ABSTRACT
The effects of duck and hen albumens at different levels (1%–4%, w/w) on proteolysis and gel properties of sardine (Sardinella albella) surimi were studied. The addition of both albumens into surimi resulted in the increases in breaking force and deformation, especially with increasing levels added (P < 0.05). Both albumens inhibited autolysis of surimi in a dose dependent manner as indicated by the lower TCA-peptide content and more retained myosin heavy chain and actin. Texture profile analysis showed that hardness, cohesiveness, springiness, gumminess and chewiness of surimi gel increased with increasing albumen levels. In general, breaking force, hardness, gumminess and chewiness of surimi gel added with duck albumen were higher than those added with hen albumen when the same level was used. Lightness and whiteness of gels added with albumen increased, particularly at higher amount of albumen. Surimi gel added with both albumens showed compact network with more connectivity and smaller voids than the control gel as visualised by scanning electron microscopy. Thus, duck albumen could be used to replace hen albumen for improvement of sardine surimi gel properties.
Introduction
Surimi is myofibrillar protein concentrate obtained by washing fish mince. It has a high commercial value with a wide range of applications, particularly for making a variety of gelling products, e.g. fish ball, imitation crab and/or lobster.[Citation1,Citation2] Surimi has commonly been produced from lean fish due to the white colour and superior gelling property. Nevertheless, sardine (Sardinella albella), a pelagic dark-fleshed fish, has been also used as a raw material for surimi production. In general, dark-fleshed fish contains high level of endogenous proteases, which cause the gel softening. This limits the use of surimi from dark-fleshed fish.[Citation2,Citation3] To conquer the gel weakening in surimi, several protein additives possessing protease inhibitory activity such as egg albumen, soy protein isolate, porcine and chicken plasma protein, etc. have been employed.[Citation4–Citation6]
Egg albumen is well-known to contain protease inhibitors that inhibit cysteine, serine, aspartyl, and metalloproteases.[Citation7] Five protease inhibitors have been reported in egg albumen. Those include ovostatin, ovomucoid, ovoinhibitor, cystatin C, and ovalbumin.[Citation8] Due to its efficacy in lowering proteolysis in surimi, hen egg white or albumen has been used to improve the textural properties of surimi gel.[Citation6] Egg albumen has been employed in surimi of lizardfish, king weakfish, Pacific whiting, Alaska pollock, etc.[Citation5,Citation9,Citation10] Additionally, egg albumen is often used as an ingredient to enhance the water-holding capacity of food products, especially in surimi.[Citation11]
Hen and duck eggs are among the most consumed avian eggs in Asian countries. Quail egg is also used for some particular cuisine.[Citation12] Egg albumen has been used in several foods and its compositions vary with species. Protein contents of lyophilised albumen from duck and quail eggs were higher than that of hen egg.[Citation12,Citation13] Moreover, ovomucoid from duck and hen albumen also showed different protease inhibitory activity in protection of salmon calcitonin breakdown by serine proteases.[Citation14] In general, hen albumen has been commonly manufactured into different forms, such as powder, pasteurised liquid albumen, etc.[Citation15] Due to the excellent functional properties such as foaming and gelling properties, hen albumen has become the crucial ingredient in a wide range of food products.[Citation16]
Recently, duck albumen has been found to exhibit high protease inhibitory activity with superior gelling property.[Citation17] Thus, duck albumen could be employed as potential protein additive, which was able to improve gel property of muscle foods, especially surimi suffering from gel weakening. Nevertheless, no information regarding the use of duck albumen in surimi exists. Therefore, this study aimed to comparatively investigate the impact of duck and hen albumens on proteolysis and gel properties of sardine surimi.
Materials and methods
Chemicals
All chemicals used in this study were of analytical grade. Bovine serum albumin (BSA) and trypsin from bovine pancreas (Type I, ~10,000 BAEE units/mg protein) were obtained from Sigma Chemical Co. (St. Louis, MO), and high molecular protein markers were purchased from GE healthcare UK Limited (Buckinghamshire, UK). Sodium dodecyl sulfate (SDS), β-mercaptoethanol (β-ME), trichloroacetic acid, Folin–Ciocalteu’s phenol reagent, tyrosine, glutaraldehyde, ethanol and Coomassie blue R-250 were obtained from Merck (Darmstadt, Germany).
Frozen sardine surimi, AA grade, was purchased from Man A Frozen Co., Ltd. (Songkhla, Thailand) and kept at −20°C until use, but not longer than two months.
Preparation of duck and hen albumens
Fresh duck and hen eggs within 24 h after laying were collected randomly at farm houses in Kantang, Trang province, Thailand and Faculty of Natural Resources, Prince of Songkla University, respectively. Eggs were broken and albumen was separated from egg yolk manually. Then, albumen was lyophilised using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). The powders obtained were placed in polyethylene bag and kept at −20°C until use but not longer than 2 months.
Study on the effect of hen and duck albumens on autolysis of sardine surimi
Autolysis assay was performed according to the method of Benjakul et al.[Citation4] Firstly, surimi (3g) was placed in 50 mL beaker. Thereafter, hen and duck albumens at various levels (0, 1, 2, 3 and 4%, w/w) were added and thoroughly mixed. Then, the mixtures were spread at the bottom of beaker and covered with aluminum foil. Samples were incubated at 60°C for 60 min. Subsequently, the reaction was terminated by adding 27 mL of cold 5% (w/v) trichloroacetic acid (TCA). The mixture was homogenised at 5000 rpm for 2 min using an IKA homogeniser Model T 25 D (IKA-Werke GmbH & Co. KG, Staufen, Germany). The homogenate was centrifuged at 8000 × g for 10 min using a centrifuge (Allegra 25R centrifuge, Beckman Coulter, Palo Alto, CA, USA). TCA-soluble peptide content in the supernatant was determined by the Lowry method[Citation18] and expressed as µmole of tyrosine equivalent/g sample.
Protein pattern of autolysed sardine surimi was determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) according to the method of Laemmli.[Citation19] Autolysis of surimi was performed in the same manner as previously described, except that 5% SDS solution (85°C) was used instead of 5% TCA. The mixture was homogenised at a speed of 5000 rpm for 2 min. The mixture was centrifuged for 20 min at 12,000 × g. The supernatant was subjected to SDS-PAGE analysis.
SDS-PAGE was carried out using 10% running gel and 4% stacking gel. The sample was mixed with sample buffer (50 mM Tris-HCl, pH 6.8, containing 4% SDS, 20% glycerol, 10% β ME). Sample (15 µg protein as determined by the method of Robinson and Hogden[Citation20]) was loaded onto the gel and subjected to electrophoresis at constant current of 15 mA/gel using electrophoresis unit (Mini-protein II; Bio-Rad Laboratories, Richmond, CA, USA). The gels were stained with Coomassie Brilliant Blue R-125 (0.125%) in 25% methanol and 10% acetic acid. Destaining was performed using 40% methanol and 10% acetic acid. Molecular weight (MW) of protein bands was estimated from the plot of MW standards and Rf. Quantitative analysis of protein band intensity was performed using a Model GS-700 Imaging Densitometer (Bio-Rad Laboratories, Hercules, CA, USA) with Molecular Analyst Software version 1.4 (image analysis systems).
Determination of trypsin inhibitory activity
Trypsin inhibitory activity was measured as per the method of Benjakul et al.[Citation4] Albumen samples with an appropriate dilution (200 µL) were incubated with 200 µL of porcine pancreas trypsin (0.05 mg/mL) at 37°C for 15 min. Then, 1000 µL of reaction buffer (50 mM Tris-HCl containing 20 mM CaCl2, pH 8.2) were added. Thereafter, 200 µL of BAPNA (2 mg/mL) were added and the mixture was incubated at 37°C for 15 min. To terminate the reaction, 200 µL of 30% acetic acid (v/v) were added. The release of ρ-nitroalaniline was monitored by measuring the absorbance at 410 nm using a spectrophotometer (UV-16001, Shimadzu, Kyoto, Japan). One unit of trypsin activity was defined as the enzyme causing an increase of 0.01 absorbance unit/min under the assay condition. One unit of trypsin inhibitory activity was defined as the amount of inhibitor, which reduced trypsin activity by one unit. Trypsin inhibitory activity of albumen was expressed as units/mg solid.
Study on the impact of hen and duck albumen on gel properties of sardine surimi
Preparation of sardine surimi gel
Surimi gel was prepared following the method of Buamard and Benjakul.[Citation3] Firstly, surimi was thawed under running water (25°C–28°C) until the core temperature reached 0°C–2°C. The surimi was chopped into small pieces and mixed with 2.5% salt in a mixer (National Model MK-5080M, Selangor, Malaysia). During chopping, hen and duck albumen powders were added into surimi paste at different levels (0%, 1%, 2%, 3% and 4% (w/w)). The temperature was maintained at 2°C–7°C during chopping. The moisture content of surimi paste was adjusted to 80% with cold distilled water. The paste was stuffed into a polyvinylidene chloride casing with a diameter of 2.5 cm. Both ends were sealed tightly and incubated at 40°C for 30 min, followed by heating at 90°C for 20 min. Thereafter, all gels were cooled in iced water for 30 min and stored at 4°C for 24 h before analyses.
Analyses
Breaking force and deformation
Breaking force and deformation of surimi gels added with hen or duck albumens at various levels were determined using a texture analyzer (Model TA-XT2, Stable MicroSystems, Surrey, UK) as described by Singh and Benjakul.[Citation21] Five cylindrical samples were cut into 2.5 cm height and 2.5 cm diameter and equilibrated at room temperature (25°C–28°C) before analyses. Sample was perpendicularly pressed by a spherical plunger of 5 mm diameter into the cut surface of gel at a constant depression speed (60 mm/min). The force to puncture into the gel (breaking force) and the distance at which the plunger punctured into the gel (deformation) were both recorded.
Texture profile analysis
Texture profile analysis of surimi gels was also performed using a texture analyzer (Model TA-XT2i, Stable Micro System, Surrey, England). The samples were compressed twice to 50% of their original height with a compression cylindrical aluminum probe (50 mm diameter). Force-distance deformation curve was recorded at a cross head speed of 3 mm/s and the recording speed was 3 mm/s. Hardness, springiness, cohesiveness, chewiness, and gumminess were evaluated. These parameters were recorded using the MicroStable software version 6 (Surrey, England).
Expressible moisture content
Expressible moisture content of surimi gel was measured according to the method of Buamard et al.[Citation22] Cylindrical gel samples were cut into a thickness of 5 mm, weighed accurately (X) and placed between three pieces of Whatman filter paper No.1 (Whatman International Ltd., Maidstone, UK) at the bottom and two pieces on the top of the sample. A standard weight of 5 kg was placed on the top of the sample for 2 min. The samples were then removed from the papers and weighed again (Y). Expressible moisture content was calculated with the following equation and expressed as percentage of sample weight:
Determination of colour
The colour of surimi gel samples was determined by a colorimeter (ColorFlex, Hunter Lab Reston, Reston, VA, USA) and reported in CIE system. L*, a*, b* and ΔE*, representing lightness, redness/greenness, yellowness/blueness and total difference of colour, respectively, were recorded.
The ΔE* was also calculated by the following formula:
where ΔL*, Δa*, Δb* are the difference between colour parameters of the samples and those of the white standard (L* = 92.82, a* = −1.24, b* = 0.50).
The whiteness of gels was also calculated[Citation23] using the following equation:
Determination of microstructure
Microstructure of surimi gels added without and with duck and hen albumen at 3% was examined by a scanning electron microscopy as described by Kaewmanee et al.[Citation23] Surimi samples were cut into small pieces (1x1x1 mm3). Samples were fixed with 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 12 h. Then, fixed samples were rinsed with distilled water for 1 h. Subsequently, samples were dehydrated sequentially using a series of ethanol (25%, 50%, 70%, 80%, 90% and 100%) for 15 min at each concentration. The dehydrated samples were subjected to critical point drying (CPD). The samples were coated with 100% gold (sputter coater SPI-Module, West Chester, PA, USA). The gel microstructure was visualised by a scanning electron microscope (JEOL JSM-5800LV, Tokyo, Japan).
Statistical analysis
All the experiments were conducted in triplicate using three lots of samples. Data were presented as mean value with standard deviation. One way variance of analysis (ANOVA) was performed. For pair comparison, T-test was used. The Duncan’s multiple range test was carried out to determine the significant difference between samples at P < 0.05 level using the statistical program (SPSS 11.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Effect of hen and duck albumens at different levels on autolysis of sardine surimi
TCA-soluble peptide content
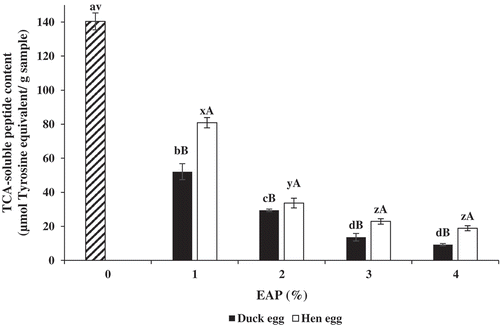
Autolysis of sardine surimi in the absence and presence of hen and duck albumens at various levels expressed as TCA-soluble peptide content is shown in . The highest TCA-soluble peptide content was observed in surimi without albumen addition (P < 0.05). When surimi paste was added with hen or duck albumens, TCA-soluble peptide content decreased as the levels of albumen increased (P < 0.05). Nevertheless, there were no differences in TCA-soluble peptide content in samples added with albumen at 3% and 4%, regardless of type of egg (P > 0.05). TCA-soluble peptides indicate the degradation of muscle proteins during heat induced gelation of surimi as the result of indigenous proteases.[Citation24] This result indicated that hen and duck albumens were able to inhibit the proteolysis of surimi in a dose-dependent manner. At the same level of albumen added, TCA-soluble peptide content of surimi incubated in the presence of hen albumen was higher than that of surimi incorporated with duck albumen (P < 0.05). Higher efficacy of duck albumen in preventing autolysis was in accordance with its higher trypsin inhibitory activity, compared to hen albumen. In the present study, trypsin inhibitory activity of duck albumen was 5880 ± 27 units/mg solid, while hen albumen had the activity of 3925 ± 22 units/mg solid. Egg albumen contains several protease inhibitors, namely ovoinhibitor, ovamacroglobulin, etc. which were able to inhibit serine protease. [Citation2] Hu et al.[Citation13] reported that duck ovostatin (ovamacroglobulin) is capable of inhibiting serine protease as well as metalloprotease, while chicken ovostatin inhibits only metalloprotease. Moreover, Shah and Khan[Citation14] proved that duck ovomucoid effectively stabilised salmon calcitonin against hydrolysis mediated by three proteases including trypsin, α-chymotrypsin and elastase. However, chicken ovomucoid could not prevent the degradation of salmon calcitonin. This result reconfirmed that duck albumen had higher inhibitory activity toward autolysis of sardine surimi. Inhibitory activity toward autolysis of both albumens was in a dose dependent manner.
Figure 1. TCA-soluble peptide content of sardine surimi incubated at 60°C for 60 min in the absence or presence of duck and hen albumens at different levels. Bars represent the standard deviation (n = 3). Different lowercase letters on the bar under the same egg albumen including the control (without albumen) indicate significant differences (P < 0.05). Different uppercase letters on the bars under the same level of albumen indicate significant differences (P < 0.05)'
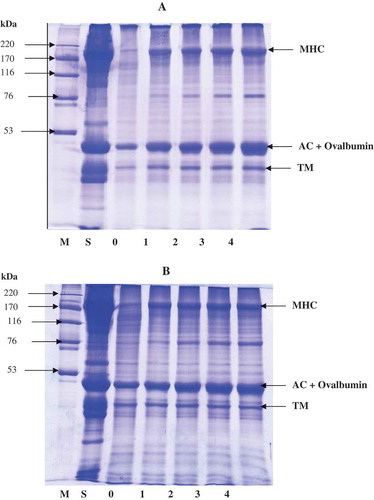
Protein pattern
Protein patterns of surimi added with hen or duck albumens at different levels and subjected to autolysis at 60°C for 60 min are shown in . The lowest intensity of myosin heavy chain (MHC) and actin bands was observed in the samples without hen or duck albumen addition. The intensity of MHC bands increased with increasing albumen levels, indicating that autolysis was inhibited more effectively as the concentration of albumen increased. This result was in agreement with the higher decrease in TCA-soluble peptide content when albumen at higher concentrations was incorporated. Surimi gel added with hen egg albumen and duck egg albumen at 3% and 4% showed similar band intensity of MHC. With addition of 3% and 4% hen egg albumen, MHC band intensity was increased by 49.3% and 51.6%, respectively, compared to that of the control gel. Surimi gel added with 3% and 4% duck egg albumen had the increase in MHC band intensity by 49.8% and 50.2%, respectively. Generally, actin and tropomyosin were resistant to proteolytic enzyme, while MHC was the main protein susceptible to degradation.[Citation24,Citation25] It was noted that band intensity of protein with MW of 43–45 kDa was increased with increasing albumen levels. It was suggested that ovalbumin from egg albumen along with actin (MW around 43–45 kDa) contributed to the increasing band intensity. The increase was more pronounced when the amount of egg albumen was increased. Egg albumen contained ovalbumin (MW: 45 kDa) as the dominant protein. [Citation11] Ovalbumin contributes around 54%–58% of egg albumen proteins. [Citation11] At the same level of albumen used, proteins including MHC were more retained when duck albumen was added, in comparison with hen albumen. This was in agreement with the higher trypsin inhibitory activity of duck albumen. As a consequence, lower degradation of muscle proteins in sardine surimi was attained when duck albumen was incorporated.
Effect of hen and duck albumen on properties of sardine surimi gel
Breaking force and deformation
Breaking force and deformation of sardine surimi gels added with hen or duck albumen at different levels are shown in . Breaking force (g) of control surimi gel (without albumen) was lowest (235.76 g) (P < 0.05). Breaking force of gel increased as the concentrations of both albumens increased (P < 0.05). At the same concentration of albumen added, the resulting gel containing duck albumen showed the higher breaking force, compared to that added with hen albumen (P < 0.05). Similar trend was found for deformation. When duck and hen albumens at the level of 3% were added, breaking force of surimi gel increased by 55% and 46%, compared to that of control gel, respectively. The increases in breaking force by 96% and 54% were obtained as duck and hen albumens at a level of 4% was incorporated, respectively. The increases in breaking force and deformation were in agreement with the decrease in TCA-soluble peptide (). Duck albumen was more effective in inhibiting proteolysis in surimi than hen albumen. MHC, the main protein components for gel formation in surimi, was more retained when protease inhibitors were present.[Citation24] Moreover, egg albumen is capable of water-holding with gel forming ability.[Citation26] Recently, Quan and Benjakul[Citation17] reported that duck albumen showed the increased gel strength, especially after storage at 4°C for 6 days. Albumen proteins plausibly formed gel along with muscle proteins, especially MHC, in surimi. As a result, strong gel network could be developed. Jitesh et al.[Citation2] also reported that the highest gel strength was obtained in lizardfish (Saurida tumbil) surimi gel added with 3% egg albumen. The result suggested that duck albumen was more sufficient for strengthening sardine surimi than hen albumen. The gel strengthening effect of both albumens was in a dose-dependent manner.
Table 1. Breaking force, deformation, and expressible moisture content of sardine surimi gels added with hen or duck albumens at different levels.
Texture profile
Texture profile of surimi gel added with duck or hen albumens at various concentrations is presented in . Hardness of surimi gel increased with increasing albumen concentrations (P < 0.05). The lowest hardness was noticeable in control gel (58.90 N), whereas the highest values (97.25 and 87.22 N) were found in the samples added with 4% duck or 4% hen albumen, in which the increases by 60% and 48% were obtained, respectively. When comparing hardness of gel added with hen or duck albumens at the same concentrations, the latter had the higher hardness (P < 0.05). Hardness is related to the strength of gel structure under compression and is the peak force during the first compression cycle.[Citation27] The addition of albumen more likely prevented the degradation of myosin in sardine muscle. Egg albumen has been known to contribute to gel strengthening via protease inhibition and its binding effect.[Citation5] Jafarpour et al.[Citation6] also reported that hardness, cohesiveness, adhesiveness and elasticity of common carp surimi gel increased as the level of egg albumen increased. Increases in hardness were in accordance with the increased breaking force of gel incorporated with albumens.
Table 2. Texture profile of sardine surimi gels added with hen or duck albumens at different levels.
The slight increase in cohesiveness of surimi gel was observed when both hen and duck albumens were added (). At the same level, higher cohesiveness was found in the samples added with hen albumen, compared to those containing duck albumen. However, there was no difference in cohesiveness among the surimi gels added with albumen in the range of 1%–4% (P > 0.05). Cohesiveness is a parameter to measure of the difficult level in breaking down the internal structure of gel.[Citation28] For springiness, no differences were obtained in all samples, regardless of albumen addition. Springiness is considered as “elasticity” or “rubberiness” of the gel in the mouth, and is a parameter to show how much the gel structure is broken down by the initial compression.[Citation28] Gumminess and chewiness of surimi gels without and with duck or hen egg albumens at various levels are shown in . In general, these values increased continuously as albumen concentrations increased (P < 0.05). The changes in gumminess and chewiness were in correlation with those of hardness. Gumminess and chewiness were calculated based on hardness, which suggests the resistance to compression force.[Citation29] At the same level of albumen, higher gumminess and chewiness were obtained in samples added with duck albumen than those containing hen albumen. The result suggested that duck albumen was found to be effective in improving the textural properties of sardine surimi gel.
Expressible moisture content
Expressible moisture contents of surimi gels added with hen and duck albumens at different levels are shown in . The highest expressible moisture content was found in the control surimi gel (6.60%). The expressible moisture content decreased as the levels of both albumens increased (P < 0.05). When the same level of albumen was used, higher expressible moisture content was observed for surimi added with hen albumen, compared to that of surimi incorporated with duck egg (P < 0.05). With the addition of 3% duck or hen albumens, the expressible moisture content decreased by approximately 39% and 30%, respectively. The expressible moisture content is indicative for the water binding properties. During thermal gelation, protein matrix was formed and water was imbibed throughout the network.[Citation30] The decrease in expressible moisture content was coincidental with the increases in breaking force and deformation of gel added with albumens. The greater amount of water was imbibed in the gel network when the order and finer network was formed. This correlated with the increased gel strength as indicated by the higher breaking force and deformation. Jafarpour et al.[Citation6] also reported that water holding capacity of surimi gel from common carp (Cyprinus carpio) increased continuously when egg albumen ranging from 1% to 3% was added. This suggested that duck albumen could be used to replace hen albumen for improvement of the gelling properties of surimi via increasing water holding capacity.
Colour and whiteness
Colour and whiteness of sardine surimi gels added with duck or hen albumens at different levels are shown in . It was observed that surimi gels added with duck or hen albumen had slightly higher lightness (L*), compared to the control surimi gel (P < 0.05). There was no difference in L*- value between surimi gels incorporated with duck and hen albumen (P > 0.05) when the same level was used. Similar trend was noticeable for the whiteness, in which the addition of both albumens at 3% or 4% yielded the gels with the highest whiteness (P < 0.05). The increase in lightness or whiteness was probably due to the white colour of albumen gel. As a result, the sardine gel with gray colour had the increased lightness and whiteness as albumen was incorporated. According to Jafarpour et al.,[Citation6] the addition of egg albumen up to 3% increased the lightness and whiteness of common carp surimi gel. Nevertheless, Benjakul et al.[Citation5] reported that the whiteness of lizardfish surimi gel was not affected by addition of egg albumen up to 3%.
Table 3. Colour and whiteness values of sardine surimi gels added with hen or duck albumens at the different levels.
The slight increases in both b*- and a*- values of surimi gels were observed as the levels of both albumens increased (P < 0.05). For a*- value, surimi gels added with hen albumen showed higher value than those incorporated with duck albumen, especially at the concentration of 2%–3%. However, there were no differences in b*- values between gels added with duck and hen albumens at the same levels. Nevertheless, Jafarpour et al.[Citation6] reported that increase in egg albumen concentration caused a decrease in the yellow colour (b* value) of common carp surimi gel. For ΔE*, the decrease in value was obtained for surimi gel added with hen and duck albumens, compared to the control. The decrease in ΔE* was coincidental with the increase in whiteness. In general, egg albumen affected the colour of sardine surimi gel to some degrees. Duck albumen could increase the lightness and whiteness of gel from sardine surimi more effectively than hen albumen.
Microstructure
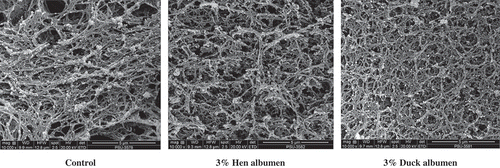
Scanning electron micrographs of sardine surimi added with hen or duck albumens at 3% and the control surimi gel are illustrated in . Surimi gel without albumen was less compact with more opened structure. Its network contained larger voids with less connectivity. However, more compact and denser gel network was observed in surimi added with hen or duck albumens at 3%. The denser and more compact structure of gel coincided with the increased breaking force () and hardness (). Albumen with protease inhibitory activity could inhibit indigenous proteases in surimi. As a result, MHC was retained and underwent aggregation, in which more ordered and finer network was developed. Furthermore, egg albumen protein also can form gel with good properties and high water-holding capacity. [Citation11,Citation17] It was noted that albumens either from duck or hen eggs at a level of 3% was sufficient to control proteolysis in surimi. Similar MHC band intensity was observed when albumen at 3% and 4% was used (). It was assumed that the increase in breaking force or hardness of gel added with 4% albumen was more likely caused by gelling ability of albumen. However, the use of egg white at high level might result in off odor, which was not desirable.[Citation31] The level of albumen up to 3% has been widely used in surimi.[Citation2,Citation5,Citation6] When comparing the microstructure of surimi added with hen and duck albumens, gel added with hen albumen showed slightly coarser network with larger voids. The less compactness was related with the lower breaking force and hardness of hen albumen added surimi gel (). The result revealed that hen and duck albumen had the impact on gel structure of sardine surimi, contributing to gel property of surimi.
Conclusions
The addition of hen and duck albumens in sardine surimi gel could reduce the degradation of muscle proteins, especially MHC. Gel strength and whiteness of surimi gel were improved. Duck albumen showed higher efficiency in enhancing the gelling properties of sardine surimi than hen albumen. Thus, duck albumen was considered to replace hen albumen and could be used as protein additive in surimi gel, where protease inhibitors from duck albumen were able to alleviate gel weakening caused by endogenous proteases.
Acknowledgments
This work was supported by the Higher Education Research Promotion and the Thailand’s Education Hub for Southern Region of ASEAN Countries Project Office of the Higher Education Commission scholarship. The TRF Distinguished Research Professor Grant was also acknowledged.
References
- Zaghbib, I.; Arafa, S.; Félix, M.; Hassouna, M.; Romero, A. Enhancement of the Gelling Properties of Sardine Surimi with Transglutaminase and Optimization of Its Activity Using Response Surface Methodology. J. Food Process. Technol. 2016, 7, 594–602.
- Jitesh, B.; Syed, M.; Hitendra, L.; Ashok, R.; Anil, S.; Balakrishnan, G. Effect of Egg Albumen (Protein Additive) on Surimi Prepared from Lizardfish (Saurida Tumbil) during Frozen Storage. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2011, 4, 306–312.
- Buamard, N.; Benjakul, S. Improvement of Gel Properties of Sardine (Sardinella Albella) Surimi Using Coconut Husk Extracts. Food Hydrocoll. 2015, 51, 146–155.
- Benjakul, S.; Visessanguan, W.; Srivilai, C. Porcine Plasma Protein as Proteinase Inhibitor in Bigeye Snapper (Priacanthus Tayenus) Muscle and Surimi. J. Sci. Food Agric. 2001, 81, 1039–1046.
- Benjakul, S.; Visessanguan, W.; Tueksuban, J.; Tanaka, M. Effect of Some Protein Additives on Proteolysis and Gel-Forming Ability of Lizardfish (Saurida Tumbil). Food Hydrocoll. 2004, 18, 395–401.
- Jafarpour, A.; Hajiduon, H.-A.; Aie, M. A Comparative Study on Effect of Egg White, Soy Protein Isolate and Potato Starch on Functional Properties of Common Carp (Cyprinus Carpio) Surimi Gel. J. Food Process. Technol. 2012, 3, 190–196.
- Réhault, S. Antiproteases. In Bioactive Egg Compounds; Huopalahti, R.; Anton, M.; López-Fandiñ, R.; Schade, R.; Eds.; Springer: Heidelberg, Germany, 2007, pp. 85–92.
- Saxena, I.; Tayyab, S. Protein Proteinase Inhibitors from Avian Egg Whites. Cell. Mol. Life Sci. 1997, 53, 13–23.
- Kuhn, C. R.; Prentice-Hernández, C.; Vendruscolo, J. L.; Soares, G. J. D. Surimi of King Weakfish (Macrodon Ancylodon) Wastes: Texture Gel Evaluation with Protease Inhibitors and Transglutaminase. Braz. Arch. Biol. Technol. 2004, 47, 895–901.
- Hunt, A.; Park, J. W.; Handa, A. Effect of Various Types of Egg White on Characteristics and Gelation of Fish Myofibrillar Proteins. J. Food Sci. 2009, 74, 683–692.
- Phillips, G. O.; Williams, P. A. Egg Proteins. In Handbook of Food Proteins; Strixner, T., Kulozik, U., Eds.; Woodhead Publishing Limited: Oxford, USA, 2011; pp. 150–209.
- Miguel, M.; Manso, M. A.; López-Fandiño, R.; Ramos, M. Comparative Study of Egg White Proteins from Different Species by Chromatographic and Electrophoretic Methods. Eur. Food Res. Technol. 2005, 221, 542–546.
- Hu, S.; Qiu, N.; Liu, Y.; Zhao, H.; Gao, D.; Song, R.; Ma, M. Identification and Comparative Proteomic Study of Quail and Duck Egg White Protein Using 2-Dimensional Gel Electrophoresis and Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Tandem Mass Spectrometry Analysis. Poult. Sci. 2016, 0, 1–8.
- Shah, R. B.; Khan, M. A. Protection of Salmon Calcitonin Breakdown with Serine Proteases by Various Ovomucoid Species for Oral Drug Delivery. J. Pharm. Sci. 2004, 93, 392–406.
- Trziszka, T.; Rózanski, H.; Polanowski, A. Eggs as A Very Promising Source of Biomedical and Nutraceutical Preparations: A Review. J. Life Sci. 2013, 7, 862-877.
- Abeyrathne, E.; Lee, H.; Ahn, D. Egg White Proteins and Their Potential Use in Food Processing or as Nutraceutical and Pharmaceutical agents—A Review. Poult. Sci. 2013, 92, 3292–3299.
- Quan, T. H.; Benjakul, S. Quality, Protease Inhibitor and Gelling Property of Duck Egg Albumen as Affected by Storage Conditions. J. Food Sci. Technol. 2017. https://doi.org/10.1007/s13197-017-2960-6 (in press).
- Lowry, O. H.; Rosebrough, N. J.; Farr, A. L.; Randall, R. J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275.
- Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Robinson, H. W.; Hogden, C. G. The Biuret Reaction in the Determination of Serum Proteins. 1. A Study of the Conditions Necessary for the Production of A Stable Color Which Bears A Quantitative Relationship to the Protein Concentration. J. Biol. Chem. 1940, 135, 707–725.
- Singh, A.; Benjakul, S. Serine Protease Inhibitors from Squid Ovary: Extraction and Its Effect on Proteolysis and Gel Properties of Surimi. J. Food Sci. Technol. 2016, 54, 267–275.
- Buamard, N.; Benjakul, S.; Konno, K. Improvement of Gel Quality of Sardine Surimi with Low Setting Phenomenon by Ethanolic Coconut Husk Extract. J. Texture Stud. 2016, 48, 47–56.
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effects of Salting Processes and Time on the Chemical Composition, Textural Properties, and Microstructure of Cooked Duck Egg. J. Food Sci. 2011, 76, 139–147.
- Oujifard, A.; Benjakul, S.; Ahmad, M.; Seyfabadi, J. Effect of Bambara Groundnut Protein Isolate on Autolysis and Gel Properties of Surimi from Threadfin Bream (Nemipterus Bleekeri). LWT-Food Sci. Technol. 2012, 47, 261–266.
- Fowler, M. R.; Park, J. W. Salmon Blood Plasma: Effective Inhibitor of Protease-Laden Pacific Whiting Surimi and Salmon Mince. Food Chem. 2015, 176, 448–454.
- Ren, Y.; Wu, J.; Renema, R. Nutritional and Health Attributes of Eggs. In Handbook of Poultry Science and Technology; Guerrero-Legarreta, I., Ed.; John Wiley & Sons, Inc: Hoboken, New Jersey, 2010; pp. 533–578.
- Chandra, M.; Shamasundar, B. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584.
- Lau, M.; Tang, J.; Paulson, A. Texture Profile and Turbidity of Gellan/Gelatin Mixed Gels. Food Res. Int. 2000, 33, 665–671.
- Yilmaz, M. T.; Karaman, S.; Dogan, M.; Yetim, H.; Kayacier, A. Characterization of O/W Model System Meat Emulsions Using Shear Creep and Creep Recovery Tests Based on Mechanical Simulation Models and Their Correlation with Texture Profile Analysis (TPA) Parameters. J. Food Eng. 2012, 108, 327–336.
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T. C. Chicken Plasma Protein Affects Gelation of Surimi from Bigeye Snapper (Priacanthus Tayenus). Food Hydrocoll. 2004, 18, 259–270.
- Ahamed, M. E.; Anjaneyulu, A.; Sathu, T.; Thomas, R.; Kondaiah, N. Effect of Different Binders on the Quality of Enrobed Buffalo Meat Cutlets and Their Shelf Life at Refrigeration Storage (4±1°C). Meat Sci. 2007, 75, 451–459.