone (FD = 64) for Y2, 2,3-butanedione and β-damascenone (FD = 256) for Y3, and 2,5-dimethylpyrazine (FD = 256) for Y4, respectively.
Table 1. Aroma-impact compounds of jujube juices detected by GC-O (FD ≥ 4).
Chemical group of aldehydes is the largest class of aroma-impact compounds of the jujube samples. Their odor threshold values are generally lower than their concentrations in the sample. Thus, those volatiles have impacted the overall aroma of samples.[Citation9] Moreover, a total of 13 aldehydes were identified in jujube samples as the key aroma-impact compounds (). Particularly, the small molecular aldehydes with six to ten carbons are perceived as having green, fatty aromas. Among these compounds, hexanal, (E)-2-hexenal, (E)-2-heptenal, (E)-2-octenal, and benzaldehyde are common to all varieties of jujube.[10,11,12] Within these detected aldehydes, (E)-2-hexenal, (E)-2-heptenal, and (E)-2-octenal are the most powerful aroma-impact compounds to contribute to the typical aroma profile of jujubes. Besides, benzaldehyde, which is considered to give an almond aroma, exhibited relatively high FD among the samples. Thus, this compound is also thought to have exerted a significant influence on the aroma profiles of jujubes.
Ketones are another important class of odor-active compounds in jujube samples. A total of five aroma-impact ketone compounds were found in the samples. They were 2,3-butanedione (butter), 2,3-octanedione (earth, sweet), 2,3-pentandione (butter), 3-hydroxy-2-butanone (butter, sweet), and β-damascenone (sweet, floral). Among these volatiles, 2,3-butanedione, 3-hydroxy-2-butanone, and β-damascenone were common aroma-impact compounds in all samples. The FD factors of these compounds varied depending on cultivars. The most powerful aroma-impact ketone compound was β-damascenone. This aroma exhibited the highest FD (256) in the Y3 sample, which might be in a large degree responsible for the sweet-like and fruity odor of the sample by GC-O.
Short-chain organic acids are another chemical class containing aroma-impact compounds found in the four cultivars of jujubes, in which three aroma-impact acids were found. Among these aroma-impact organic acids, that is, butanoic acid, hexanoic acid, and octanoic acid, were the most powerful aroma-impact compounds contributing to the aroma profile of jujubes. Butanoic acid has been known for its acidic, cheese, and sweaty odors,[Citation13] which presented the highest DF (16) among the acids in Y2 sample similar phenomenon was observed in regard to the hexanoic acid and octanoic acid in the Y2 sample. Therefore, those compounds were considered to contribute characteristic acidic note to the Y2 and other jujube sample.
In addition, a total of 5 aroma-impact pyrazine derivatives were found in the samples. These were 2-methylpyrazine (roasted), 2,5-dimethylpyrazine (nutty, roasted), 2,6-dimethylpyrazine (nutty, roasted), 2-ethylpyrazine (nutty, roasted), and 2,3,5-trimethylpyrazine (nutty, roasted), which were found in all jujube samples with significantly different concentrations (). Generally, pyrazine-derived compounds present nutty and roasted aromas. During the industrial processing, the jujubes need to be dried in order to remove a large amount of water so as to decrease the water content and water activity to extend the shelf-life of jujube products. As a result, various pyrazine derivatives were formed through the Maillard reaction.[Citation13] In this study, the most powerful aroma-impact pyrazine derivatives were 2-methylpyrazine (FD=64) for Y1 and Y4, 2,5-dimethylpyrazine for Y1 (FD=64) and Y4 (FD=256), 2,6-dimethylpyrazine for Y1 (FD=64) and Y4 (FD=64), and 2,3,5-trimethylpyrazine for Y4 (FD=64), respectively.
Table 2. Result of recovery (%) and RSD (%) of two internal standards in four filtered jujube juices.
Identification of volatile compounds in jujubes by GC-MS
A recovery study of internal standards was performed to investigate the validity of the extraction method (SDE). From the , recoveries ranged from 81.5% to 93.2% for these two compounds with relative standard deviations (RSD) below 10%. The result demonstrated that this method was reliable.
Table 3. Average values (mean ± standard deviation) (μg/kg) of volatile compounds detected in filtered jujube juices.
Furthermore, the volatile compounds identified in jujube juices are presented in . Differences were observed in terms of the number of volatile compounds depending on the jujube cultivars that were harvested in different regions. A total number of 31, 31, 32, and 32 volatile compounds were identified in the four samples, respectively. Among those compounds, (E)-2-hexenal presented the highest amounts in Y1 (304.72 μg/kg), Y2 (216.15 μg/kg), and Y3 (233.61 μg/kg). While hexanal presented the highest amount in Y4 (369.05 μg/kg) samples.
According to the report by Francisca,[Citation14] only sixteen compounds were identified in four cultivars jujubes. The major compounds were aldehyde compounds, such as hexanal, octanal, and nonanal. Similarly, aldehyde compounds were considered as major volatile compounds in jujubes.[Citation15] Among those compounds, hexanal, trans-2-hexenal, and benzaldehyde presented largest amounts in jujubes. These results were mainly consistent with this research, while high-carbon acids and esters were identified as major volatile compounds in jujubes in other research.[16] The amounts of small-molecular aldehyde compounds were low. The results were different with our research. The difference might be attributed to the cultivars, climate, or soil condition. As being well known, volatile aromas with high concentrations do not always indicate that to contribute the characteristic aromas of samples, rather, the threshold values of the volatile chemicals are more critical to make the aromatic contribution.
Sensory analysis of four jujubes
Sensory evaluation was conducted by the analyses of organoleptic quality of four cultivars of jujubes, using six descriptors including “roast,” “sweet,” “green,” “sour,” “fruity,” and “mellow” attributes. The statistical analysis demonstrated that those samples showed dramatic differences in all attributes (p < 0.05) (). Remarkable differences of all sensory attributes amongst these samples also validated the differences of individual flavoring intensities in those samples, which are shown in . Nevertheless, it is worth to mention that the panelists are also a significant factor to influence the overall flavor perception, which is not unusual in the characteristic descriptive analysis. The panelists are very possibly to perceive different intensities of the same aroma, but react upon physiological diversities in personal preference such as giving central or extreme ratings, even though the panelists were trained before the sensory evaluation.[Citation7]
Figure 2. Aroma profiles of jujube juices obtained from Y1, Y2, Y3, and Y4 samples. In sensorial parameters indicated with an (*), a difference among some trials is verified for p < 0.05.
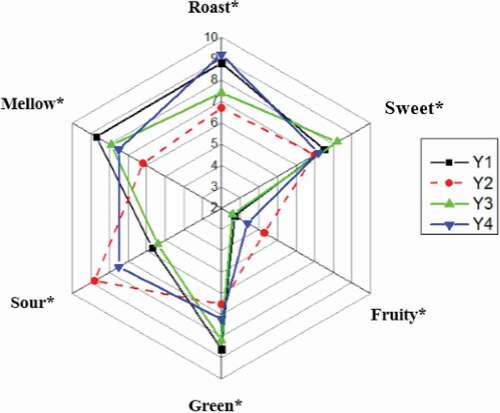
The Y1 and Y4 were accompanied by a stronger “roast” attribute than that in other samples. The major compounds involved in this aroma category included 2,5-dimethylpyrazine, 2,6-dimethylpyhhhrazine, 2-ethylpyrazine, and 2,3,5-trimethylpyrazine. It is well known that 2,5-dimethylpyrazine and 2-ethylpyrazine are typically associated with roast aromas and could be produced by the Maillard reaction during the food processing. A previous study found that these compounds are derived from amino acids and sugars.[Citation17]
The Y3 sample was accompanied by a stronger “sweet” attribute than other jujubes. This observation implied that the Y3 jujube sample might have the highest amounts of compounds able to release the “sweet” aroma. Since the “sweet” attribute is an indispensable part of a desirable flavor of jujubes, it is often an important indicator of the jujubes quality. In this study, the major aroma-impact compounds of jujubes in the “sweet” category included the following volatile chemicals, that is, 3-hydroxy-2-butanone and β-damascenone, all of which have the sweet and fruity aromas (see ).
The Y1 and Y3 samples were rated with the top two highest scores of the “green” attribute, while the Y2 sample presented the lowest score in this note. The same phenomenon was observed in “mellow” attribute. Traditionally, the “green” attribute is also considered to be typically associated with a fresh grassy or earthy flavor. It is mainly composed of aldehydes, such as hexanal, (E)-2-hexenal, (E)-2-heptenal, and (E)-2-octenal. Previous studies found that thermal processing could cause remarkable changes of volatile components in terms of their aroma profiles and quantities, particularly the loss of green aromas. Thus, some drying parameters, such as temperature and time of thermal processing, are critical in developing high quality jujubes.[Citation11]
“Sour” attribute is also a typical aroma note in jujubes. Its highest aroma intensity was found in Y2 (8.8) and the lowest one in Y3 (5.4). It was mainly composed of short-chain acidic compounds, such as butanoic acid, hexanoic acid and octanoic acid. Undoubtedly, these compounds could impact the overall aroma profile of jujubes. However, it is worth to point out that the volatile organic acids are often not positively correlated with the desirable quality of jujubes because these compounds are typically perceived as offensive and confer a negative sensory contribution to the aroma profile of jujubes, particularly when the amounts of those compounds were too high.[Citation18]
The Y1 and Y4 samples were detected to have the highest values of “fruity” attribute, while the Y2 and Y3 samples showed lower corresponding sensorial scores. Generally, the “fruity” attribute is closely related to the volatile esters, such as ethyl hexanoate and ethyl octanoate, which were detected and listed in .
Conclusion
The result presented 35, 29, 34, and 33 aroma-impact compounds (FD ≥ 4) in the Y1, Y2, Y3, and Y4 samples, respectively. The compounds bearing the highest FD factor were (E)-2-hexenal, (E)-2-heptenal (FD = 256) for Y1, 2,3-butanedione, (E)-2-hexenal, 2,5-dimethylpyrazine, (E)-2-heptenal and β-damascenone (FD = 64) for Y2, 2,3-butanedione and β-damascenone (FD = 256) for Y3, and 2,5-dimethylpyrazine (FD = 256) for Y4. In addition, sensory evaluation was performed for jujubes by using six descriptors including the “roast,” “sweet,” “green,” “sour,” “fruity,” and “mellow” attributes, by which the result showed that the Y1 was accompanied by the “green” and “mellow” attribute, Y2 with “fruity” and “sour” attribute, Y3 with a “sweet” attribute, and Y4 with a “roasted” attribute.
Funding
The research is supported in part by the Shanxi Provincial Key Scientific and Technological Project (No. 20150311020-2) and Shanxi International Collaborative Research Program (No. 2015081024) and Natural Science Foundation of Shanghai Institute of Technology (No. YYY-11067).
Additional information
Funding
References
- Gao, Q.H.; Wu, C.S.; Wang, M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. Journal of Agricultural and Food Chemistry 2013, 61, 3351−3363.
- Zheng, H.; Lu, H.F.; Zheng, Y.P.; Lou, H.Q.; Chen, C.Q. Automatic Sorting of Chinese Jujube (Zizyphus jujuba Mill. cv. ‘hongxing’) Using Chlorophyll Fluorescence and Support Vector Machine. Journal of Food Engineering 2010, 101, 402–408.
- Chen J.P.; Chan P.H.; Lam C. T.W.; Li Z.G.; Lam K. Y.C.; Yao P.; Dong T.; Lin H.Q.; Lam H.; Tsim K.W.K. Fruit of Ziziphus jujuba (Jujube) at Two Stages of Maturity: Distinction by metabolic profiling and biological assessment. Journal of Agricultural and Food Chemistry 2015, 63, 739–744.
- Jridi M.; Souissi N.; Ben Salem, M.; Ayadi M.A.; Nasri, M.; Azabou, S. Tunisian date (Phoenix dactylifera L.) by-products: Characterization and potential effects on sensory, textural and antioxidant properties of dairy desserts. Food Chemistry 2015, 188, 8–15.
- Hammouda H.; Cherif J.K.; Trabesi-Ayadi M.; Baron A.; Guyot S. Detailed polyphenol and tannin composition and its variability in Tunisian Dates (Phoenix dactylifera L.) at different maturity stages. Journal of Agricultural and Food Chemistry 2013, 61, 3252–3263.
- Manjeshwar S.B.; Bantwal R. V.B.; Shaun M.K.; Harshith P.B.; Praveen K.V. A review of the chemistry and pharmacology of the jujube fruits (Phoenix dactylifera L.). Food Research International 2015, 44, 1812–1822.
- Zhu J.C.; Chen F.; Wang L.Y.; Niu Y.W.; Shu C.; Chen H.X.; Xiao Z.B. Comparison of Aroma-Active Compounds and Sensory Characteristics of Durian (Durio zibethinus L.) Wines Using Strains of Saccharomyces cerevisiae with Odor Activity Values and Partial Least-Squares Regression. Journal of Agricultural and Food Chemistry 2015, 63, 1939–1947.
- Cheng H.; Qin Z.H.; Guo X.F.; Hu X.S.; Wu J.H. Geographical origin identification of propolis using GC-MS and electronic nose combined with principal component analysis. Food Research International 2015, 51, 813–822.
- Guth H. Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry 1997, 45, 3027–3032.
- Deng H.; Wang Y.Z.; Shi L.W.; He X.H.; Meng Y.H.; Guo Y.R. Analysis and identification of aroma components for the Qingjian date. Food Research And Development 2013, 12, 201–205.
- Mu Q.Y.; Chen J.P. Variation of volatile compounds of Chinese dates during toast. Transaction of the Chinese Society of Agriculture Engineering 2001, 17, 99–101.
- Mu Q.Y.; Chen J.P.; Zhang B.S. Identification of volatile fragrant compounds of Chinese dates by gas chromatography-mass spectrometry (GC-MS) analysis. Transaction of the Chinese Society of Agriculture Engineering 1999, 15, 251–255.
- Lee S.J.; Ahn B. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE) with sensory characterisation. Food Chemistry 2009, 114, 600–609.
- Hernández, F.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. Journal of the science of food and agricultural 2016, 96, 2682–2691.
- Galindo, A.; Noguera-Artiaga, L.; Cruz, Z.N.; Burlo, F.; Hernandez, F.; Torrecillas, A.; Carbonell-Barrachin A.A. Sensory and physicochemical quality attributes of jujube fruits as affected by crop load. LWT - Food Science and Technology 2015, 63, 899–905.
- Lu, Y.; Zhao, Z.H.; Liu, M.J. Influences of drying on the volatile compounds in Chinese jujube. Asian Journal of Chemistry 2013, 25, 3765–3768.
- Burbank H.M.; Qian M.C. Volatile sulfur compounds in Cheddar cheese determined by headspace solid-phase microextraction and gas chromatograph-pulsed flame photometric detection. Journal of Chromatography A 2005, 1066, 149–157.
- Xiao Z.B.; Liu J.H.; Che F.; Wang L.Y.; Niu Y.W.; Feng T.; Zhu J.C. Comparison of aroma-active volatiles and their sensory characteristics of mangosteen wines prepared by Saccharomyces cerevisiae with GC-olfactometry and principal component analysis. Natural Product Research 2015, 29, 656–662.