?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of the present study was to investigate the physicochemical characteristics and storage stability of water-in-oil-in-water (W/O/W) emulsions and spray-dried red ginseng extract capsules (RGEC) prepared using different secondary coating materials such as maltodextrin (MD), whey protein concentrate (WPC), or arabic gum (AG). The particle sizes of W/O/W emulsions and spray-dried RGEC coated with MD were considerably lower than those of W/O/W emulsions and spray-dried RGEC coated with WPC or AG. The zeta-potential value (−36 mV) of spray-dried RGEC coated with MD was significantly higher than that of spray-dried RGEC coated with WPC or AG (p < 0.05), indicating that spray-dried RGEC coated with MD were much more stable than spray-dried RGEC coated with WPC and AG. In the storage stability study, it was found that the refrigerator temperature (4°C) was preferred for storing spray-dried samples for a long time. Based on these results, it was confirmed that MD could be the most efficient coating material for W/O/W emulsions and nano-sized spray-dried RGEC.
Introduction
Ginseng is one of the most commonly used herbal foods and medicines, owing to its unique health benefits and functional properties in the world, especially in Asian countries today.[Citation1] Red ginseng, which is prepared by steaming and drying fresh ginseng, is claimed to have more bioactive substances than both fresh and white ginseng due to the chemical transformation of ginsenosides that takes place during processing.[Citation1,Citation2] Ginsenosides are the known main active components in ginseng, and they are typically used as marker compounds for evaluation of the quality properties.[Citation2–Citation4] Ginsenosides are dammarane-type triterpene glycosides and they can be classified into the following three groups: the protopanaxadiols, protopanaxatriols, and oleanolic acid derivatives. Among the commercial red ginseng products, the red ginseng extract has highly concentrated forms of ginsenosides, with potentially greater health benefits including antidiabetic and antiviral properties.[Citation5–Citation7] Although the presence of a number of active ingredients in red ginseng extracts is noteworthy,[Citation8,Citation9] the level of bioavailability of these substances needs to be further improved so that they can be utilized for providing health benefits.
Micro- or nanocapsules can be developed using encapsulation techniques for the protection and controlled release of the phyto-bioactive ingredients or other sensitive substances.[Citation10,Citation11] In the medical and food industries, encapsulation is used to deliver a wide range of phyto-bioactive compounds, as these substances need to be protected against heat, moisture, oxidation, and pH until they reach the desired site in the body after ingestion.[Citation12–Citation14] The encapsulated material is generally called a capsule, which consists of a core substance surrounded by a continuous matrix of the wall materials. The application of micro- or nanocapsules in the medical and food industries is a novel and innovative process. However, during the formulation of capsules, it is essential to strictly control several factors, such as the particle size and morphology, compatibility of the polymer, and the physicochemical properties of the vehicle.[Citation15–Citation17]
Water-in-oil-in-water (W/O/W) emulsions and spray-dried nanocapsules can enhance the bioavailability of the nutraceutical ingredients.[Citation15,Citation17–Citation19] Spray-drying technology is widely practiced to improve the storage quality of the encapsulated functional bioactive materials. Recently, spray-drying technology has been frequently used to manufacture micro- or nanocapsules in the medical and food industries.[Citation15] This technique involves the rapid conversion of liquid substances into powders, enabling instant drying of the thermo-sensitive products without any degradation.[Citation15] The core part of this process is the dissolution of core materials in the dispersion of the coating matrix, which is sprayed in hot air to remove water and help accumulate the powder in the outlet. To maintain a high encapsulation yield as well as stability of the micro- or nanocapsules in the spray-drying process, selection of suitable coating materials is very important.[Citation16,Citation17,Citation20–Citation22] In addition to being highly soluble in water, an ideal coating material should have a good emulsifying ability, crystallinity, low viscosity, film-forming ability, and cost effectiveness. Coating materials typically used in the spray-drying process are arabic gum (AG), maltodextrin (MD), milk protein, soy protein, modified starch, and so on.
Accordingly, the production of W/O/W emulsions and spray-dried red ginseng extracts capsules could be a valuable tactic to increase the absorption of its active components in various disease treatments.[Citation15,Citation19] However, to the best of the authors’ knowledge, none of the studies have apparently reported about the production of W/O/W emulsions and red ginseng extract capsules (RGECs), nor have they elucidated the physicochemical properties and storage stability of the final products. In the present study, W/O/W emulsions of red ginseng extracts were prepared with the high-energy emulsification-evaporation method, and the obtained W/O/W emulsions were used to produce spray-dried red ginseng extract micro- or nanocapsules. The main objective of the present study was to investigate the physicochemical characteristics and storage stability of W/O/W emulsions and spray-dried RGECs prepared using different secondary coating materials such as MD, WPC, or AG.
Materials and methods
Materials
Red ginseng extract as a core material was provided by Korea Bio Red Ginseng Co., Ltd. (Geumsan, Korea). Medium-chain triglyceride (MCT) as a primary coating material was purchased from Wellga Co., Ltd. (Seongnam, Korea). As secondary coating materials, MD (DE 18, Samyang Genex, Seoul, Korea), AG (Samchun Pure Chemical Co., Ltd., Pyeongtaek, Korea), or whey protein concentrates (WPCs) (Davisco Foods International, Eden Prairie, MN, USA) were used. As emulsifiers, polyglycerol polyricinoleate (PGPR, HLB 0.6) and polyoxyethylene sorbitan monolaurate (PSML, HLB 16.7) (95.0% purity) were obtained from Il-Sin Co., Ltd. (Seoul, Korea). Ethyl acetate (99.5% purity), n-hexane (95.0% purity), and sodium benzoate (99.0% purity) were purchased from Samchun Chemicals Co., Ltd (Pyeongtaek, Korea). Oil Red O was obtained from Sigma–Aldrich (St. Louis, MO, USA) and all chemical solvents were of reagent grade unless otherwise specified.
Preparation of W/O/W emulsion and spray-dried RGECs
To produce spray-dried RGEC, W/O/W emulsions of red ginseng extracts were first prepared using the high-energy emulsification-evaporation method. Red ginseng extracts (the core material) were dissolved in distilled water (0.8 g/mL), filtered through a membrane paper (Whatman No. 4), and combined with 0.02% sodium benzoate to prevent the degradation of red ginseng extracts. The primary emulsions (W/O) of red ginseng extracts were produced using MCT (the primary coating material). To produce the W/O emulsions, the ratio of the core to the coating material was 2:8, and 10% PGPR (HLB 0.6) was used as the primary emulsifier. The coating material was mixed with the emulsifier at 400 rpm and 50°C for 30 min by using a hotplate stirrer (HSD 150-03P; Misung Scientific Co., Ltd, Yangju, Korea), and then the core material and ethyl acetate (as a solvent in the coating material and surfactant mixture) were added. The mixture was homogenized using a high-speed homogenizer (Matsushita Electric Industrial Co. Ltd., Tokyo, Japan) at 12,000 rpm for 5 min to produce the W/O emulsion.
Secondary emulsions (W/O/W) were prepared with W/O emulsions and 10% MD, AG, or WPC as the secondary coating materials. To produce W/O/W emulsions, the ratio of the W/O emulsion to the coating material was 1:16, and 10% PSML (HLB 16.7) was used as the secondary emulsifier. The secondary coating materials were mixed with emulsifiers at 400 rpm and 50°C for 30 min using a hotplate stirrer, and then the W/O emulsions were added. The mixture was homogenized to form W/O/W emulsions using a high-speed homogenizer at 12,000 rpm for 5 min. Finally, ethyl acetate was removed by a rotary evaporator (EYELA, Tokyo Rikakikai Co., Ltd, Tokyo, Japan) at 50°C for 30 min.
Finally, to produce spray-dried RGEC, W/O/W emulsions were spray-dried using a two-way nozzle-spray dryer (EYELA SD-1000, drying capacity 1,500 mL/h; Tokyo Rikakikai Co. Ltd., Tokyo, Japan) in which the inlet and outlet temperatures, feed rate, and inlet air volume were controlled. In this dryer, the W/O/W emulsion was fed by a peristaltic pump and sprayed into the drying chamber. Inlet- and outlet-air temperatures of the spray dryer were fixed at 170°C and 85°C, respectively. Cyclone-separated spray-dried RGECs were prepared with the W/O/W emulsion from the air–powder mix. The dried powder was collected and stored in opaque airtight containers at 4°C until further analysis.
Particle size
Particle size distribution of the W/O/W emulsions and spray-dried RGEC was determined by a particle size analyzer (Delsa™ Nano Particle Size, Beckman Coulter, Inc., Fullerton, CA, USA), which employs laser diffraction for size measurement. Briefly, W/O/W emulsions and spray-dried RGEC were combined with 10 mL of deionized water at a ratio of 1:100 and mixed using a magnetic stirrer at 25°C. The suspension was poured into a cuvette and its particle size was measured at 25°C at a fixed scattering angle of 90°. All samples were measured in triplicate.
Moisture content and encapsulation yield
Spray-dried RGECs were analyzed for their moisture content using the AACCI Approved Method 44-15A (AACCI, 2000).[Citation23] To measure the yield of spray-dried RGEC indirectly, the encapsulation yield of spray-dried RGEC was determined according to the modified method proposed by Baik et al.[Citation24] The yield was determined, using Oil Red O as the marker compound, by UV–visible spectrophotometry (Beckman Coulter Inc., Fullerton, CA, USA). A wavelength of 500 nm was used to detect the absorbance of Oil Red O, which was prepared with mixtures of the spray-dried RGEC containing Oil Red O in hexane. The experiment was performed by suspending samples (5 g) in 10 ml of hexane. The suspensions were then mixed using a magnetic stirrer (HSD 150-03P; Misung Scientific Co., Ltd, Yangju, Korea) at 250 rpm for 5 min. The resulting suspensions were centrifuged at 3,000 rpm and 20°C for 10 min, and the supernatants were collected for UV analysis. The yield was determined by the following equation:
where A is the absorbance of Oil Red O isolated from the spray-dried RGEC layer, and B is the absorbance of Oil Red O added in MCT.
Zeta-potential
Zeta-potential was determined to assess the electrical stability of W/O/W emulsions and spray-dried RGEC using a particle size analyzer. A sample of 0.3 g was diluted with 30 mL of deionized water and 1 mL of the diluted sample was injected into a flow cell. All measurements were performed in triplicate at a fixed temperature of 25°C and an angle of 15°, respectively. Zeta-potential was measured at the following four different cell positions: 0.7, 0.35, −0.35, and −0.7 .
Storage stability
Storage stability (%) is the relative amount of red ginseng extract remaining in the powder with respect to the initial amount in the powder during storage. To determine the storage stability of spray-dried RGEC, the method proposed by Hardas et al. was used.[Citation25] Around 2 g of each sample was poured into a 4 mL brown vial and stored in a temperature-controlled incubator with 20% RH (Programmable Temperature Chamber, EN-STC-150, Koyangsi, Kyunggido, Korea) at 4°C, 25°C, 45°C, and 60°C for 0, 2, 4, 6, 8, 10, and 12 weeks. After the desired temperature and storage time were reached, 1 g of the sample in each vial was collected for subsequent analysis. The storage temperature-related stability of spray-dried RGEC was analyzed according to the yield of spray-dried RGEC during storage. All samples were measured in triplicate.
Statistical analysis
Analysis of variance (ANOVA) and Duncan’s multiple test were performed to analyze the differences between groups. Data was expressed as mean±SD and statistical significance was set at p < 0.05. All statistical analyses were performed using SAS 9.0 (SAS Institute Inc., Cary, NC, USA) and the graphical representations were performed using Sigmaplot 10.0 (Systat Software Inc., San Jose, CA, USA).
Results and discussion
Particle size
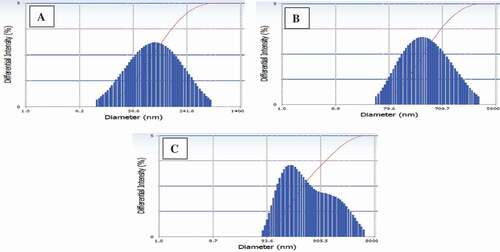
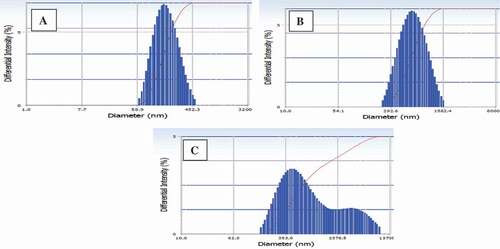
The particle sizes of W/O/W emulsions and spray-dried RGEC prepared using different secondary coating materials (MD, WPC, or AG) are illustrated in and , respectively. Prior to the spray-drying process, the particle size distributions of W/O/W emulsions coated with MD showed the nano-size only among all the samples (). The particle size distributions of spray-dried RGEC coated with MD, WPC, and AG ranged from 100 to 500 nm, 100 nm to 1 µm, and 300 nm to 3 µm, respectively (). The present study confirmed that the particle size distribution of W/O/W emulsions and spray-dried RGEC coated with MD showed nanoparticles only. The present results were in agreement with those presented by Saloko et al.,[Citation26] who indicated that the nanocapsules coated with MD showed a smaller size (14 nm) than the nanocapsules coated with a mixture of chitosan and MD. In addition, the capsules coated with MD had a higher stability of the core ingredient than the capsules coated with AG due to the smaller particle size of the capsules coated with MD.[Citation27] Therefore, in the present study, it was suggested that MD can be the most efficient secondary coating material among all the samples due to the smaller particle size and improved stability.
Moisture content and encapsulation yield
Moisture contents of spray-dried RGEC coated with the different secondary coating materials are listed in . The moisture contents of spray-dried RGEC coated with MD, WPC, and AG were 1.80%, 1.36%, and 2.62%, respectively. The moisture contents of spray-dried RGEC coated with MD and WPC were significantly lower than that of spray-dried RGEC coated with AG (p < 0.05). Similarly, Hoffmeister et al.[Citation28] reported that the moisture content of the spray-dried capsules coated with MD was 1.7 ± 0.3%. Therefore, in the present study, spray-dried RGECs coated with MD and WPC were more stable than spray-dried RGECs coated with AG due to a decrease in the moisture content.
Table 1. Moisture content and encapsulation yield of spray-dried RGEC coated with different secondary coating materials
The encapsulation yields of spray-dried RGECs coated with MD, WPC, and AG were 93.05 ± 1.51%, 97.33 ± 1.03%, and 86.67 ± 1.35%, respectively (). Chegini and Ghobadian[Citation29] reported that the encapsulation yield of orange juice after the spray-drying process depends on the operating variables such as inlet air temperature, feed ratio, atomizer speed, and so on. In the study by Gonnissen et al.,[Citation30] the yield of the spray-drying process of encapsulated acetaminophen by MD was about 82% and a non-sticky powder was obtained. Li et al.[Citation15] also indicated that the yields of the spray-dried particles coated with 10% whey protein and 1% AG were 89.4% and 74.7%, respectively.
Zeta-potential
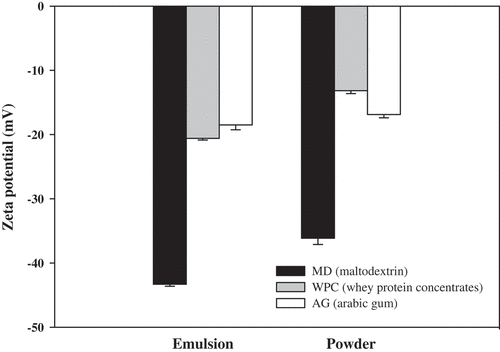
Zeta-potential can be a critical and generally accepted parameter to specify the stability of a colloid system. That is, all of the particles get charged either negatively or positively probably because of the selective adsorption of ions in the colloidal system.[Citation31] The values of the zeta-potential for assessing the electrical stability of W/O/W emulsions and spray-dried RGEC were obtained by the dynamic light scattering method, as shown in . In the zeta-potential analysis, the dividing line between stable and unstable suspensions is generally taken at either +30 mV or −30 mV. Particles with zeta-potentials more positive than +30 mV or less negative than −30 mV are normally considered as stable micro- or nanocapsules.[Citation32] In the present study, the zeta-potential values of the W/O/W emulsion coated with MD, WPC, and AG were −43 mV, −20 mV, and −18 mV, respectively, and the values of spray-dried RGEC coated with MD, WPC, and AG were −36 mV, −13 mV, and −16 mV, respectively. Therefore, W/O/W emulsions and spray-dried RGEC coated with MD were much more stable than the W/O/W emulsions and spray-dried RGEC coated with WPC and AG. Based on the findings of the particle size and zeta-potential obtained in the present study, it was apparently observed that the optimum secondary coating material for the production of W/O/W emulsions and spray-dried RGEC can be MD, and therefore, MD was used for subsequent studies on the storage stability of spray-dried RGEC.
Storage stability
The storage stability of spray-dried RGEC coated with MD at different storage temperatures (4°C, 25°C, 45°C, and 60°C) during 12 weeks is presented in . In all the samples studied, storage stability significantly decreased with an increase in the storage period from 0 to 12 weeks (p < 0.05). Similarly, Ersus and Yurdagel[Citation33] indicated that the contents of anthocyanin in a microencapsulated powder coated with MD were decreased by 11% and 33% during the storage period of 64 days at 4°C and 25°C, respectively. In the present study, spray-dried RGEC stored at a temperature of 4°C for 12 weeks exhibited the highest value (78.69%) of storage stability, followed by the temperatures of 25°C, 45°C, and 60°C. Thus, the present study demonstrated that lower temperatures are preferred for storing spray-dried RGEC for a long time.
Table 2. Effect of temperature on the stability of spray-dried RGEC coated with maltodextrin (MD) during 12 weeks
Conclusions
This study confirmed that W/O/W nanocapsules of RGEC coated with MD can be efficiently prepared using the spray-drying technique. In the particle size analysis, spray-dried RGEC coated with MD ranged from 100 to 500 nm. Furthermore, it was indicated that MD was the most efficient secondary coating material for spray-dried RGEC because RGEC coated with MD exhibited the highest zeta-potential value (−36 mV). In the storage stability study, it was found that the refrigerator temperature (4°C) can be more effective in enhancing the storage stability of the capsules coated with MD. Based on the results obtained from the present study, spray-dried RGEC coated with MD exhibited a nanosize and high stability during storage at a lower temperature (4°C). Therefore, W/O/W emulsions and spray-dried RGEC coated with MD could be used to load other bioactive compounds and active food ingredients for various applications.
References
- Chang, Y. H.; Ng, P. K. W. Extraction of Ginsenosides from a Blend of Wheat Flour and Ginseng Powder. Food. Chem. 2009, 115, 1512–1518. DOI: 10.1016/j.foodchem.2009.01.039.
- Chang, Y. H.; Ng, P. K. W. Effects of Extrusion Process Variables on Extractable Ginsenosides in Wheat-Ginseng Extrudates. J. Agric. Food. Chem. 2009, 57, 2356–2362. DOI: 10.1021/jf8031827.
- Mi, J.; Zhang, M.; Ren, G.; Zhang, H.; Wang, Y.; Hu, P. Enriched Separation of Protopanaxatriol Ginsenosides, Malonyl Ginsenosides and Protopanaxadiol Ginsenosides from Panax Ginseng Using Macroporous Resins. J. Food. Eng. 2012, 113, 577–588. DOI: 10.1016/j.jfoodeng.2012.06.027.
- Corbit, R. M.; Ferreira, J. F. S.; Ebbs, S. D.; Murphy, L. L. Simplified Extraction of Ginsenosides from American Ginseng (Panax Quinquefolius L.) for High-Performance Liquid Chromatography-Ultraviolet Analysis. J. Agric. Food. Chem. 2005, 53, 9867–9873. DOI: 10.1021/jf051504p.
- Lee, M. H.; Lee, B. H.; Jung, J. Y.; Cheon, D. S.; Kim, K. T.; Choi, C. Antiviral Effect of Korean Red Ginseng Extract and Ginsenosides on Murine Norovirus and Feline Calicivirus as Surrogates for Human Norovirus. J. Ginseng. Res. 2011, 25, 429–435. DOI: 10.5142/jgr.2011.35.4.429.
- Park, M. Y.; Lee, K. S.; Sung, M. K. Effects of Dietary Mulberry, Korean Red Ginseng, and Banaba on Glucose Homeostasis in Relation to PPAR-alpha, PPAR-gamma, and LPL mRNA Expressions. Life. Sci. 2005, 77, 3344–3354. DOI: 10.1016/j.lfs.2005.05.043.
- Hong, Y. J.; Kim, N.; Lee, K.; Sonn, C. H.; Lee, J. E.; Kim, S. T.; Baeg, I. H.; Lee, K. M. Korean Red Ginseng (Panax Ginseng) Ameliorates Type 1 Diabetes and Restores Immune Cell Compartments. J. Ethnopharmacol. 2012, 144, 225–233. DOI: 10.1016/j.jep.2012.08.009.
- An, Y. E.; Ahn, S. C.; Yang, D. C.; Park, S. J.; Kim, B. Y.; Baik, M. Y. Chemical Conversion of Ginsenosides in Puffed Red Ginseng. LWT Food Sci. Technol. 2011, 44, 370–374. DOI: 10.1016/j.lwt.2010.09.013.
- Wang, H. Y.; Hua, H. Y.; Liu, X. Y.; Liu, J. H.; Yu, B. Y. In Vitro Biotransformation of Red Ginseng Extract by Human Intestinal Microflora: Metabolites Identification and Metabolic Profile Elucidation Using LC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2014, 98, 296–306. DOI: 10.1016/j.jpba.2014.06.006.
- Chang, M. W.; Stride, E.; Edirisinghe, M. Stimulus-Responsive Liquids for Encapsulation Storage and Controlled Release of Drugs from Nano-Shell Capsules. J. Royal. Soc. Interface. 2011, 8, 451–456. DOI: 10.1098/rsif.2010.0428.
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M. J.; Ros, G.; Lagaron, J. M.; López-Rubio, A. Encapsulation of Folic Acid in Food Hydrocolloids through Nanospray Drying and Electrospraying for Nutraceutical Applications. Food. Chem. 2015, 168, 124–133. DOI: 10.1016/j.foodchem.2014.07.051.
- Augustin, M. A.; Hemar, Y. Nano- and Micro-Structured Assemblies for Encapsulation of Food Ingredients. Chem. Soc. Rev. 2009, 38, 902–912. DOI: 10.1039/B801739P.
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y. D. Casein Micelle as a Natural Nano-Capsular Vehicle for Nutraceuticals. Food. Hydrocollods. 2007, 21, 936–942. DOI: 10.1016/j.foodhyd.2006.09.006.
- Fathi, M.; Martin, A.; McClements, D. J. Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems. Trends. Food. Sci. Technol. 2014, 39, 18–39. DOI: 10.1016/j.tifs.2014.06.007.
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T. F. Nanoparticles by Spray Drying Using Innovative New Technology: The Büchi Nano Spray Dryer B-90. J. Controlled. Release. 2010, 147, 304–310. DOI: 10.1016/j.jconrel.2010.07.113.
- Lee, S. H.; Heng, D.; Ng, W. K.; Chan, H. K.; Tan, R. B. H. Nano Spray Drying: A Novel Method for Preparing Protein Nanoparticles for Protein Therapy. Int. J. Pharm. 2011, 403, 192–200. DOI: 10.1016/j.ijpharm.2010.10.012.
- Schafroth, N.; Arpagaus, C.; Jadhav, U. Y.; Makne, S.; Douroumis, D. Nano and Microparticle Engineering of Water Insoluble Drugs Using a Novel Spray-Drying Process. Colloids. Surf. B. 2012, 90, 8–15. DOI: 10.1016/j.colsurfb.2011.09.038.
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients-A Review. Food. Bioprocess. Technol. 2012, 5, 3–14. DOI: 10.1007/s11947-011-0638-z.
- Sosnik, A.; Seremeta, K. P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid. Interface. Sci. 2015, 223, 40–54. DOI: 10.1016/j.cis.2015.05.003.
- Sham, J. O. H.; Zhang, Y.; Finlay, W. H.; Roa, W. H.; Löbenberg, R. Formulation and Characterization of Spray-Dried Powders Containing Nanoparticles for Aerosol Delivery to the Lung. Int. J. Pharm. 2004, 269, 457–467. DOI: 10.1016/j.ijpharm.2003.09.041.
- Patel, R. P.; Patel, M. P.; Suthar, A. M. Spray Drying Technology: An Overview. Indian. J. Sci. Technol. 2009, 2, 44–47.
- Sollohub, K.; Cal, K. Spray Drying Technique: II. Current Applications in Pharmaceutical Technology. J. Pharm. Sci. 2010, 99, 587–597. DOI: 10.1002/jps.21963.
- American Association of Cereal Chemists (AACC). Approved Method of the AACC, Methods 44-15A 10th ed; AACC International: St. Paul, MN, USA, 2000.
- Baik, M. Y.; Suhendro, E. L.; Nawar, W. W.; McClements, D. J.; Decker, E. A.; Chinachoti, P. Effects of Antioxidants and Humidity on the Oxidative Stability of Microencapsulated Fish Oil. J. Am. Oil. Chemists. Soc. 2004, 81, 355–360. DOI: 10.1007/s11746-004-0906-7.
- Hardas, N.; Danviriyakul, S.; Foley, J.; Nawar, W.; Chinachoti, P. Accelerated Stability Studies of Microencapsulated Anhydrous Milk Fat. LWT Food Sci. Technol. 2000, 33, 506–513. DOI: 10.1006/fstl.2000.0696.
- Saloko, S.; Darmadji, P.; Setiaji, B.; Pranoto, Y. Antioxidative and Antimicrobial Activities of Liquid Smoke Nanocapsules Using Chitosan and Maltodextrin and Its Application on Tuna Fish Preservation. Food. Biosci. 2014, 7, 71–79. DOI: 10.1016/j.fbio.2014.05.008.
- Barbosa, M. I. M. J.; Borsarelli, C. D.; Mercadante, A. Z. Light Stability of Spray-Dried Bixin Encapsulated with Different Edible Polysaccharide Preparations. Food. Res. Int. 2005, 38, 989–994. DOI: 10.1016/j.foodres.2005.02.018.
- Hoffmeister, C. R.; Durli, T. L.; Schaffazick, S. R.; Raffin, R. P.; Bender, E. A.; Beck, R. C.; Pohlmann, A. R.; Guterres, S. S. Hydrogels Containing Redispersible Spray-Dried Melatonin-Loaded Nanocapsules: A Formulation for Transdermal-Controlled Delivery. Nanoscale. Res. Lett. 2012, 7, 1–13. DOI: 10.1186/1556-276X-7-251.
- Chegini, G. R.; Ghobadian, B. Effect of Spray-Drying Conditions on Physical Properties of Orange Juice Powder. Dry. Technol. 2005, 23, 657–668. DOI: 10.1081/DRT-200054161.
- Gonnissen, Y.; Remon, J. P.; Vervaet, C. Development of Directly Compressible Powders via Co-Spray Drying. Eur. J. Pharmaceutics. Biopharmaceutics. 2007, 67, 220–226. DOI: 10.1016/j.ejpb.2006.12.021.
- Lin, C. H.; Chaudhury, M. K. Using Electrocapillarity to Measure the Zeta Potential of a Planar Hydrophobic Surface in Contact with Water and Nonionic Surfactant Solutions. Langmuir. 2008, 24, 14276–14281. DOI: 10.1021/la8027572.
- Couvreur, P.; Barratt, G.; Fattal, E.; Legrand, P.; Vauthier, C. Nanocapsule Technology: A Review. Crit. Rev. Ther. Drug. Carrier. Syst. 2002, 19, 99–134. DOI: 10.1615/CritRevTherDrugCarrierSyst.v19.i2.10.
- Ersus, S.; Yurdagel, U. Microencapsulation of Anthocyanin Pigments of Black Carrot (Daucus Carota L.). By Spray Drier. Journal of Food Engineering. 2007, 80, 805–812. DOI: 10.1016/j.jfoodeng.2006.07.009.