ABSTRACT
We extracted soluble dietary fiber from yellow (YSDF) and purple (PSDF) fleshed potatoes by-product to compare their physicochemical properties and structural characteristics. YSDF and PSDF showed similar monosaccharide compositions. YSDF gave a higher oil-holding capacity and swelling capacity (15.6 ± 1.1 g/g, 18.7 ± 0.8 mL/g) than PSDF (12.5 ± 0.9 g/g, 13.5 ± 1.0 mL/g). Emulsifying activity and emulsion stability of YSDF (33.8 ± 1.4 mL/100 mL and 31.5 ± 0.3 mL/100 mL, respectively) were significantly higher than those of PSDF (24.8 ± 1.1 mL/100 mL and 19.6 ± 1.8 mL/100 mL, respectively). Although PSDF showed a stronger DPPH (1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl) and ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonate)) free radical-scavenging activities (0.18 ± 0.01 and 2.39 ± 0.20 mg Trolox/g SDF), FRAP (ferric-reducing antioxidant power) free radical-scavenging activity was similar to that of YSDF. Furthermore, molecular weight of YSDF (3967.3 Da) was lower than that of PSDF (4462.8 Da). Thermal property was different between YSDF and PSDF, and YSDF presented more rough, irregular, and porous surface. It can be concluded that SDFs from yellow fleshed potatoes are more suitable to use as fiber-rich functional ingredients.
Introduction
Dietary fiber (DF), indigestible carbohydrate of plant origin, is known as the seventh important nutrient for human. It is very beneficial to our health for it can reduce risks of cancer, cardiovascular diseases, diabetes, and asthma.[Citation1,Citation2] Furthermore, DF also has some physicochemical properties, including hydration properties, oil-holding capacity (OHC) and swelling capacity (SC), as well as emulsifying activity (EA) and emulsifying stability (ES).[Citation3–Citation5] The physicochemical properties are beneficial to the food industry as DFs can improve food properties (texture, color, water holding, and gel capacities) of fruits, cereals, and meat products.[Citation6] DFs can be classified as soluble dietary fibers (SDFs) or as insoluble dietary fibers (IDFs), according to their solubility in water.[Citation6] Additionally, several intervention studies have shown that SDFs were more beneficial to human health than IDFs.[Citation7]
Potato (Solanum tuberosum L.) is the dominant tuber crop worldwide and is consumed as a good source of energy for high content of carbohydrates traditionally. With the increasing demand for health life and diet, there is an increasing interest in cultivating potato with colored flesh.[Citation8–Citation10] Purple-fleshed potato, a kind of potatoes, has attracted a great attention from the public due to high levels of anthocyanins in it.[Citation11] For purple-fleshed potato, more attention of researchers and the public is being focused on its beneficial effects on health for it has abundant anthocyanins.[Citation12] While DF is also rich in purple-fleshed potato, which is same as the content of crude protein.[Citation11] Additionally, DF content is in the range of 1 and 2 g/100 g fresh weight in raw potato.[Citation13] In food industry, potato is mainly used to produce starch, and the peels as well as residues are usually discarded or used as fodders. Additional values of the by-products with this processing way are very low and bad for the environment. Because potato is quantitatively important in the diet, it is a significant source of DFs.[Citation13]
Recently, few papers have reported the application of potato DFs in food industry. Curti et al.[Citation14] used potato fibers to improve bread physicochemical properties during storage and concluded that potato fiber addition in bread contributed to reduce bread stalling. Sudha et al.[Citation15] demonstrated that potato peel was rich in DFs, a by-product from potato industry, and used as a source of DFs in bread making. However, a comprehensive study on physicochemical and functional properties as well as structural characteristics of potatoes DFs has not been reported.
Therefore, SDFs were extracted from by-products of yellow and purple fleshed potatoes using an enzymatic method. Their physicochemical, functional, thermal properties, and the chemical compositions as well as structural features were determined. The aims of this study were to comprehensively investigate SDFs from potatoes by-products and establish their potential application as functional ingredients and compare the differences between the SDFs from yellow and purple fleshed potatoes by-products.
Materials and methods
Samples and reagents
Fresh yellow and purple fleshed potatoes were harvested in October 2016, and the by-products were provided by Lanzhou Shenrong Co., Ltd., Gansu Province, China. α-Amylase, trypsin, and amyloglucosidase were obtained from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals mentioned were purchased from Sinopharm Chemical Reagent (Shanghai, China).
Sample preparation
The by-products were dried and milled to obtain the powder, and then defatted with ethyl ether in a Soxhlet. SDFs were extracted from the dried powder using the AOAC (Association of Official Analytical Chemists) method with slight modification (AOAC 991.43, 1996).[Citation16] One gram of the powder was dispersed in a beaker with 40 mL buffer (MES-Tris, pH 8.2). Then, 50 μL α-amylase solution was added to hydrolyze the powder at 95°C for 35 min. When the temperature was cooled to 60°C, trypsin (100 μL, 50 mg/mL) was added to hydrolyze. Then, 300 μL of amyloglucosidase solution was added to react for 30 min (pH 4.5). Through centrifuging at 10,000 rpm for 10, supernatant of the hydrate was collected and then condensed to 5 mL. SDFs were obtained using ethanol deposition method, and the obtained powder was named as YSDF and PSDF, respectively.
Chemical analysis of SDFs
Moisture and ash contents were measured using the AOAC method 2001.12 (AOAC 2001).[Citation17] Monosaccharide compositions was determined using the method of Xie and others (2017), with some modifications. SDFs (5 mg) were hydrolyzed using 2 M TFA (trifluoroacetic acid) at 120°C for 3 h. Then alditol acetates, acetic anhydride, and pyridine were added into the hydrolysate, and the mixture was reacted at 90°C for 30 min. Quantification was performed in a GC-MS system (Agilent 7890-5875, Agilent Technology, USA). Glucose, xylose, galactose, arabinose, rhamnose, and mannose were used as the authentic standards.
Physicochemical and functional properties of SDFs
OHC and SC
OHC (g oil/g SDF) and SC (mL/g SDF) were determined according to the methods described by Xie et al.[Citation18] and Wang et al.[Citation19]
EA and ES
EA and ES were determined following Lan et al.[Citation20] Briefly, 25 mL of 2 g/100 mL fibrous suspension in a graduated centrifuge tube was homogenized with 25 mL of corn oil for 5 min. After centrifuging at 1200 rpm for 5 min, the EA was calculated using the equation
where V1 is the volume of emulsifying layer, and V is the total volume. Then the prepared emulsion was heated at 80°C for 30 min to calculate the ES using the equation
where V1 is the volume of emulsifying layer, and V is the total volume.
Antioxidant activities
Antioxidant activities were determined using DPPH/ABTS/FRAP assay.[Citation21] Preparation of DPPH free radical solution was conducted by dissolving 19.7 mg of DPPH in 100 mL of ethanol. Then the stock solution was diluted with ethanol to get an absorbance of 0.70 ± 0.05 at 525 nm to obtain the working solution of DPPH radical. ABTS solution (5 mL 7 mM) was mixed with and 88 μL 140 mM of potassium persulphate solution to obtained the ABTS free radical solution. The stock solution was diluted with ethanol to obtain the working solution, and the absorbance value was at 0.70 ± 0.02 at 734 nm. FRAP solution was composed of Na-Ac buffer (500 mL, 300 mM, pH 3.6), TPTZ (2, 3, 5-triphenyl-2h-tetrazolium,chloride) (50 mL 10 mM), and 50 mL 20 mM of FeCl3.
Afterward, 4.9 mL of the working solution and 0.1 mL distilled water were added into a centrifuge tube containing 10 mg of SDFs to start the reaction under rigorous shake for 30 min. Then, the mixture was centrifuged to obtain the supernatant at 9000 rpm for 10 min. The absorbance was recorded using a UV-2802S spectrophotometer at 525/734/593 nm respectively (Unico, Shanghai, China).
Trolox equivalent antioxidant activity (TEAC) was used to compare the free radical-scavenging activity of each sample. Briefly, 0.1 mg/mL of trolox solution (0–100 μL) and water (100–0 μL) were added to 4.9 mL of the working solution (total volume 5 mL). TEAC of the samples (per gram SDF) was calculated using the standard calibration curves and the equation
where a is the slope, b represents the intercept of trolox calibration curves, and m is the SDF amount.
Molecular weight distribution analysis of SDFs
The gel permeation chromatography (HLC-8320GPC, TOSOH Bioscience, Tokyo, Japan) with a 7.8 × 300 mm column (G4000PWXL, TOSOH Bioscience, Tokyo, Japan) was employed to determine the molecular weight distribution of SDFs. The average molecular weight was determined using the external standard method with dextran as the standard, and distilled water was used as the mobile phase.[Citation22]
Thermal analysis of SDFs
Thermogravimetry (TG) and differential scanning calorimetry (DSC) of the SDFs were recorded by a simultaneous DSC-TGA (Q600, TA, USA) according to the method of Xie et al.[Citation18] The condition was as follows: the temperature was increased from 20°C to 600°C with a liner heating rate 10°C/min, nitrogen atmosphere (75 mL/min), the weight of sample was approximately 3–5 mg, and empty crucible was used as the reference.
Fourier transforms infrared spectroscopy of SDFs
The Fourier transforms infrared (FT-IR) spectra of the SDFs were recorded using an VERTEX-70 Fourier transform infrared spectroscopy (Bruker Co., Germany). Wavelength was set in the range of 4000 to 600 cm−1 at a resolution of 0.44 cm−1. KBr was used to dilute the samples (1:100, v/v) before acquisition.
Scanning electron microscopy of SDFs
The morphological characteristics of the SDFs were observed using a high-resolution field emission scanning electron microscopy (FE-SEM) Sirion 200 (FEI Company, USA) with 500-, 4000-, 8000-, and 30,000-fold magnification.[Citation18] The SDFs were fixed on a specimen holder and coated with gold powder.
Statistical analysis
All experimental results were expressed as means ± standard deviation (n = 3). Statistical analysis was conducted using SPSS software (SPSS for Windows, 16.0, 2007, SPSS Inc., USA). Significant differences were analyzed using Turkey test at the level of P < 0.05.
Results and discussion
Chemical composition analysis of SDFs
lists the moisture, ash, and monosaccharide compositions of YSDF and PSDF. The moisture of PSDF was significantly higher than that of YSDF, but no significant difference was found in ash content. Monosaccharide composition of YSDF and PSDF was similar, which were mainly composed of arabinose, mannose, glucose, galactose, and rhamnose. Furthermore, galactose was the major monosaccharide of YSDF (95.77% ± 3.11%) and PSDF (94.19% ± 4.02%). This result was in agreement with previous reports on non-starch polysaccharide residues of potato DFs, which demonstrated that glucose, galactose, arabinose, and mannose were the major monosaccharide composition.[Citation23] Cheng et al.[Citation24] also found that glucose and galactose were the main monosaccharides of SDFs from potato pulp. The results indicate that chemical compositions of YSDF were same as those of PSDF.
Table 1. Chemical compositions of SDFs (% dry matter).
Physicochemical and functional properties of SDFs
OHC and SC
Chemical and structural natures as well as particle size of DFs determine OIHC and SC.[Citation25] The OHC and SC of the SDFs are listed in . YSDF showed a slightly higher OHC (15.6 ± 1.1 g/g) than that of PSDF (12.5 ± 0.9 g/g). Likewise, a significant difference (P < 0.05) was observed in SC between YSDF (18.7 ± 0.8 mL/g) and PSDF (13.5 ± 1.0 mL/g). These results demonstrated that physicochemical properties of YSDF were better than PSDF; the reason could be due to the differences in chemical and structural natures or particle size of the SDFs.[Citation5] The OHC and SC values of the SDFs were higher than those reported in some vegetables, including carrot (5.5 and 18.3 mL/g, respectively) and onion (2.4–4.3 and 4.0–22.5 mL/g, respectively),[Citation3,Citation26] suggesting that DFs from yellow or purple fleshed potatoes by-product are a good DF resource.
Table 2. Physiochemical properties of SDFs.
EA and ES
EA refers to a molecule’s ability to dissolve or disperse two immiscible liquids. ES is the ability to hold an emulsion and its resistance to disruption.[Citation19,Citation20] The EA and ES of SDFs from yellow and purple fleshed potatoes by-products were evaluated. As can be seen in , the EA and ES of YSDF (33.8 ± 1.4 mL/100 mL and 31.5 ± 0.3 mL/100 mL, respectively) were significantly higher than those of PSDF (24.8 ± 1.1 mL/100 mL and 19.6 ± 1.8 mL/100 mL, respectively). According to the literature, emulsifying ability is related with molecular weight and particle size of the emulsifier, thus the different emulsifying ability of the SDFs may be due to the differences in molecular weight and particle size.[Citation27,Citation28] The results indicate that SDFs from yellow and purple fleshed potatoes by-products have good emulsion properties, but YSDF is more useful in food industry to obtain emulsion formation or to prolong shelf life of foods. The emulsifying ability, a functional property, relates to the ability to inhibit bile acids adsorption in the small intestine, leading to cholesterol degradation in the liver and further reducing its level in the blood.[Citation20,Citation29] Therefore, the addition of the SDFs in foods might be beneficial for food quality and also be better for our health.
Antioxidant activities
Antioxidant activity is one of the known mechanisms by which antioxidants inhibit lipid peroxidation through the scavenging of so-called free radicals.[Citation30] Free radical species in human body can induce cardiovascular and inflammatory diseases and also participate in neurodegenerative disorders, cancer, and aging. DFs rich in antioxidants may help to prevent these pathologies.[Citation21,Citation31] Hence, it is important to evaluate the antioxidant activity of YSDF and PSDF. The results were expressed as the TEAC (mg Trolox/g SDF) and are listed in . As expected, PSDF showed a stronger DPPH free radical-scavenging activity (0.18 ± 0.01 mg Trolox/g SDF) than YSDF (0.04 ± 0.01 mg Trolox/g SDF). Furthermore, ABTS free radical-scavenging activity of PSDF was slightly higher (2.39 ± 0.20 mg Trolox/g SDF) than that of YSDF (1.96 ± 0.09 mg Trolox/g SDF). However, no significant difference (P > 0.05) was found for FRAP free radical-scavenging activity between YSDF and PSDF (0.22 ± 0.02 mg Trolox/g SDF and 0.19 ± 0.02 mg Trolox/g SDF, respectively). It is interesting to find that PSDF did not show a higher FRAP free radical-scavenging activity, although there are a lot of anthocyanins in purple fleshed potatoes, a kind of polyphenolics. The reason could be due to that the FRAP determines only the reducing capability of phenolics to reduce Fe(3+) to Fe(2+) in an aqueous reaction system.[Citation32] In addition, it is also interesting to find that the TEAC values of the SDFs followed the order of ABTS > FRAP > DPPH, which could be explained by the ABTS assay can be used to determine both hydrophilic and lipophilic antioxidant capacities of extracts in multiple media for ABTS can dissolve in both aqueous and organic solvents.[Citation33] Hence, the value of ABTS was almost 10-fold than that of DPPH or FRAP. For DPPH assay is suitable for measuring the antioxidant capacity in the presence of the ethanol/methanol solvent used, the values of DPPH assay were the lowest.[Citation34]
Table 3. Functional properties of DFs.
Molecular weight distribution of SDFs
Molecular weight of SDFs was varied largely in the sight of its intricate compositions, for example, pectin, glucan, gums, and mucilage.[Citation35] We next evaluated the molecular weight of SDFs, and the corresponding results are listed in . It can be seen that the main molecular weights of SDFs from yellow fleshed potato by-products (3967.3 and 1859.3 Da, respectively) were significantly smaller than that of SDFs from purple fleshed potatoes by-products (4462.8 and 2677.2 Da, respectively). It was inferred that a smaller particle size of YSDF was associated with the increased porosity and capillary attraction of SDFs and consequently improve its physicochemical properties, including OHC, EA, and ES. Additionally, polydispersity of YSDF was higher than that of PSDF, suggesting that SDFs from purple fleshed potatoes by-product had a narrow polydispersity.
Table 4. Molecular weight of SDFs.
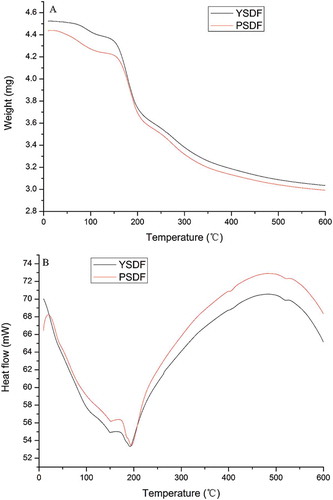
Thermal properties of SDFs
The thermal properties of SDFs from yellow and purple fleshed potatoes by-product were determined and are presented in and . The TG curve profiles of the SDFs were similar, with two mass losses, including water release (50–130°C) and DFs degradation processes (200–500°C). The DTG curves were recorded to determine the temperatures and mass losses during the heating process. In , it can be observed that YSDF gave a higher νmax value (6.9%/min) as compared with that for PSDF (0.87%/min). Likewise, a higher Ton value (57.8°C) was measured for YSDF than that for PSDF (40.3°C). Furthermore, a higher changed mass was observed in PSDF (4.44%). Additionally, the DSC curves allowed calculation of the Ton and enthalpy (∆H). PSDF showed higher Ton (55.8°C) and ∆H values (217.7 J/g) compared with those of YSDF (52.0°C and 65.7 J/g, respectively). These results indicate that water molecules were relatively easily released from SDFs from purple fleshed potatoes by-product. It can be ascribed to a larger molecular weight, in the sight of the relatively easy release of water molecule in the macromolecule of SDFs.[Citation36]
Table 5. Thermal properties of SDFs.
Other than the water release process, the SDFs showed a similar onset temperature (YSDF 223.6°C, PSDF 223.9°C) of the TG curves during the SDFs degradation process. Moreover, PSDF gave a lower νmax values (145.7%/min) than YSDF (219.4%/min). In addition, a significant difference was observed in enthalpy between YSDF (3955 J/g) and PSDF (4309 J/g) on the DSC curves, indicating that thermal stability and homogeneity of PSDF were better than those of YSDF. These differences can be explained by the degradation of large molecule needing more energy.[Citation37]
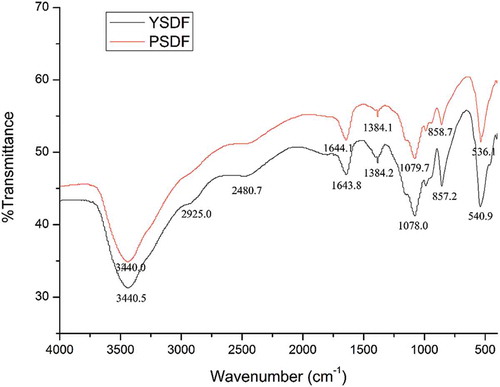
FT-IR spectra of SDFs
The FT-IR spectra of the SDFs from yellow and purple fleshed potatoes by-product were tested and are displayed in . The O–H stretching band at 3440 cm−1 in the FT-IR spectra of the SDFs was attributed to vibrations of the hydrogen bound to the hydroxyl group of glucan.[Citation38] The peaks at 2925 cm−1 were attributed to the C–H stretching bands of the methylene groups of polysaccharides.[Citation35] The strong peak near 1645 cm−1 was attributed to the aromatic benzene. The peak near 1380 cm−1 belonged to the stretching of the carboxyl functional groups.[Citation39] The peak at 1080 cm−1 was a signal of polysaccharides formation associated with C–C stretching vibrations.[Citation40] The FT-IR spectra of YSDF and PSDF were similar, indicating that the chemical compositions and bonds of SDFs from yellow and purple fleshed potatoes by-product were similar, consistent with the monosaccharide compositions result.
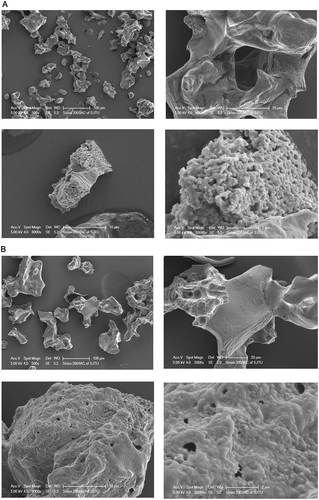
Scanning electron microscopy of SDFs
The particle morphologies of the SDFs from yellow and purple fleshed potatoes by-products were observed by SEM. As can be seen in , YSDF and PSDF showed a rough, irregular, and porous surface, but the surface of YSDF was more uneven than that of PSDF. Interestingly, a special structure, composed of a lot of granules, like a spongy bulk, was observed for the SDFs. Links between the granules were loose, and intervals could be found in the structure of YSDF. But the links were tighter in the surface of PSDF, and the surface was more compact. The differences in structure between YSDF and PSDF could be associated with the differences in the physicochemical properties, including OHC, SC, EA, and ES.[Citation5]
Conclusion
With this study, we found that monosaccharide compositions of the DFs from yellow and purple fleshed potatoes by-product were similar. But physicochemical and functional properties were different between YSDF and PSDF. YSDF gave a higher OHC and SC (15.6 ± 1.1 g/g, 18.7 ± 0.8 mL/g) than PSDF (12.5 ± 0.9 g/g, 13.5 ± 1.0 mL/g). EA and ES of YSDF (33.8 ± 1.4 mL/100 mL and 31.5 ± 0.3 mL/100 mL, respectively) were significantly higher than those of PSDF (24.8 ± 1.1 mL/100 mL and 19.6 ± 1.8 mL/100 mL, respectively). Although PSDF showed stronger DPPH and ABTS free radical-scavenging activities (0.18 ± 0.01 and 2.39 ± 0.20 mg Trolox/g SDF), FRAP free radical-scavenging activity was similar to that of YSDF. Furthermore, molecular weight of YSDF (3967.3 Da) was lower than that of PSDF (4462.8 Da). The FT-IR spectra illustrated chemical compositions and bonds of YSDF were similar to those of PSDF. The TG–DSC curves also demonstrated that YSDF and PSDF gave different thermal properties. YSDF presented a more irregular, rough, and porous surface, and PSDF exhibited an even surface, and the surface was relatively flat and compact. Accordingly, this study provides a comparative understanding of the SDFs from yellow and purple fleshed potatoes, and the differences in physicochemical and functional properties as well as structural characteristics between YSDF and PSDF. It can be concluded that the obtained SDFs have good physicochemical properties, but YSDF is more suitable to use as a fiber-rich ingredient in functional foods.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Funding
References
- Brownlee, I. A.;. The Physiological Roles of Dietary Fibre. Food Hydrocoll. 2011, 25, 238–250.
- Chen, J.; Zhao, Q.; Wang, L.; Zha, S.;, et al. Physicochemical and Functional Properties of Dietary Fibers from Maca (Lepidium Meyenii Walp.) Liquor Residue. Carbohydr. Polym. 2015, 132, 509–512.
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.;, et al. Dietary Fibre and Fibre-Rich By-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421.
- Tosh, S. M.; Yada, S.; Bassett, C.; Boye, J.;, et al. Dietary Fibres in Pulse Seeds and Fractions: Characterization, Functional Attributes, and Applications. Food Res. Int. 2010, 43, 450–460.
- Xie, F.; Wang, Y.; Wu, J.; Wang, Z. Functional Properties and Morphological Characters of Soluble Dietary Fibers in Different Edible Parts of Angelica Keiskei. J. Food Sci. 2016, 81, C2189–C2198.
- Tao, B.; Ye, F.; Li, H.; Hu, Q.;, et al. Phenolic Profile and in Vitro Antioxidant Capacity of Insoluble Dietary Fibers Powders from Citrus (Citrus Junos Sieb. Ex Tanaka) Pomace as Affected by Ultrafine Grinding. J. Agric. Food Chem. 2014, 62, 7166–7173.
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of Insoluble Dietary Fibres in Soya Bean Okara and Their Physicochemical Properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613.
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial Phytochemicals in Potato — A Review. Food Res. Int. 2013, 50, 487–496.
- Kotíková, Z.; Šulc, M.; Lachman, J.; Pivec, V.;, et al. Carotenoid Profile and Retention in Yellow-, Purple- and Red-Fleshed Potatoes after Thermal Processing. Food Chem. 2016, 197, 992–1001.
- Linderborg, K. M.; Salo, J. E.; Kalpio, M.; Vuorinen, A. L.;, et al. Comparison of the Postprandial Effects of Purple-Fleshed and Yellow-Fleshed Potatoes in Healthy Males with Chemical Characterization of the Potato Meals. Int. J. Food Sci. Nutr. 2016, 67, 581–591.
- Tian, J.; Chen, J.; Lv, F.; Chen, S.;, et al. Domestic Cooking Methods Affect the Phytochemical Composition and Antioxidant Activity of Purple-Fleshed Potatoes. Food Chem. 2015, 197, 1264–1270.
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.;, et al. Extraction and Purification of Anthocyanins from Purple-Fleshed Potato. Food & Bioproducts Processing. 2016, 99, 136–146.
- Thed, S. T.; Phillips, R. D. Changes of Dietary Fibers and Starch Composition of Processed Potato Products during Domestic Cooking. Food Chem. 1995, 52, 301–304.
- Curti, E.; Carini, E.; Diantom, A.; Vittadini, E. The Use of Potato Fibre to Improve Bread Physico-Chemical Properties during Storage. Food Chem. 2015, 195, 64–70.
- Sudha, M. L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fibers and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692.
- AOAC. AOAC Official Method 991.43. Total, Soluble, and Insoluble Dietary Fibers in Foods; Association of Official Analytical Chemists: Washington DC, 1996.
- AOAC. AOAC Official Method 12. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington DC, 2001.
- Xie, F.; Wang, Y.; Wu, J.; Wang, Z. Insoluble Dietary Fibers from Angelica Keiskei By‐Product and Their Functional and Morphological Properties. Starch‐Stärke. 2017. DOI: 10.1002/star.201600122.
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and Physicochemical Properties of Soluble Dietary Fibers from Orange Peel Assisted by Steam Explosion and Dilute Acid Soaking. Food Chem. 2015, 185, 90–98.
- Lan, G.; Chen, H.; Chen, S.; Tian, J. Chemical Composition and Physicochemical Properties of Dietary Fibers from Polygonatum Odoratum as Affected by Different Processing Methods. Food Res. Int. 2012, 49, 406–410.
- Zhou, Y.; Xie, F.; Zhou, X.; Wang, Y.;, et al. Effects of Maillard Reaction on Flavor and Safety of Chinese Traditional Food: Roast Duck. J. Sci. Food Agric. 2015, 96, 1915–1922.
- Xie, F.; Gong, S.; Zhang, W.; Wu, J.; Wang, Z. Potential of Lignin from Canna Edulis Ker Residue in the Inhibition of α-D-glucosidase: Kinetics and Interaction Mechanism Merging with Docking Simulation. Int. J. Biol. Macromol. 2016, 95, 592–602.
- Graham, H.; Rydberg, M. B. G.; Aaman, P. Extraction of Soluble Dietary Fibers. J. Agric. Food Chem. 2002, 36, 494–497.
- Cheng, L.; Zhang, X.; Hong, Y.; Li, Z.;, et al. Characterisation of Physicochemical and Functional Properties of Soluble Dietary Fibrefrom Potato Pulp Obtained by Enzyme-Assisted Extraction. Int. J. Biol. Macromol. 2017, 101, 1004–1011.
- Chau, C. F.; Wang, Y. T.; Wen, Y. L. Different Micronization Methods Significantly Improve the Functionality of Carrot Insoluble Fibre. Food Chem. 2007, 100, 1402–1408.
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M. A.; Aguilera, Y.;, et al. Effect of Sterilisation on Dietary Fibre and Physicochemical Properties of Onion By-Products. Food Chem. 2011, 127, 501–507.
- Guo, X.; Zhao, W.; Pang, X.; Liao, X.; Hu, X.; Wu, J. Emulsion Stabilizing Properties of Pectins Extracted by High Hydrostatic Pressure, High-Speed Shearing Homogenization and Traditional Thermal Methods: A Comparative Study. Food Hydrocoll. 2014, 35, 217–225.
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion Stabilizing Properties of Depolymerized Pectin. Food Hydrocoll. 2002, 16(3), 249–256.
- Zhang, J.; Wang, Z. Soluble Dietary Fibers from Canna Edulis Ker By-Product and Its Enzymatic and Antioxidant Activities. Food Biotechnol. 2011, 25, 336–350.
- Briones-Labarca, V.; Venegascubillos, G.; Ortizportilla, S.; Chacanaojeda, M.; Maureira, H. Effects of High Hydrostatic Pressure (HHP) on Bioaccessibility, as Well as Antioxidant Activity, Mineral and Starch Contents in Granny Smith Apple. Food Chem. 2011, 128, 520–529.
- Briones-Labarca, V.; Muñoz, C.; Maureira, H. Effect of High Hydrostatic Pressure on Antioxidant Capacity, Mineral and Starch Bioaccessibility of a Non Conventional Food: Prosopis Chilensis Seed. Food Res. Int. 2011, 44, 875–883.
- Roginsky, V.; Lissi, E. A. Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food. Food Chem. 2005, 92(2), 235–254.
- Prior, R. L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53(10), 4290–4302.
- MacDonald‐Wicks, L. K.; Wood, L. G.; Garg, M. L. Methodology for the Determination of Biological Antioxidant Capacity in Vitro: A Review. J. Sci. Food Agric. 2006, 86(13), 2046–2056.
- Wang, H.; Huang, T.; Tu, Z. C.; Ruan, C. Y.; Lin, D. The Adsorption of lead(II) Ions by Dynamic High Pressure Micro-Fluidization Treated Insoluble Soybean Dietary Fibers. J. Food Sci. Technol. 2016, 53, 25–32.
- Zhang, J.; Wang, Z. W.; Yu, W. J.; Wu, J. H. Pectins from Canna Edulis Ker Residue and Their Physicochemical Characterization. Carbohydr. Polym. 2013, 83, 210–216.
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion Process Improves the Functionality of Soluble Dietary Fibers in Oat Bran. J. Cereal Sci. 2011, 54, 98–103.
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural Characteristics and Functional Properties of Rice Bran Dietary Fibers Modified by Enzymatic and Enzyme-Micronization Treatments: Food Science Technology. Science Technologie Alimentaire. LWT Food Sci. Technol. 2016, 75, 344–351.
- Park, K. H.; Lee, K. Y.; Lee, H. G. Chemical Composition and Physicochemical Properties of Barley Dietary Fibers by Chemical Modification. Int. J. Biol. Macromol. 2013, 60, 360–365.
- Ma, M.; Mu, T. Modification of Deoiled Cumin Dietary Fibers with Laccase and Cellulase under High Hydrostatic Pressure. Carbohydr. Polym. 2016, 136, 87–94.