ABSTRACT
The barberry fruits are used as food flavoring. Berberis integerrima seeds are used as waste product in barberry’s processes. This study describes the potential use of B. integerrima seed oil (BSO) as a natural antioxidant. 2,2-diphenyl-1-picryl hydrazyl scavenging activity and ferric-reducing antioxidant power of the oil alcoholic extract were determined. Protective effect of BSO in stabilizing soybean oil was further evaluated based on the peroxide values, thiobarbituric acid values, conjugated dienes and trienes, and Rancimat test during storage. Results of gas chromatography (GC-FID) showed that more than 90% of the fatty acids were linolenic (ω-3), linoleic (ω-6), and oleic (ω-9) acids. Total phenolic compounds were 323.0 mg Gallic acid/kg oil. Chromatographic separation of their sterols and tocopherols (HPLC) demonstrated that β-sitosterol, campesterol, stigmasterol cholesterol, sitostanol, ∆5-avenasterol, ∆7-avenasterol, clerosterol, and α-tocopherol and γ-tocopherol were present in the BSO, respectively. The IC50 of the oil alcoholic extract was less than the commercial antioxidants, including butylated hydroxyanisole and butylated hydroxytoluene. The statistical results indicated that BSO has significant effects on soybean oil stability on most days.
Introduction
The healthful effects of minor components available in vegetable oils have become a subject of renewed interest in recent years. Currently, an increased interest is observed for valuable vegetable oils such as berry seed oils due to their health properties, which are related to their high value of polyunsaturated fatty acids (PUFAs) and antioxidants. Different types of barberry are well known around the world due to various benefits such as medical, ornamental, and food uses. Two important barberry species in Iran are Berberis vulgaris (poloei) and Berberis integerrima (abi). The precedent of zereshk slew in Iran dates back to ancient times (about two centuries ago). In commercial scale, Iran is the only country that generates seedless barberries.[Citation1] B. integerrima is a thorny shrub with fragile branches to a height of 1–3 m. The fruits of this type of barberry are, small, red, ellipsoid, 7–10 mm long, and 3–4 mm in diameter, with a mild sour taste. In addition, there are two, three, or even five small fusiform seeds inside the fruit.[Citation2]
The barberry’s sour taste makes the fruit a favorite for flavoring and seasoning. A lot of Berberis L. species are used to diminish insomnia, bronchial diseases, urinary and gastrointestinal inconveniences, liver disorder, and are also used as an antibacterial, antifungal, antipyretic, and antirheumatic agent in traditional medication.[Citation3] A significant part of human diet contains vegetable oils and fats. Oxidation of lipids occurs in food during technological processing. Oxidation of oils not only causes off-flavor, off–odor, and color changes but also decreases safety and nutritional quality of products, resulting in harmful effects on human health.[Citation4] Consequently, several studies have been undertaken with the aim of enhancing the stability of oils and lipids.
Synthetic antioxidants such as tertiary butylhydroquinone (TBHQ), butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA) are widely used in food products in order to avoid or decrease oxidative effects. Recent studies have shown that such components can cause some health problems such as toxicosis and carcinogenesis. Accordingly, there have been numerous interests to use natural antioxidants available in spices and plant substances as promising substitutes recently.[Citation5] The use of natural antioxidants in food plants has the following advantages: they are accepted by the consumers, they are considered safe, they do not need safety tests, and they have functional and acceptable sensory characteristics.[Citation6]
The antioxidant properties of plants have been reported to be efficient in delaying the development of rancidity in fats and oils. It is well known that a lot of plants and their natural extracts are stable for auto-oxidation due to the presence of natural phenolic compounds.[Citation7–Citation10] Negligible previous studies have yet been conducted on the B. integerrima seed oil (BSO). This report can serve as a milestone toward development of newer natural antioxidants with improved oxidative stability and nutritional value.
In the present study, cold-pressed filtered oils from B. integerrima seeds are studied and chemical composition of the whole seeds and physicochemical properties of the crude oil are determined. In addition, valuable compounds in the oil such as the fatty acid composition, including Essential Fatty Acids (EFAs), total phenolic content, and tocopherol fraction are identified and the antioxidant effectiveness of BSO is determined. The objective of this study was to evaluate the antioxidant capacity of alcoholic extract of BSO using ferric-reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picryl hydrazyl (DPPH·) scavenging ability and to examine the ability of BSO at different levels in delaying soybean oil oxidation as compared to commercial antioxidants.
Materials and methods
Oil and seeds
B. integerrima fruits were purchased from garden of Firoozkuh in northeast of Tehran. For the preparation of materials, all waste substances including stones, thorns, branches, and leaves were carefully removed from fruits. Then, purged fruits were packed in plastic bags and stored at −80°C (JalTeb Lab Equipment, Type J D 300 L) until they were used for testing. For oil extraction, it is necessary to remove the excess water from the fruits. Therefore, the fruits were dried in an oven at 50°C for 48 h to diminish effects of drying process on the extracted oil.[Citation11] Seeds were manually removed from the dried fruits and were ground by Moulinex grinder (Type DPA 1, CMMF 800W, France). All solvents and chemicals used in this study were purchased from Merck (Darmstadt, Germany), Sigma Aldrich (St. Louis, MO), and Rankem (New Delhi, India).
Oil extraction
Barberry seed oil was extracted by the solvent method. In summary, to separate the flesh of dried fruits and grind the seeds, the solvent (petroleum benzene; boiling point: 30–40°C) was added to the barberry seed powder in a closed container and shacked continuously; the ratio of solids to solvent was 1:4 at 25°C for 16 h. Then, the solution was filtered and the remaining solvent was evaporated by using a rotary evaporator below 60°C. The pure oil was transferred into small vials and maintained at −30°C until it was used for testing. Finally, the oil content of barberry seeds (g oil/100 g barberry seed) was calculated.[Citation12]
Physicochemical analysis of crude oil
American Oil Chemists' Society (AOCS) official methods Cc 10a-25 and Cc 7-25[Citation13] were used to determine the density and refractive index (RI) of the extracted oils, respectively. RI was measured by a refractometer (RX-7000a; Atago Co., Japan). Free fatty acid (FFA) content and acid value were determined by titration methods, Ca 5a-40 and Cd 3d- 63, respectively. Saponification number and iodine value were carried out according to Cd 3-25 and Cd 16-87, respectively, defined in AOCS official methods.[Citation13] The unsaponifiable matters in the oil were measured by AOCS method Ca 6b-53.[Citation13] The color of oil was determined by a Lovibond Tintometer (Tintometer Ltd., Salisbury, UK) using a 1-inch cell. Specific extinctions at 232 nm (K232) and 270 nm (K270) were determined according to the AOCS official method Ch5-91[Citation13] using UV-Vis Spectrophotometer (model UVS-2100Shinco, Southern Korea).
Fatty acid composition
To prepare fatty acid methyl esters, 0.1 g oil was placed in a screw cap vial and then 1 mL hexane and 5 mL methanolic NaOH (0.5 N) were added. After heating vial in a boiling water bath for 10 min, it was allowed to cool to room temperature. Then, 2.175 mL of boron trifluoride was added as catalyst and the vial was heated in a boiling water bath for 3 min. After that, the vial was cooled and 1 mL of saturated sodium chloride solution and 1 mL of hexane were added and shaken completely. The vial was settled for 5 min and then 0.2 mL aliquot of the top (hexane) layer was injected into the gas chromatography instrument (GC; Unicam 4600, UK) and analyzed using the conditions defined by Metcalf et al.[Citation14]
Fatty acid profile of barberry seed oil was specified by GC equipped with a flame ionization detector. A fused-silica BPX-70 column (30 m × 0.25 mm i.d., 0.25 μm film thickness, SGE, Melbourne, Australia) was used with helium as the carrier gas (20 psi) to separate the fatty acids. A split injector at 250°C and an flame ionization detector (FID) at 270°C were also used during the separation. The initial temperature of column was 160°C for 6 min and rose to 180°C at a rate of 6°C/min. After 9 min, heating rate enhanced to 20°C/min until it reached to 200°C. Fatty acid quantification was determined by the internal standard method (using C15 as an internal standard).
Alcoholic extraction of oil
In summary, 5000 µL of the BSO was mixed with 500 µL of ethanol. The mixture was vigorously stirred for 1 h, further centrifuged at 3000g for 5 min and kept in −25°C for 30 min to better separate both phases.[Citation15] The alcoholic extract was immediately used for the tests.
DPPH radical scavenging activity
DPPH· radical scavenging activity (RSA) was determined according to the method of Brand-Williams et al.[Citation16] In summary, 2 mL of various concentrations of alcoholic extract was added to 1 mL of methanolic DPPH· solution (0.2 mM) and then mixed. The reactions were carried out in the dark and at room temperature for 1 h. The absorbance value was read at 517 nm against water-DPPH· solution (2:1 V/V) and methanol-DPPH· solution (2:1 V/V) as aqueous and alcoholic control, respectively. The RSA % was calculated according to the following equation:
The results were demonstrated by comparison of IC50 values (the concentration of the antioxidant required to scavenge 50% of DPPH· provided in the test) of ascorbic acid, BHA, BHT, and TBHQ as standards with IC50 values of BSO alcoholic extract.
Ferric reducing antioxidant power (FRAP)
The FRAP assay was performed according to the Benzie and Strain report.[Citation17] This procedure is based on an enhancement of absorbance at 593 nm owing to the formation of tripyridyl-S-triazine complexes with Fe2+ [TPTZ-Fe (II)] in the presence of a reductive agent. The FRAP reagent was prepared from 2.5 mL of TPTZ solution (10 mmol/L) in hydrochloric acid (40 mmol/L) and 2.5 mL of FeCl3 solution (20 mmol/L) mixed with 25 mL of acetate buffer (0.3 mol/L, pH = 3.6). For the designation of the antioxidant capacity, the FRAP reagent (2.08 mL) was mixed with 240 mL of water and 80 mL of the appropriately diluted sample or standard solution of FeSO4·7H2O (25–750 mM). The mixture was allowed standing for 5 min at room temperature before the absorption was measured at 593 nm (Unicam Helios b, Spectronic Unicam, Cambridge, UK). FRAP values, derived from triplicate analyses, were calculated according to the calibration curve for FeSO4.7H2O as follows and expressed as mmol of Fe2+ equivalent per 100 g oil.[Citation17]
where, y = absorbance at 593 nm, x = concentration of FeSO4.7H2O in mM, R2 = 0.9988.
Total phenol contents
Determination of total phenolic content in BSO was performed based on the method described by Fuentes et al.[Citation18] Oil sample (2.5 g) was weighed and dissolved in 5 mL hexane. Phenolic compounds were extracted by 3 mL of 60% (v/v) methanol/water by vortex system for 2 min. The two phases were separated by centrifugation (for 10 min at 3500 rpm), and the hexane phase was extracted in a similar way again. The methanolic extracts were pooled. An aliquot (0.2 mL) of the methanolic phase was removed, and its final volume reached 2.5 mL with water. Then, 0.25 mL of Folin–Ciocalteu reagent was added to this solution, and the solution was settled for 3 min at room temperature. Finally, 0.5 mL of sodium carbonate solution (35%, w/v) was added to this solution, stirred, and diluted with water to 5 mL. The resulting solution was kept for 2 h at room temperature. The absorbance of the final solution was measured at a wavelength of 725 nm. The calibration curve was prepared using standard solutions of Gallic acid within the range of 2–200 mg/L.
Determination of sterols
Sterols were determined using AOCS method Ch 6-91.[Citation13] Trimethylsilyl) derivatives of sterols were prepared by mixing 100 µL each of bis (trimethylsilyl)-trifluoroacetamide, 1% trimethylchlorosilane, and pyridine and heating at 60°C for 30–60 min. GC analysis was carried out on Agilent (Little Falls, DE, USA) gas chromatograph equipped with an FID, a split-splitless injector, and a Supelco SPB-5 (30 m × 0.25 mm i.d., 0.25 µm of film thickness, BSO on oxidative stability of soybean oil; Supelco, Inc. Bellefonte, PA, USA). The velocity of helium, as a carrier gas, was 20 cm/s. The chromatographic conditions employed were injection in the split mode with a split flow of 25 mL/min with a split ratio of 1:22; pressure at column head, 200 kPa; injector temperature, 295°C; detector temperature, 300°C; initial oven temperature, 265°C; initial time, 35 min; final temperature, 300°C; final time, 5 min; and injection volume, 1.0 µL. Peaks were identified by comparing the retention times of sterols with those of the standards. Quantification of all sterols was based on an internal standard method using α-cholestanol (5–750 mg).
Determination of tocopherols
The tocopherol composition of oil was determined based on the AOCS method Ce 8-89.[Citation13] Tocopherols were determined by HPLC Agilent Technologies L1200, normal phase silica column YMC-Pack SIL (250 mm × 46 mm i.d. and 5 µm particle size). The chromatographic system included a UV-Vis detector set at 292 nm. Separation of all tocopherols is based on isocratic elution when the solvent flow rate is set at 1 mL/min. The solvent system selected for tocopherol elution was acetonitrile/methanol/water (5:47.5:47.5 v/v). Prior to HPLC analysis, 2 g of oil was diluted with 100 mL of hexane and filtered (0.45 mm nylon syringe filter). Then, 20 µL samples were injected. The isomers were identified by retention time, co-chromatography, and UV-Vis absorption spectrum as compared to standards [α-, β-, γ-, and δ-tocopherol (purity ≥ 95%)] analyzed in the same conditions. The quantification was carried out by external calibration curves for α-, β-, γ-, and δ-tocopherol.
Determination of β-carotene content
The β-carotene content of the oil sample was analyzed according to the method of Lianhe et al.[Citation19] Briefly, 5 mL of acetone–hexane solution (4:6, v/v) was added to 200 mg of the oil and vigorously shaken for 1 min. Then, the absorbance of the solution was determined at 453, 505, 645, and 663 nm. The amount of β-carotene in BSO was calculated according to the following equation:
β-carotene (mg/100 mL) = 0.216 *A663 – 1.220 * A645 – 0.304 * A505 + 0.452 * A453(3)
BSO on oxidative stability of soybean oil
Antioxidative effects of the BSO on lipid peroxidation were evaluated in soybean oil. The BSO was added to soybean oil without antioxidant at 2% and 5% levels. Oxidative stability of oils was evaluated during 0, 2, 5, 10, and 14 days by analyzing the peroxide values (PVs), thiobarbituric acid (TBA), conjugated dienes (CD), conjugated trienes (CT), and Rancimat indices against a control sample (soybean oil without any antioxidant = CO) and soybean oil containing 200 ppm of TBHQ as synthetic antioxidant. Each sample contains 50 g of oil. They were stored in sealed dark glass containers at room temperature.
Peroxide value and thiobarbituric acid
PVs (mEq of O2/kg) were measured by AOCS cd 8-53 official method.[Citation13] TBA was determined using the method of AOCS Cd 19-19.[Citation13] This procedure allowed the direct determination of TBA in oils and fats without preliminary isolation of secondary oxidation products.
Conjugated dienes and trienes
The absorption values at 232 nm (for CD) and 270 nm (for CT) were recorded by spectrophotometry (model UVS-2100Shinco, Southern Korea), following the analytical methods described by International Union of Pure and Applied Chemistry (IUPAC) , method II.D.23.[Citation20] The results were expressed as absorbance unit (AU).
Rancimat
An automated Metrohm Rancimat apparatus (model 743) was used to determine oxidative stability index (OSI) of the oils according to the procedure described by Anwar et al.[Citation21] Briefly, 3 g oil was carefully weighed into each of the reaction vessels and analysis was performed at 120°C at an airflow rate of 20 L/h. The OSI of the samples was automatically recorded corresponding to the break point in the plotted curves.
Statistical analysis
The data are presented as the means ± standard deviations from the three replicates. The data were analyzed using a one-factor analysis of variance, and means were compared by the least significant difference (LSD) test with the Statistical Analysis System (SAS) software. The standard level of significance used to justify a claim of a statistically significant effect is 0.05 (p < 0.05).
Results and discussion
Fatty acids composition of BSO
The oil content of B. integerrima seed was found to be 11.4% ± 0.7%. According to , three major fatty acids, including linolenic, linoleic, and oleic, were found in the BSO. These unsaturated fatty acids constituted more than 91% of the total amount. Since the barberry seed oil has large amounts of linolenic and linoleic, it is a rich source of ω-3 and ω-6 fatty acid. n-6/n-3 ratio in this oil is close to 1, which is important from a nutritional physiological point of view. According to other studies, linolenic, linoleic, and oleic fatty acids are the main fatty acids in other berry seed oils,[Citation22] but their amount varies in the oils studied here. The barberry seed oil contained about 8% saturated fatty acids, mainly palmitic acid (5.9%) and stearic acid (1.9%). Also, the amount of other fatty acids in the barberry seed oil was very low, according to the results reported in the literature.[Citation23]
Table 1. Fatty acid composition and physicochemical characteristics of the B. integerrima seed oil.
Physicochemical characterization of barberry seed oil
Results of some physicochemical properties of the BSO are presented in . Physical attributes of lipids are determined by chemical structures and functional groups and greatly impress the role of lipids in foods. The barberry seed oil was yellowish-brown in color and liquid at room temperature (25°C ± 1°C), even in a refrigerator. The density of oil was 0.821 ± 0.004 g/cm3. RI is used to measure the unsaturation changes during hydrogenation of oil or fat. The barberry seed oil showed an RI of 1.4780 ± 0.0001 at 25°C, which was higher than that reported for pumpkin oil.[Citation24]
K232 and K270 are simple and helpful parameters for evaluation of the oil oxidation. K232 is usually considered as an indicator of the primary oxidation products, CD. K270 measures the presence of CT as secondary oxidation products of oil, ketones, and aldehydes and primary oxidation products of linolenic acid.[Citation24] As shown in , the K232, K270, and R-value (K232/K270) of barberry seed oil were 3.9 ± 0.2, 2.2 ± 0.1, and 1.8 ± 0.0, respectively.
The FFA% and acid value for BSO were measured about 0.7% ± 0.01% as oleic acid and 1.4 ± 0.02 mg KOH/g oil, respectively. So, this oil is suitable for edible aims, and its acid value did not exceed the maximum limit of 4.0 (mg KOH/g oil) according to the New Zealand Food Regulation (1984) and Codex Alimentarius Commission (1999).[Citation25] Van Hoed et al.[Citation23] have studied five seed oils of berry species. They reported all oils, which had an FFA value between 0.5% and 1.5% as oleic acid equivalents.
The BSO had an iodine value about 180.0 ± 0.9 (). The high iodine value in B. integerrima seed was an indicative of the presence of a higher number of unsaturated bonds. The saponification value (SV) is an indicator of the average length of fatty acid. It is inversely proportional to the molecular weight of the lipid. According to , the SV of BSO was 197.2 ± 2.0 (mg KOH/g oil). Unsaponifiable matters in the vegetable oils are various nonglyceridic bioactive materials containing aldehydes, pigments, hydrocarbons, alcohols, ketones, fat-soluble vitamins, and sterols that may form naturally or may be formed during the process or degradation of oils.[Citation24] As shown in , the content of unsaponifiable matters was 2.3% ± 0.2% for the oil.
β-Carotene is profitable for long-term storage of oils since it is a secondary or preventive antioxidant acting as a singlet oxygen quencher.[Citation26] In this study, the amount of β-carotene was 48.9 ± 0.2 mg/kg oil, which is lower than those reported for palm seed oil.[Citation19] Color of extracted crude oil exhibited red unit 4.9, yellow unit 18.6, and blue unit 1.9, respectively. The BSO was yellowish-brown in color.
Total phenol contents
Lately, there has been an increasing interest in the study of phenolic compounds in the oil seeds because they stand for potentially health-promoting matters and have industrial applications.[Citation24] These naturally occurring compounds are known to prevent lipid oxidation and have a great effect on the stability, sensitivity, and nutritional attributes of oil and its products.[Citation27] BSO is a very rich source of phenolic compounds since its level (323.0 ± 3.1 mg Gallic acid/kg oil) is much higher than those reported for various vegetable oils (). This is within the range reported by Lianhe et al. for olive oil.[Citation19]
Table 2. Comparison of phenolic content of B. integerrima seed oil with other oils.
Sterols
Phytosterols, as natural matters of vegetable oils, have received special attention due to their ability to lower serum cholesterol contents in humans, and, as a result, the risk of heart diseases will be reduced.[Citation28] The information is lacking about the content of phytosterols in BSO. In the current study, the total sterol content was found to be 762.3 mg/100 g oil. The total sterol reported in literature ranges from 404 to 692 mg/100 g oil content for five different berry seed oils.[Citation23] The main sterol in this sample was β-sitosterol (71.8%), similar to most vegetable oils. In the BSO, other phytosterols were campesterol (15.8%), following the ∆5-avenasterol (7%) and stigmasterol (3.1%) ().
Table 3. Sterol, tocopherol, and β-carotene contents of B. integerrima seed oil.
Tocopherols
The determination of tocopherol homologues in the BSO is important owing to their antioxidative effects, which provide some protection against oil oxidation by terminating free radicals, and their positive nutritional effects in human metabolism as biological antioxidants.[Citation27] As seen from , α- and γ-tocopherol contents of the oil were 94.0 and 17.1 mg/100 g, respectively. Β- and δ-tocopherol were not detected in the sample tested. α-Tocopherol is the most important lipid-soluble antioxidant in human body.[Citation22] The content of α-tocopherol in BSO was close to the level found in sea buckthorn seed oil and higher than the amount found in the raspberry and blackcurrant seed oils.[Citation22]
Evaluation of antioxidant activity of BSO ethanolic extracts
DPPH· scavenging activity
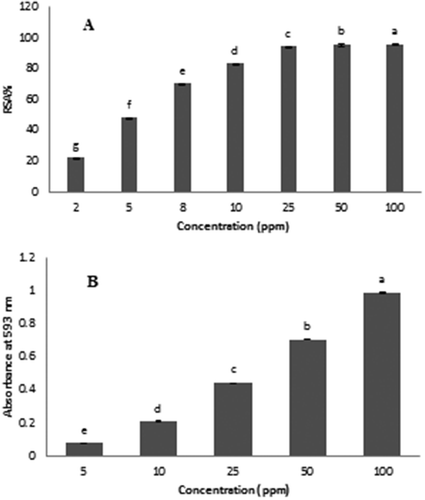
shows the relationship between DPPH· RSA and concentration of 2–100 mL/L of BSO alcoholic extract. As seen from , DPPH· RSA increases when the BSO concentration increases. Phenolic compounds are common among secondary metabolites that can be derived from plants in pentose phosphate, shikimate, and phenylpropanoid cycles. Phenols have high scavenging ability of radicals owing to the presence of hydroxyl groups in their structure. Hence, it has been reported that phenol compounds are associated with antioxidant activity and play a major role in preventing fat and oil peroxidation.[Citation29] As explained above, BSO had high amounts of phenolic compounds. The IC50 index is the concentration of substance, which is able to have 50% scavenging effect on radical. Using this test, IC50 of BSO ethanolic extract was determined to be about 5.47 ± 0.01 mL/L. IC50 of BSO ethanolic extract was lower than BHT and BHA synthetic antioxidants.
Ferric-reducing antioxidant power
The reducing power (RP) in this study was determined as the Fe3+ to Fe2+ transformation, and RP increased with increasing the concentration of phenolic compounds in the BOS extract. According to this test, the RP of BSO was determined to be 5.68 µmol Fe (II) per gram of oil. RP is generally associated with the presence of reducing substances, which have been revealed to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom.[Citation30] The results of RP are in good agreement with the content of phenolic compounds (). Koca and Karadeniz[Citation31] observed a linear relationship between FRAP values and total phenolic compounds for blueberries.
Effect of BSO on lipid oxidation in soybean oil
Peroxide value
Hydroperoxides are the primary product of lipid oxidation. Therefore, PV determination can be used as oxidative index during the initial stage of lipid oxidation.[Citation31] The PVs were calculated for soybean oil without any antioxidant as control sample (CO), soybean oil with 2% BSO (B2), soybean oil with 5% BSO (B5), and soybean oil with 200 ppm TBHQ (TBHQ) during 14 days at room temperature and are shown in . The initial PV of CO was 5 mEq O2/kg oil. By adding BSO (such as TBHQ) to the soybean oil resulted in fixing their PV during storage, thus showing enhancement of the oxidative stability of CO. Our results indicate that PV of CO increases during storage period as follows: the initial PV of CO was 5 mEq O2/kg oil and PV was 10, 14, 12, and 12 mEq O2/kg oil, respectively, after 2, 5, 10, and 14 days. At the end of storage period, CO had the highest PV (12 mEq/kg) and was oxidized rapidly, while the PV for B2, B5, and TBHQ was stable and did not have any changes during storage period, suggesting that BSO delays the onset of primary oxidation. The PV results demonstrate that the BSO was an effective antioxidant in this test as TBHQ.
Table 4. Effect of the BSO in soybean oil oxidation during 14 days in room temperature.
Thiobarbituric acid
The reaction between TBA and malonaldehyde produced by lipid hydroperoxide decomposition is employed as the basis of measurement of secondary oxidation products.[Citation32] TBA values reveal that BSO at 2% and 5% significantly inhibit soybean oil oxidation (p < 0.05). Our results indicate that BSO at 5% prevents soybean oil oxidation better than BOS at 2% (). In general, the percentage inhibition of soybean oil oxidation was concentration-dependent. However, BOS at both levels effectively controlled soybean oil oxidation. TBHQ was significantly better than other samples to prevent soybean oil oxidation during storage period (p < 0.05), probably due to its purification (). Furthermore, the TBA value of CO was significantly higher than the B2, B5, and TBHQ samples ().
Ultraviolet absorption (conjugated dienes and trienes)
Lipids containing methylene-interrupted dienes and polyenes exhibit a shift due to their double bond position during oxidation. The resulting CD reveals an intense absorption at 232 nm and has been well associated with PV; similarly, CT absorbs at 270 nm. The greater the levels of CD and CT in oil, the lower will be the oxidative stability.[Citation31]
Absorption at 232 and 270 nm was observed in all samples. This revealed a pattern similar to that of PV due to the formation of primary and secondary products of lipid oxidation (). Absorption at 232 nm increased progressively with increasing time due to the formation of CD (). Formation of ketones, aldehydes, and other oxidation products leads to an increase in absorption at 270 nm ().
The variation of absorption at 232 and 270 nm may indicate that the oxidation products have been formed or broken down. Our results show that the rate of formation of oxidation products in B5, B2, and TBHQ was lower than CO. So, BSO could develop oxidative stability such as TBHQ. In all days, CO has maximum CD and CT levels. B5 and TBHQ did not have significant difference to prevent CD and CT formation during storage period (p < 0.05). The amount of CD and CT in B2 was lower than CO (p < 0.05). There was no significant difference between B2, B5, and TBHQ except in fifth day for CD and fourth day for CT. Our results reveal that B5 (such as TBHQ) contains the lowest conjugated oxidation products, and these results are in accordance with the PV.
Rancimat
The OSI clearly shows that the oxidative stability of all samples decreases when the storage time increases (). In all days, TBHQ exhibited maximum oxidative stability. When the oxidative stability of CO has the least value, the result is statistically significant (p < 0.05). The BSO at 2% level was more effective than 5% to extend oxidation stability of soybean oil. This can be attributed to high amount of its linoleic and linolenic acids (more than 75% of total fatty acids). This study also indicates that the effect of BSO to improve oxidative stability of soybean oil at both levels (2% and 5%) is significant (). These findings are in agreement with the results of Van hoed et al.,[Citation23] who reported that berry seed oils have a rather low oxidative stability and thus require careful packaging and storage. There was no literature available for BSO.
Conclusion
There is a growing consumer consciousness for healthy food products. From the present study, it is concluded that BSO can be considered as an antioxidant to the edible oils. BSO showed antioxidative potency in a descending order when it was added to soybean oil. In this study, the protective effect of the BSO is compared with synthetic antioxidant TBHQ. Thus, it could be prepared and added to the vegetable oils as a natural antioxidant and a suitable alternative for synthetic antioxidants such as TBHQ.
References
- Alemardan, A.; Asadi, W.; Rezaei, M.; Tabrizi, L.; Mohammadi, S. Cultivation of Iranian Seedless Barberry (Berberis integerrima ‘Bidaneh’): A Medicinal Shrub. Ind. Crops Prod. 2013, 50, 276–287.
- Berenji Ardestani, S.; Sahari, M. A.; Barzegar, M.; Abbasi, S. Some Physicochemical Properties of Iranian Native Barberry Fruits (Abi and Poloei): Berberis integerrima and Berberis Vulgaris. J. Food Pharm. Sci. 2013, 1, 67–74.
- Hosseinzadeh, H.; Ramezani, M.; Shafaei, H.; Taghiabadi, E. Anticonvulsant Effect of Berberis integerrima L. Root Extracts in Mice. J. Acupunct. Meridian Stud. 2013, 6(1), 12–17.
- Yang, Y.; Songa, X.; Suia, X.; Qia, B.; Wanga, Z.; Lia, Y.; Jiang, L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crops Prod. 2016, 80, 141–147.
- Stojiljkovi´C, D.; Arsi´Cb, I.; Tadi´, V. Extracts of Wild Apple Fruit (Malus sylvestris (L.) Mill., Rosaceae) as a Source of Antioxidant Substances for Use in Production of Nutraceuticals and Cosmeceuticals. Ind. Crops Prod. 2016, 80, 165–176.
- Jorge, N.; Médici Veronezi, C.; Vieira Del, R. P. Antioxidant Effect of Thyme (Thymusvulgaris L.) And Oregano (Origanumvulgare L.) Extracts in Soybean Oil under Thermoxidation. J. Food Process. Preservation. 2015, 39, 1399–1406.
- Zaborowska, Z.; Przygoński, K.; Bilska, A. Antioxidative Effect of Thyme (Thymus vulgaris) in Sunflower Oil. Acta Scientiarum Polonorum. Technol. Alimentaria. 2012, 11, 283–291.
- Wang, J.; Shahidi, F. Oxidative Stability of Marine Oils as Affected by Added Wheat Germ Oil. Int. J. Food Properties. 2017. DOI: 10.1080/10942912.2017.1286507.
- Keramat, M.; Golmakani, M. T.; Aminlari, M.; Shekarforoush, S. S. Comparative Effect of Bunium persicum and Rosmarinus officinalis Essential Oils and Their Synergy with Citric Acid on the Oxidation of Virgin Olive Oil. Int. J. Food Properties. 2016, 19(12), 2666–2681.
- Saravana Babu, P. A.; Aafrin, B. V.; Archana, G.; Sabina, K.; Sudharsan, K.; Sivarajan, M.; Sukumar, M. Effects of Polyphenols from Caralluma fimbriata on Acrylamide Formation and Lipid oxidation—An Integrated Approach of Nutritional Quality and Degradation of Fried Food. Int. J. Food Properties. 2017, 20(6), 1378–1390.
- Motalleb, G.; Hanachi, P.; Kua, S. H.; Fauziah, O.; Asmah, R. Evaluation of Phenolic Content and Total Antioxidant Activity in Berberis Vulgaris Fruit Extracts. Int. J. Biol. Sci. 2005, 5, 648–653.
- Zarringhalami, S.; Sahari, M. A.; Barzegar, M.; Hamidi Esfehani, Z. Changes in Oil Content, Chemical Properties, Fatty Acid Composition and Triacylglycerol Species of Tea Seed Oil during Maturity Period. J. Food Biochem. 2011, 35, 1161–1169.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; Press: Champaign, IL, United states, 1997.
- Metcalf, L. C.; Schmirz, A. A.; Pelka, J. R. Rapid Preparation of Methyl Esters from Lipid for Gas Chromatography. Anal. Chem. 1966, 38, 514–515.
- Espín, J. C.; Soler-Rivas, C.; Wichers, H. J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2, 2-Diphenyl-1-Picrylhydrazyl Radical. J. Agr. Food Chem. 2000, 8, 648–656.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30.
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76.
- Fuentes, E.; Báez, M. E.; Bravo, M.; Cid, C.; Labra, F. Determination of Total Phenolic Content in Olive Oil Samples by UV–Visible Spectrometry and Multivariate Calibration. Food Anal. Methods. 2012, 5, 1311–1319.
- Lianhe, Z.; Xing, H.; Li, W.; Zhengxing, C. Physicochemical Properties, Chemical Composition and Antioxidant Activity of Dalbergia odorifera T. Chen Seed Oil. J. Am. Oil Chem. Soc. 2012, 89, 883–890.
- IUPAC. Standard Methods for the Analysis of Oils and Fats and Derivatives; Press: Pergamon, Canada, 1979.
- Anwar, F.; Bhanger, M. I.; Kazi, T. G. Relationship between Rancimat and Active Oxygen Method Values at Varying Temperatures for Several Oils and Fats. J. Am. Oil Chem. Soc. 2003, 80, 151–155.
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and Antioxidative Activities of Supercritical CO2-extracted Oils from Seeds and Soft Parts of Northern Berries. Food Res. Int. 2011, 44, 2009–2011.
- Van Hoed, V.; Clercq, N. D.; Echim, C.; Jelcovic, M.; Leber, E.; Dewettinck, K.; Verhe, R. Berry Seeds: A Source of Specialty Oils with High Content of Bioactives and Nutritional Value. J. Food Lipids. 2009, 16, 33–49.
- Gohari Ardabili, A.; Farhoosh, R.; Haddad Khodaparast, M. H. Chemical Composition and Physicochemical Properties of Pumpkin Seeds (Cucurbita pepo Subsp. Pepo Var. Styriaka) Grown in Iran. J. Agric. Sci. Technol. 2011, 13, 1053–1063.
- Teh, S. S.; Birch, J. Physicochemical and Quality Characteristics of Cold-Pressed Hemp, Flax and Canola Seed Oils. J. Food Comp. Anal. 2013, 30, 26–31.
- Castelo-Branco, V. N.; Torres, A. G. Potential Application of Antioxidant Capacity Assays to Assess the Quality of Edible Vegetable Oils. Lipid Technol. 2009, 21, 152–155.
- Kasote, D. M.; Badhe, Y. S.; Hegde, M. V. Effect of Mechanical Press Oil Extraction Processing on Quality of Linseed Oil. Ind. Crops Prod. 2013, 42, 10–13.
- Li, T. S. C.; Beveridge, T. H. J.; Drover, J. C. G. Phytosterol Content of Sea Buckthorn (Hippophae rhamnoides L.) Seed Oil: Extraction and Identification. Food Chem. 2007, 101, 1633–1639.
- Saoudi, S.; Chammem, N.; Sifaoui, I.; Bouassida-Beji, M.; Jiménez, I. A.; Bazzocchi, I. L.; Silva, S. D.; Hamdi, M.; Bronze, M. R. Influence of Tunisian Aromatic Plants on the Prevention of Oxidation in Soybean Oil under Heating and Frying Conditions. Food Chem. 2016, 212, 503–511.
- Wu, Y.; Huib, D.; Eskinc, N. A. M.; Cuid, S. W. Water-Soluble Yellow Mustard Mucilage: A Novel Ingredient with Potent Antioxidant Properties. Int. J. Biol. Macromol. 2016, 91, 710–715.
- Koca, I.; Karadeniz, B. Antioxidant Properties of Blackberry and Blueberry Fruits Grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450.
- Vaisali, C.; Belur, P. D.; Regupathi, I. Comparison of Antioxidant Properties of Phenolic Compounds and Their Effectiveness in Imparting Oxidative Stability to Sardine Oil during Storage. LWT Food Sci. Technol. 2016, 69, 153–160.