ABSTRACT
Cloud stability of the juice is affected by pectin methylesterase (PME) activity. Come up time (CUT) of thermal treatment showed inactivation effect on PME. In this study, lacunarity and fractal dimension (FD) were used to evaluate the effect of CUT on PME activity and appearance of the sour orange juice. The CUT lasted 2.68, 2.58, 2.51, 2.46, 2.43, 2.33, and 2.28 min at 60, 65, 70, 75, 80, 85, and 90°C, respectively. The fractal dimension and lacunarity were estimated using the box counting method. PME activity, cloud value and lightness were measured according to Kimball, spectroscopy methods, and image processing, respectively. Higher PME inactivation and cloud value and lower lightness of the juice were obtained at a higher temperature. FD showed a high correlation with changes in PME activity, cloud, and lightness of the juice. Lacunarity was used to describe the shape and gap distribution within the image. As the combination of FD and lacunarity was useful in the study of the image, these factors could be applied to evaluate the effect of CUT on some of the physical and chemical properties of fruit juice.
Introduction
Sour orange (Citrus aurantium L), also known as bitter, marmalade, Seville or bigarade orange, is commonly grown in different parts of Asia like Iran. Despite the bitter and sour taste, its juice is used as a seasoning in different food products and salads. Sour orange, like other citrus, is an important source of nutritional compounds including antioxidants such as ascorbic acid, flavonoids, and phenolic compounds.[Citation1] Antioxidants have an important role in human body protection against a variety of diseases, particularly cardiovascular ones.[Citation2] Hence, daily consumption of the citrus product has positive effects on body health.[Citation3]
Turbidity or cloudy appearance positively influences colour and organoleptic properties of the citrus juice.[Citation4] The membrane and high molecular weight compounds, such as pectin, are suspended in the juice as the endocarp cells are ruptured during the mechanical extraction methods.[Citation5] Pectin, a sugar-acid derivative polymer, has a small portion of the cloud material, but its effect on the cloud stability of the juice is undeniable. Pectin is rich in galacturonic acid units linked together via glycoside bonds with side chains of sugars. Lots of the carboxyl groups are esterified with methanol to form methoxy groups.[Citation5] Pectin methylesterase (PME), also known as pectinase, pectin esterase, and pectin methoxylase, naturally exists in the citrus juice.[Citation6] This enzyme de-esterifies the methoxylated pectin which causes the cloud loss in juice. Pasteurisation treatment at temperature between 60°C to 100°C is required to guarantee the safety of high acid food products (pH 4.5) such as citrus juice.[Citation7] Pasteurisation condition is sufficient to control the microbial and enzymatic spoilage of these kinds of food products. An index is needed to make sure about quality and safety of the pasteurised foodstuffs. Besides the effect on cloud stability, PME is introduced as pasteurisation index in citrus products due to its higher thermal resistance than target microorganisms.[Citation8]
Inactivation of microorganisms and enzymes such as PME generally occurs during come up time (CUT) and holding time. Ball (Citation1923) suggested that 42% of CUT has this inactivation effect.[Citation9] Tajchakavit and Ramaswamy (1997) calculated the effectiveness percentage of CUT on PME inactivation between 46 and 58% during thermal processing of orange juice at 70 to 90°C.[Citation10] Hence, considering the effect of this stage in thermal processing is necessary to prevent from overheating of the product and subsequently energy wasting, organoleptic and nutritional losses to enhance the consumers-touting.
Visual appearance is the first aspect of the food quality which affects the consumer’s judgement to buy a product.[Citation11] The colour is one of the important visual properties of food products that would be changed by physicochemical processes during thermal treatments like non-enzymatic browning[Citation12,Citation13] and enzymatic reactions.[Citation14] As the colour of food product is a concern for food manufacturers and customers, its changes can be used as an indicator to evaluate the extent of deterioration and quality of the food during different processing.[Citation14] In addition, the colour measurement is simpler and faster than lots of chemical analyses. Physical indicators based on the Hunter Lab parameters are beneficial to study the colour changes of food products. Considering environmental conditions and personal health, evaluation of the colour changes based on human eye vision is not accurate.[Citation15] So, computer vision is an effective method in visual colour prediction during different food processing situations.[Citation16,Citation17] Occurrence of chemical reactions and changes in physical aspects influence the colour of food;[Citation18] hence, this method is also useful to evaluate the correlation between colour parameters of the food product and its physicochemical properties.[Citation12] For example, researchers found that thermal processing resulted in a decrement in lightness (L*-value) due to changes in PME content in pineapple puree, pineapple juice, blended orange–carrot juice, and grapefruit juice.[Citation14,Citation16,Citation17,Citation19]
Fractal is another useful method which is used to quantify the food colour and structural changes.[Citation20–Citation23] In the 1960s, Mandelbrot used the ‘fractal’ to indicate objects whose complex geometry can’t be simply described by Euclidean dimension.[Citation24] Fractal dimension (FD) shows the degree of ruggedness and geometric complexities of both natural and synthetic particles.[Citation25] The more heterogeneous or irregular structure has the higher FD, but the more homogeneous or smooth one has the lower FD. Lacunarity describes the homogeneity of an image, and it is defined as ‘gappiness’ and visual texture in the morphological analysis which characterises the difference between two images having similar FD.[Citation26] In fact, FD just describes the filled space in the image; while, lacunarity describes the distribution of the sizes of gaps or lacunae of the image. Both of these factors (FD and lacunarity) are used to identify an appropriate food processing condition by considering the physical and microstructural changes of the products. Study on FD and lacunarity of pre-sliced pork ham revealed irregularity and complexity of texture dependent on its formulation and processing conditions.[Citation23] FD was used to study the changes in the intensity of the redness colour in meat during oxidation as an indicator of alterations in the non-homogenous distribution of the colours associated with redness intensity and content of metmyoglobin and Thiobarbituric acid reactive substances (TBARS).[Citation25] It was also found that FD could be applied to describe the enzymatic browning of avocado puree and slices of pear, apple, banana, and avocado.[Citation20–Citation22,Citation27,Citation28] The formed fractal structures by aggregation of colloidal particles were also applied to understanding the structure, aggregation, and stability of particles that are responsible for the cloudy appearance of apple juice.[Citation29] FD and lacunarity were studied to evaluate the structural changes in quality of the fresh papaya sliced during environmental conditions.[Citation30] Rising in FD and reduction in lacunarity were found due to exposure of the sliced papaya to environmental conditions and breakdown of tissue after cutting the fruit which resulted in physicochemical changes such as carotene stability reduction.[Citation30] These factors were also applied in quantifying the muskmelon epidermis netting and introduced as appropriate tools in characterising the heterogeneity of fruit epidemic netting.[Citation31]
Since there is no evidence for the effect of CUT on FD and lacunarity changes in sour orange juice during thermal processing and also by considering the importance of PME inactivation and cloud stabilisation as an essential technological step in citrus juice processing, the aim of this study was to investigate the changes of FD and lacunarity as result of PME inactivation, cloud stability, and also colour changes of sour orange juice in CUT.
Materials and methods
Samples preparation
Sour orange (Citrus aurantium L.) was purchased from a local market (Gorgan, Iran) and kept at 4°C until the experiments were carried out. The juice was extracted from the washed and squeezed fruits by using a domestic juicer. It was then filtered using a sieve with mesh size 170 (pore size of 0.09 mm) to remove large size particles.
Thermal processing
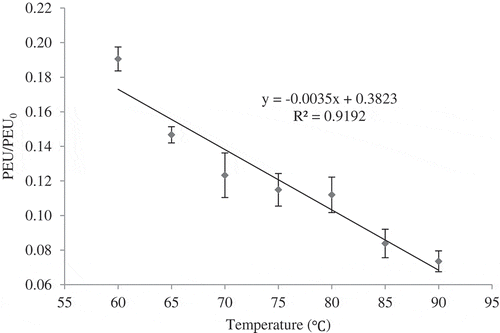
Thermal treatments were carried out in a water bath (WNB-22, Memmert, Germany) at selected temperatures. The CUT lasted 2.68, 2.58, 2.51, 2.46, 2.43, 2.33, and 2.28 min at 60, 65, 70, 75, 80, 85, and 90°C, respectively. 15 ml of the prepared juice sample was sealed in a test tube (15 mm outer diameter, 160 mm length, and 1 mm thickness). Time–temperature data of sample during heating were recorded using a data logger (TC-08, Pichotechnology Co, UK) and a 1 mm diameter copper–constant thermocouple (T-type) placed in the cold point of the test tube containing the juice. Finally, the sample was cooled to 25°C in an ice-water bath to minimise the effect of cooling time on the enzyme inactivation.
Physical chemical analyses
Total acidity, pH, soluble solids, moisture content, and total ash were determined in triplicate.[Citation32] Total acidity was determined by titration the juice with NaOH 0.1 N solution using phenolphthalein as an indicator and expressed as citric acid percentage. pH was also measured using pH-meter (W3B, BEL, Italy) at 25°C. Total soluble solids were determined by a refractometer (UV/VIS80 + T, PG Instrument, US) at 25°C that expressed as °Brix. The moisture content of sour orange juice was measured by drying the sample to a constant weight at 105°C in the oven (FD53, Binder, Germany). The ash content was measured by burning the juice samples in hot air furnace in a silica crucible at 525°C up to a constant weight. The ash content was calculated by the difference in weight and expressed in percentage based on a dry basis. Percentage of pulp content was measured according to Kimball (Citation1991) method by measuring the pulp volume of centrifuged juice at 1500 rpm for 10 min.[Citation5]
Measurement of PME activity
To measure the PME activity, 5 ml of sour orange juice was mixed with 20 ml of 1% pectin-salt solution (10 g pectin and 15.3 g NaCI diluted in 1 L distilled water) and incubated at 30°C. The pH of the solution was adjusted to 7 with NaOH (2 N). Then, the pH of the solution was readjusted to 7.7 with NaOH (0.05 N). Finally, 0.1 ml of NaOH (0.05 N) was added, and the time taken to regain pH 7.7 was recorded.[Citation5] The enzyme activity unit (PEU) was calculated according to Eq. (1) :[Citation5]
Measurement of cloud value
5 ml of sour orange juice was centrifuged at 3000 rpm for 10 min at room temperature. Cloud value was measured as supernatant absorbance at 660 nm (T-80, UV/VIS Double Beam Spectrophotometer).[Citation33] The absorbance of distilled water was considered as a blank.
Measurement of visual colour
Colour changes of sour orange juice at the end of the CUT were analysed by image processing method. 15 ml of processed juice was filled in the plate (with 1 cm height and 9 cm diameter), and its picture was taken by a scanner (Scanjet G2710, HP, USA), which was completely by a black cover. The taken pictures were saved as JPEG format and 600dpi resolution (RGB colour). The pictures were rectangularly cropped (950 × 950 pixel) and analysed by Image J software (version 1.47) to evaluate the lightness of the sample (L*-value).
Analysis of fractal dimension and lacunarity
The fractal dimension of each image was calculated by the box counting method via the Image J software (version 1.47).[Citation34] The box-counting method, the most frequently used method, was applied to compute the FD of a signal. FD is measured by counting the number of boxes needed to cover the 2-D image with a range of boxes of size. By plotting the log of the number of required boxes to cover the signal completely versus the log of the actual box size, the FD is obtained from the slope of log-log plot as Eq. (2):
Where the spatial location (x, y) of the image is covered by N(r) number of boxes having r size.[Citation30] To compare the changing values of the fractal dimension of a sample in CUT, the normalised fractal dimensions were reported as Eq. (3):[Citation35]
Where FD0 and FD are the fractal dimensions of fresh and processed juice sample, respectively. Lacunarity studied using Image J software was based on box counting method. This was calculated as the standard deviation ( divided by the square of mean (
) for size of boxes (r). In this study, it was evaluated with a combination of the boxes having fixed size (2–128 pixels).[Citation30]
Statistical analysis
All experiments were performed in triplicate, and the presented results are the mean of the obtained values. The average of three experiments values at each test condition was used for regression analysis (Excel, Microsoft Office 2010). All statistical analyses were conducted using the SAS software (version 9.1). Results were submitted to analysis of variance (ANOVA) of the two factors (time and temperature) with a significance level of P < 0.05.
Result and discussion
Effect of CUT on PME activity
shows the properties of fresh sour orange juice. Since this juice is categorised in high acid food products (pH = 3.01), the temperature below 100°C is adequate to inactivate the vegetative bacteria (spoilage and pathogens types) and enzyme to guarantee its safety. PME activity in fresh sour orange juice was 0.00045 PEU. As shows, the ratio of PME activity (PEU/PEU0) decreased insignificantly (P > 0.05) and linearly by a high coefficient of determination (R2 > 0.91) as the temperature rose in CUT. Similar to most of the other enzymes, PME has protein structure so it is sensitive to thermal treatment. In the beginning of the thermal processing, around 80.94% reduction in PME activity was observed by rising in the temperature from 25°C to 60°C. Thereafter, by 5°C change in temperature (between 60°C and 90°C), PME inactivation continued gradually. These changes could be the result of breaking up the hydrogen bonds, unfolding the tertiary protein structure and thermal deamidation of amino acid in the enzyme configuration, since protein denaturation mainly takes place at about 40°C.[Citation36] Our results are in agreement with other studies that showed a decrement in PME activity during thermal processing of different citrus juice.[Citation6,Citation8]
Table 1. Some physicochemical properties of fresh sour orange juice.
Effect of CUT on cloud stability
shows the absorbance at 660 nm of sour orange juice measured at the end of CUT. The absorbance of fresh sour orange juice was 0.444. Generally, by rising in temperature of CUT, the turbidity of sour orange juice increased without any order (P > 0.05), since the de-esterification of the methoxylated pectin decreased as a result of PME inactivation. It was reported that the orange juice became more turbid by PME inactivation.[Citation37] Also, during thermo-sonication treatment of grapefruit juice, the cloud value was promoted because the breaking up of the larger cloud particles into the smaller ones.[Citation6] The smaller particles tend to being suspended in the juice and pass the light less through the juice; hence, the higher absorbance is recorded using spectrophotometer.[Citation38] Researchers also mentioned that the cloud stability of the treated orange juice depends not only on PME inactivation but is also affected by the changes in pectin structure and size reduction of cloud particles.[Citation4,Citation6,Citation39]
Table 2. Effect of CUT on cloud value of sour orange juice at different temperature.
Effect of CUT on lightness of the colour
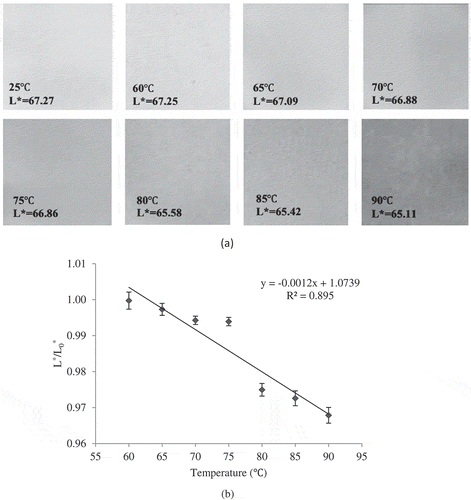
In this study, only lightness parameter was studied to characterise the changes of turbidity or cloudy appearance of sour orange juice. L*-value varies from 0 to 100 and represents the light–dark spectrum. As shown in , the lightness of the fresh juice was 67.27. By increasing in temperature, the lightness ratio (L*/L*0) of colour of the juice reduced insignificantly (P > 0.05) which reflected that the juice became darker. The alteration in this colour aspect was linear (R2 > 0.89), as shown in b. Studies showed that the lightness of the blended orange–carrot juice decreased during thermal treatment.[Citation19] Also, the thermally pasteurised grapefruit juice exhibited lower lightness than fresh juice.[Citation40] In another study, during thermal treatment of pineapple juice at 65 to 95°C, decreasing in lightness was observed. [Citation16]
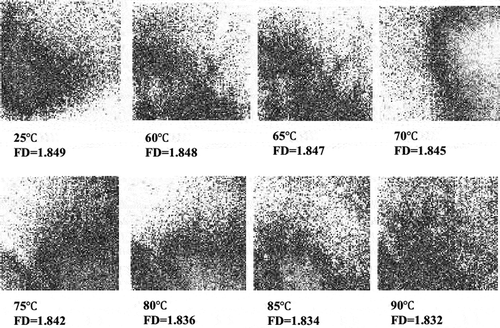
Figure 2. a) Sample images of lightness of fresh and heated sour orange juice and b) the effect of CUT on ratio of lightness of juice at different temperatures.
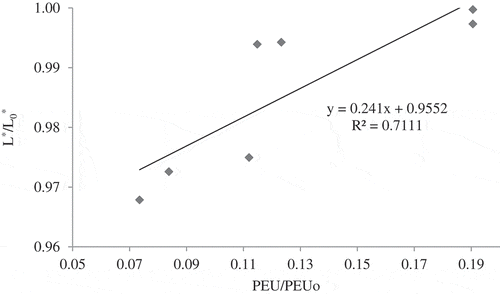
shows the relationship between ratio of PME activity and ratio of L*-value of sour orange juice at the end of CUT with different temperatures. It reveals that the changes of L*-value had a linear and direct relation with inactivation of PME in CUT (R2 > 0.71). It could be concluded that as the temperature increased due to the rising in inactivation of PME and cloud value, sour orange juice became darker and more turbid. These results are in agreement with other researches on the relationship between pectin de-esterification and cloudy appearance of the citrus juice. [Citation6,Citation39]
Changes in fractal dimension and lacunarity
Calculated FD of fresh sour orange juice was 1.849 (). FD value ratio (FD/FD0) reduced (P > 0.05) linearly (R2 > 0.97) by rising in temperature during CUT (). Many of the esterified carboxyl groups in the structure of pectin prevent the occurrence of some reactions such as precipitation. [Citation6] If there are enough de-esterified carboxyl groups available in pectin by PME activity, precipitation occurs via link formation between galacturonic acids and cations such as calcium to produce insoluble pectate; these phenomena cause cloud loss of citrus juice. [Citation4] In other words, having a turbid and cloudy citrus juice is possible if the colloid particles are small enough to retain suspension in the juice. [Citation39]
Figure 5. (a) Changes of fractal dimension ratio, (b) relationship between FD and inactivation of PME in sour orange juice at the end of different CUT.
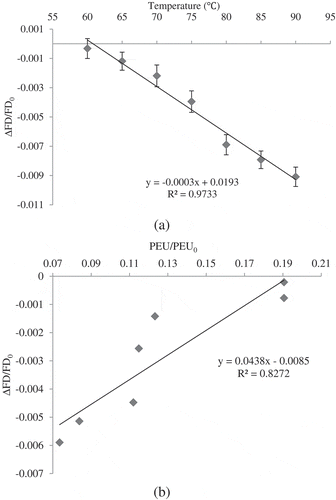
b shows a linear and direct relationship between the inactivation of PME and FD changes (R2 > 0.82). Considering the positive slope of the curve, it can be concluded that greater FD was estimated when the higher PME inactivation occurs. It means that by increasing in temperature of CUT, FD reduction (improvement in homogeneity of the sour orange juice) could be attributed to the thermal inactivation of PME that prevents pectate formation and also increasing the size of cloud particles. [Citation4,Citation6] Unlike citrus juice, in apple juice suspended cloud particles should be removed to make a clear juice by different methods like enzyme clarification. [Citation29,Citation41] It was reported that the aggregation of cloud particles took place immediately after pectin de-esterification by adding pectolytic enzymes to the turbid apple juice.[Citation29] It was also mentioned that a de-esterified colloid particle diffuses until it meets another particle to create a ‘fractile cluster’ or an ‘island structure’ or joins an existing island to extend it. This island structure is in agreement with self-similarity properties of a fractal structure.[Citation29,Citation41]
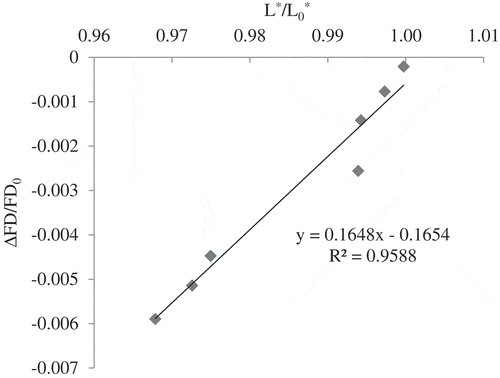
It was found that the L*-value and the FD reduced as the temperature increased. Lightness had a direct relation with PME activity (). So it can be concluded that the L*-value changes were highly dependent on the size of cloud particles and altered continuously by changing in size of pectin. As expected, there was a direct and positive relation between the lightness and DF ratio () with a high coefficient of determination (R2 > 0.95). As a result, the analysis of FD could be introduced as an indicator for PME content and the appearance of the sour orange juice. The fractal derived from the images of banana peel increased during storage so fractal was introduced as an effective indicator of the senescence process of the banana peel.[Citation27] The enzymatic browning kinetics of sliced pear, sliced apple, and sliced and pureed avocado were studied by calculating L*-value and FD. Obtained results showed that fractal method was more sensitive in difference detection where colour patterns have a non-homogenous colour surface.[Citation20,Citation22,Citation28] The FD value was used as an indicator of changes in the non-homogenous distribution of the surface colours of meat during oxidation.[Citation25]
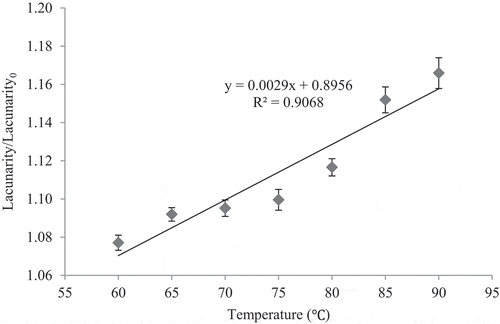
Lacunarity of the sample images was estimated at the end of CUT at different temperatures. As shown in , in contrast to the FD, the lacunarity ratio linearly increased (R2 > 90) at higher temperatures (P < 0.05). Considering that FD and lacunarity complement each other, these factors can be used in characterising the non-homogenous images and providing more information about the spatial distribution of intensity pixels.[Citation23,Citation30] Lacunarity characterises the spatial organization of the juice and estimates the space filling capacity and homogeneity.[Citation23] Increasing of the lacunarity revealed that the treated juice at higher temperatures consisted of the tight clumps structures including larger void gaps.[Citation23] During the study of the image processing method in quantifying the muskmelon epidermis netting, it was found that FD had an inverse relation with lacunarity. In other words, FD reduced during four different growth stages of the fruit, while lacunarity increased as the forming netting occupied less space.[Citation31]
Conclusion
CUT is an initial stage in thermal pasteurisation in which the inactivation of lots of enzymes like PME occurs. The effect of CUT on the inactivation of PME, as a pasteurisation index in high acid juice, was evaluated. Some juice physical properties such as variation in lightness, cloud stability, FD, and lacunarity were changed as PME was thermally inactivated. By rising in applied temperature during CUT, the PME activity reduced, which led to more turbidity or cloudy appearance and lower lightness of the sour orange juice due to a reduction in the size of cloud particle, especially pectin. FD could be effectively used to predict the PME activity as FD decreased linearly by an increase in PME inactivation. Hence, estimation of FD as a physical factor can prevent time and cost consumption of the chemical experiments to measure PME activity. Increasing of lacunarity revealed that the treated juice at higher temperature consisted larger void gaps. Therefore, a combination of FD and lacunarity is useful in the study of the irregularity, heterogeneity, roughness, and complexity of the images.
References
- Jayaprakasha, G.; Patil, B. S. In Vitro Evaluation of the Antioxidant Activities in Fruit Extracts from Citron and Blood Orange. Food Chemistry 2007, 101(1), 410–418. doi:10.1016/j.foodchem.2005.12.038.
- Antunes, M. D.; Dandlen, S.; Cavaco, A. M.; Miguel, G. Effects of Postharvest Application of 1-MCP and Postcutting Dip Treatment on the Quality and Nutritional Properties of Fresh-Cut Kiwifruit. Journal of Agricultural and Food Chemistry 2010, 58(10), 6173–6181. doi:10.1021/jf904540m.
- Nowak, D.; Gośliński, M.; Wojtowicz, E. Comparative Analysis of the Antioxidant Capacity of Selected Fruit Juices and Nectars: Chokeberry Juice as a Rich Source of Polyphenols. International Journal of Food Properties 2016, 19(6), 1317–1324. doi:10.1080/10942912.2015.1063068.
- Tiwari, B.; Muthukumarappan, K.; O’donnell, C.; Cullen, P. Inactivation Kinetics of Pectin Methylesterase and Cloud Retention in Sonicated Orange Juice. Innovative Food Science & Emerging Technologies 2009, 10(2), 166–171. doi:10.1016/j.ifset.2008.11.006.
- Kimball, D. A. Citrus Processing: Quality Control and Technology. Van Nostrand Reinhold; 1991.
- Aadil, R. M.; Zeng, X. A.; Zhang, Z. H.; Wang, M. S.; Han, Z.; Jing, H.;; et al. Thermosonication: A Potential Technique that Influences the Quality of Grapefruit Juice. International Journal of Food Science & Technology 2015, 50(5), 1275–1282. doi:10.1111/ijfs.12766.
- Chen, C.S.; Shaw, P.E.; Parish, M.E. Orange and Tangerine Juices. Fruit Juice Processing technology. In: S. Nagy, C. S. Chen, P. E. Shaw (Eds.). Fruit Juice Processing Technology. Agscience Inc, Auburndale, FL; 1993, 110–165.
- Polydera, A.; Galanou, E.; Stoforos, N.; Taoukis, P. Inactivation Kinetics of Pectin Methylesterase of Greek Navel Orange Juice as a Function of High Hydrostatic Pressure and Temperature Process Conditions. Journal of Food Engineering 2004, 62(3), 291–298. doi:10.1016/S0260-8774(03)00242-5.
- Ball, C. O. Thermal Process Time for Canned Food; Bull. 37, National Research Council: Washington, DC, 1923.
- Tajchakavit, S.; Thermalvs, R. H. Microwave Inactivation Kinetics of Pectin Methylesterase in Orange Juice under Batch Mode Heating Conditions. LWT-Food Science and Technology 1997, 30(1), 85–93. doi:10.1006/fstl.1996.0136.
- Nambi, V. E.; Thangavel, K.; Shahir, S.; Chandrasekar, V. Color Kinetics during Ripening of Indian Mangoes. International Journal of Food Properties 2016, 19(10), 2147–2155. doi:10.1080/10942912.2015.1089281.
- Vikram, V.; Ramesh, M.; Prapulla, S. Thermal Degradation Kinetics of Nutrients in Orange Juice Heated by Electromagnetic and Conventional Methods. Journal of Food Engineering 2005, 69(1), 31–40. doi:10.1016/j.jfoodeng.2004.07.013.
- Xu, Z.; Lin, T.; Wang, Y.; Liao, X. Quality Assurance in Pepper and Orange Juice Blend Treated by High Pressure Processing and High Temperature Short Time. Innovative Food Science & Emerging Technologies 2015, 31, 28–36. doi:10.1016/j.ifset.2015.08.001.
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J. S.; Lemmens, L.; Panozzo, A.;; et al. Colour and Carotenoid Changes of Pasteurised Orange Juice during Storage. Food Chemistry 2015, 171, 330–340. doi:10.1016/j.foodchem.2014.09.007.
- Iqbal, S. M.; Gopal, A.; Sankaranarayanan, P.; Nair, A. B. Classification of Selected Citrus Fruits Based on Color Using Machine Vision System. International Journal of Food Properties 2016, 19(2), 272–288. doi:10.1080/10942912.2015.1020439.
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of Thermal Processing on the Quality Loss of Pineapple Juice. Journal of Food Engineering 2005, 66(2), 259–265. doi:10.1016/j.jfoodeng.2004.03.016.
- Chutintrasri, B.; Noomhorm, A. Color Degradation Kinetics of Pineapple Puree during Thermal Processing. LWT-Food Science and Technology 2007, 40(2), 300–306. doi:10.1016/j.lwt.2005.11.003.
- Su, G.; Zhu, S.; Xu, M.; Ramaswamy, H. S.; Lin, Y.; Yu, Y. Pressure Degradation Kinetics of Anthocyanin Pigment and Visual Color of Chinese Bayberry Juice. International Journal of Food Properties 2016, 19(2), 443–453. doi:10.1080/10942912.2015.1038562.
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and Heat Pasteurization on the Physical–Chemical Characteristics of Blended Orange and Carrot Juice. LWT-Food Science and Technology 2006, 39(10), 1163–1170. doi:10.1016/j.lwt.2005.07.002.
- Quevedo, R.; Díaz, O.; Caqueo, A.; Ronceros, B.; Aguilera, J. Quantification of Enzymatic Browning Kinetics in Pear Slices Using Non-Homogenous L∗ Color Information from Digital Images. LWT-Food Science and Technology 2009, 42(8), 1367–1373. doi:10.1016/j.lwt.2009.03.011.
- Quevedo, R.; Díaz, O.; Ronceros, B.; Pedreschi, F.; Aguilera, J. M. Description of the Kinetic Enzymatic Browning in Banana (Musa Cavendish) Slices Using Non-Uniform Color Information from Digital Images. Food Research International 2009, 42(9), 1309–1314. doi:10.1016/j.foodres.2009.04.004.
- Quevedo, R.; Jaramillo, M.; Díaz, O.; Pedreschi, F.; Aguilera, J. M. Quantification of Enzymatic Browning in Apple Slices Applying the Fractal Texture Fourier Image. Journal of Food Engineering 2009, 95(2), 285–290. doi:10.1016/j.jfoodeng.2009.05.007.
- Valous, N. A.; Mendoza, F.; Sun, D.-W.; Allen, P. Texture Appearance Characterization of Pre-Sliced Pork Ham Images Using Fractal Metrics: Fourier Analysis Dimension and Lacunarity. Food Research International 2009, 42(3), 353–362. doi:10.1016/j.foodres.2008.12.012.
- Lopes, R.; Betrouni, N. Fractal and Multifractal Analysis: A Review. Medical Image Analysis 2009, 13(4), 634–649. doi:10.1016/j.media.2009.05.003.
- Quevedo, R.; Valencia, E.; Cuevas, G.; Ronceros, B.; Pedreschi, F.; Bastias, J. Color Changes in the Surface of Fresh Cut Meat: A Fractal Kinetic Application. Food Research International 2013, 54(2), 1430–1436. doi:10.1016/j.foodres.2013.10.006.
- Al-Kadi, O. S.; Watson, D. Texture Analysis of Aggressive and Nonaggressive Lung Tumor CE CT Images. IEEE Transactions on Biomedical Engineering 2008, 55(7), 1822–1830. doi:10.1109/TBME.2008.919735.
- Quevedo, R.; Mendoza, F.; Aguilera, J.; Chanona, J.; Gutiérrez-López, G. Determination of Senescent Spotting in Banana (Musa Cavendish) Using Fractal Texture Fourier Image. Journal of Food Engineering 2008, 84(4), 509–515. doi:10.1016/j.jfoodeng.2007.06.013.
- Quevedo, R.; Ronceros, B.; Garcia, K.; Lopéz, P.; Pedreschi, F. Enzymatic Browning in Sliced and Puréed Avocado: A Fractal Kinetic Study. Journal of Food Engineering 2011, 105(2), 210–215. doi:10.1016/j.jfoodeng.2011.02.012.
- Benítez, E.; Lozano, J.; Genovese, D. Fractal Dimension and Mechanism of Aggregation of Apple Juice Particles. Food Science and Technology International 2010, 16(2), 179–186. doi:10.1177/1082013209353240.
- Cáez Ramírez, G.; Téllez-Medina, D. I.; García-Armenta, E.; López, G. F. G. Digital Image Analysis and Fractal Metrics as Potential Tools to Monitor Colour Changes in Fresh-Cut Papaya (Carica Papaya L.). International Journal of Food Properties 2017, 1–13. doi:10.1080/10942912.2017.1293090.
- Li, L.; Chang, L.; Ke, S.; Huang, D. Multifractal Analysis and Lacunarity Analysis: A Promising Method for the Automated Assessment of Muskmelon (Cucumis Melo L.) Epidermis Netting. Computers and Electronics in Agriculture 2012, 88, 72–84. doi:10.1016/j.compag.2012.06.006.
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, Ed 19, 2012.
- Versteeg, C.; Rombouts, F.; Spaansen, C.; Pilnik, W. Thermostability and Orange Juice Cloud Destabilizing Properties of Multiple Pectinesterases from Orange. Journal of Food Science 1980, 45(4), 969–971. doi:10.1111/jfds.1980.45.issue-4.
- Russell, D. A.; Hanson, J. D.; Ott, E. Dimension of Strange Attractors. Physical Review Letters 1980, 45(14), 1175. doi:10.1103/PhysRevLett.45.1175.
- Kerdpiboon, S.; Devahastin, S. Fractal Characterization of Some Physical Properties of a Food Product under Various Drying Conditions. Drying Technology 2007, 25(1), 135–146. doi:10.1080/07373930601160973.
- Tornberg, E.;. Effects of Heat on Meat proteins–Implications on Structure and Quality of Meat Products. Meat Science 2005, 70(3), 493–508. doi:10.1016/j.meatsci.2004.11.021.
- Iftikhar, T.; Wagner, M. E.; Rizvi, S. S. Enhanced Inactivation of Pectin Methyl Esterase in Orange Juice Using Modified Supercritical Carbon Dioxide Treatment. International Journal of Food Science & Technology 2014, 49(3), 804–810. doi:10.1111/ijfs.12368.
- Kubo, M. T. K.; Augusto, P. E.; Cristianini, M. Effect of High Pressure Homogenization (HPH) on the Physical Stability of Tomato Juice. Food Research International 2013, 51(1), 170–179. doi:10.1016/j.foodres.2012.12.004.
- Lacroix, N.; Fliss, I.; Makhlouf, J. Inactivation of Pectin Methylesterase and Stabilization of Opalescence in Orange Juice by Dynamic High Pressure. Food Research International 2005, 38(5), 569–576. doi:10.1016/j.foodres.2004.11.010.
- Igual, M.; Contreras, C.; Camacho, M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage Conditions on the Physical and Sensory Properties of Grapefruit Juice. Food and Bioprocess Technology 2014, 7(1), 191–203. doi:10.1007/s11947-013-1088-6.
- Beveridge, T.;. Letter to the Editor: Fractile Images and Apple Juice Haze. Food Research International 1998, 31(5), 411–414.