ABSTRACT
The American cranberry (Vaccinium macrocarpon) is one of the fruits containing antioxidants in great quantity and of high quality. From recent research, it is evident that both cranberry and its products, when consumed chronically or acutely, boost the antioxidant effect. Likewise, most studies revealed the anti-inflammatory potential of the cranberry polyphenols. Both effects exert direct action mechanisms, revealed by the ability of the polyphenols to remove the reactive oxygen species, as well as indirect effects, represented by the action of these phytochemicals on the cell signaling pathways and genetic expression. A limited number of articles that evaluated the effects of cranberry on mitochondrial damages are available. However, an enhancement in the functions of this organelle was confirmed by the increased production of adenosine triphosphate (ATP). Therefore, further studies are required to demonstrate the benefits credited to the use of cranberry, as well as to describe the action mechanisms of the polyphenols.
Introduction
Cranberry (Vaccinium macrocarpon) along with blueberry occupies the top spot in the fruit ranking, revealing the highest quantity and quality of its constituent antioxidants. It also naturally contains fewer carbohydrates and more vitamins, minerals, and phenolic compounds compared to other fruits.[Citation1,Citation2] Cranberry is consumed more in the dried form than in natura, and it is used in several products including juices, shakes, cereal bars, cheeses, and chocolates.[Citation3] Currently, it is most widely used in the powder and extract forms in various food products and nutraceutical supplements.[Citation4] Cranberries possess a distinctive composition of phenolic compounds, including three classes of flavonoids (flavonoids, anthocyanins, and proanthocyanidins), catechins and an assortment of phenolic acids that induce various biological effects, such as antioxidant, enzyme activity modulation, and gene expression regulating effects.[Citation4–Citation7] However, the phenolic compounds of cranberry vary according to the type of fruit processing (juice, dehydrated, extract, lyophilized), storage time, and method of analysis used to phenolic compounds identification and quantification.[Citation3,Citation8]
From several in vitro studies, it is evident that high concentrations of cranberry polyphenols have been associated with antibacterial, antiviral, antimutagenic, anticarcinogenic, antiangiogenic, antiinflammatory, and antioxidant properties.[Citation5,Citation7,Citation9–Citation13] Animal models reveal that cranberry extracts can reduce C-reactive protein and inflammatory interleukins, increase nitric oxide synthesis, decrease Helicobacter pylori infection, and raise the sensitivity of the β-pancreatic cells to glucose.[Citation14–Citation16] Clinical studies highlight the fact that cranberry products can reduce LDL cholesterol (LDL-C), total cholesterol[Citation17], and LDL-C oxidation[Citation18,Citation19], increase HDL cholesterol (HDL-C), improve endothelial function[Citation20,Citation21], and enhance the plasma antioxidant capacity.[Citation1,Citation18,Citation22]
At present, an interdependent relationship has been recognized between oxidative stress and inflammation.[Citation23] The presence of excessive free radicals has been identified as the main stimulus for the onset of the inflammatory process, which in turn, induces the immune system cells to produce free radicals.[Citation24–Citation27] Besides, the free radicals also rank among the major causes of mitochondrial damage and thus consequently reduce output of adenosine triphosphate (ATP).[Citation25,Citation28] Hence, the polyphenolic food sources are highlighted for their antioxidant action and potential in helping to prevent as well as control oxidative stress and its effects.[Citation29]
The high polyphenolic content naturally present in cranberry supports the scientific evidence of the innumerable beneficial health effects it offers. Thus, the objective of this review was to evaluate the benefits of using cranberry in various forms - lyophilised, juiced, or dried - as a source of phenolic compounds and its effects on oxidative stress, inflammation, and mitochondrial damage.
Cranberry antioxidant property
Reactive oxygen species (ROS) is constantly being produced in the body during normal cellular events, such as energy production, detoxification of the body or through physical exercise and exposure to environmental pollutants.[Citation30] Along with the nitrogen and hydrogen reactive species, the ROS forms free radicals which can alter the biomolecular integrity of the lipids, proteins, and DNA. These changes increase the stimulation of the inflammatory processes, consequently raising the risk of chronic non-communicable diseases, such as cardiovascular diseases and some types of cancers.[Citation31–Citation33]
Bioactive compounds able to retard or prevent the oxidative effects are termed antioxidants. They are effective when they block the free radical-initiated chain reactions and eliminate them by preventing the occurrence of oxidative damage.[Citation34,Citation35] Apart from the endogenous protection provided by the antioxidant enzymes, like catalase, superoxide dismutase, glutathione reductase and glutathione peroxidase, antioxidant intake through the diet is an additional protective factor in maintaining cellular redox balance. This complex endogenous and exogenous antioxidant protection system interacts and acts synergistically to neutralise the free radicals.[Citation36,Citation37]
Scientific evidence indicates that cranberry is a particularly rich source of polyphenols, with associated antioxidant properties. However, the wide variety of methods that it has been used in different studies has revealed substantial differences in the findings, particularly with respect to the cranberry product used in the intervention and its polyphenolic content. Besides, a few studies in the literature discuss the quantity of the antioxidant to be ingested, to achieve maximum health benefits. Among the studies evaluated in this review, 364 mg/day was the lowest recommended concentration of polyphenols, which was effective in reducing oxidative stress and protein and lipid oxidation.[Citation2] However, Duthie et al., (2006) did not observe any change in the oxidative stress even when the polyphenols were ingested at 850 mg/day for two weeks ().[Citation22]
Table 1. Effects of cranberry consumption on oxidative stress, inflammation, and mitochondrial damages.
Oxidative stress represents an early event in the pathophysiology of chronic non-communicable diseases.[Citation38] Under such conditions, therapeutic antioxidant use influences the progression and control of these diseases, proving effective in attenuating the free radical-induced oxidative damage.[Citation39] For example, a review on the relevance of polyphenols with regards to human health revealed that cranberry inhibited lipid oxidation and prevented the formation of peroxidation products.[Citation40,Citation41] These results are credited to the action of the cranberry antioxidants in the elimination of free radicals, like hydroxyl radicals, superoxide radicals and singlet oxygen, culminating in preventing the oxidation of the biomolecules.[Citation9] Other studies that evaluated oxidative stress through the assessment of protein, lipid, and DNA oxidation products reported a decline in these markers, reaffirming the antioxidant capacity of the cranberry.[Citation2,Citation6,Citation18,Citation42,Citation43]
The development of metabolic syndrome (MS) too includes oxidative stress as a causal factor, and not only as a consequence or an independent risk factor.[Citation44] Therefore, some studies have utilized cranberry as a food supplement to mitigate the oxidative damages related to MS and its comorbidities and have reported promising results.[Citation2,Citation42] Basu et al. (2011) evaluated the effect of cranberry juice on lipid peroxidation and the antioxidant capacity in women with MS. They observed a 33% of reduction in the concentration of oxidized low-density lipoprotein (oxLDL) and a 50% decline in the production of malondialdehyde (MDA), besides a 47% increase in the total plasma antioxidant capacity. In this study, the volunteers presented low consumption of other polyphenolic sources, enabling the benefits observed to be attributed exclusively to the consumption of cranberry juice.[Citation42]
Experimental studies also highlight the antioxidant potential of cranberry.[Citation15,Citation45,Citation46] Kim et al. (2014) observed that despite the lipopolysaccharide (LPS)-induced oxidative stress, hypercholesterolemic rats fed on a cranberry-powder enriched diet showed reduced lipid and protein oxidation. The diet included 10% cranberry powder, which contained 122.6 mg/100 g of polyphenols, and its effectiveness was attributed to the high concentration of cranberry phenolic compounds, like flavonoids.[Citation45] This same researcher group repeated the experiment using diabetic rats as a model and recorded a 79% reduction in TBARS (Thiobarbituric acid reactive substances) in the animals fed on a cranberry-enriched diet in comparison with those provided with the standard diet.[Citation15]
Studies that evaluated the impact of cranberry consumption on antioxidant enzymes reported controversial results, irrespective of dose, time, and form of supplementation.[Citation6,Citation15,Citation22] Duthie et al. (2006) observed that the antioxidant capacity of the healthy subjects remained unchanged even after they consumed 750 mL cranberry juice every day, for two weeks.[Citation22] Similarly, Valentová et al. (2006) also found no change in the erythrocyte concentrations of superoxide dismutase, glutathione peroxidase, and glutathione reductase after a daily consumption of 2 or 6 capsules of lyophilized cranberry juice.[Citation43] On the other hand, Mathison et al. (2014), who also evaluated healthy subjects, observed a rise in the plasma glutathione concentration and increased activity of the superoxide dismutase enzyme after acute cranberry consumption.[Citation6] In an experimental study, diabetic mice with LPS-induced oxidative stress showed a restoration of superoxide dismutase concentrations post consuming a diet supplemented with lyophilized cranberry.[Citation15] Results from an in vitro study also observed that both the juice and lyophilized cranberry extract raised the glutathione peroxidase levels.[Citation47]
Although the antioxidant effect of cranberry is well established, the exact mechanism by which its polyphenols promote these benefits has not yet been fully elucidated. However, regardless of the source, the polyphenols have been observed to exhibit similar action mechanisms. Thus, although studies in cranberry do not attempt to investigate the antioxidant action mechanisms, their polyphenols act similar to the manner of polyphenols drawn from other fruits. Accordingly, two action mechanisms have been extensively described in the literature - a direct one, where the polyphenols act in eliminating the radical superoxide and other reactive oxygen species of the organism; and an indirect one, where the polyphenols can stimulate endogenous antioxidant defense by stimulating the transcription factor NF-E2 related factor 2 (Nrf2), responsible for regulating the expression of the antioxidant enzymes, like catalase and glutathione.[Citation48,Citation49]
In a study to determine the pharmacokinetics of the cranberry polyphenols, it was found that four hours after the intake of cranberry juice containing 835 mg of polyphenols, the plasma concentrations ranged from 0.56 to 4.64 nmol/L, demonstrating the rapid removal of these compounds from the organism. Thus, the authors suggested that at these concentrations, the polyphenols would be insufficient to alter the cellular redox state through free radical neutralization (direct mechanism), but could affect the gene expression (indirect mechanism), which would potentially increase the expression and synthesis of the antioxidant enzymes.[Citation49]
The molecular targets of the polyphenols in regulating the cell signaling pathways continue to remain obscure.[Citation50–Citation52] The Mitogen-activated protein kinases (MAPK) and phosphatidylinositol-3-kinase (PI3K/AKT) pathways transmit a multiplicity of extracellular stimuli by phosphorylation and activation of the downstream transcription factors that enable the cell to respond to stress with the increased or decreased expression of the critical genes.[Citation47,Citation52] When the potential influence of the juice and cranberry extract was evaluated in terms of the expression of the components of the MAPKs and PI3K/AKT pathways, it was observed that the preincubation of the HepG2 cells with cranberry was insufficient to reverse or prevent the overexpression of these pathways after exposure to 400μM tert-Butyl hydorperoxide, except for the c-Jun-N-terminal kinases (JNK) pathway, which showed normal expression after preincubation with cranberry juice.[Citation47]
Considering the low bioavailability of cranberry, the capability of its polyphenols to interfere in gene expression may be the plausible reason for its positive effect on oxidative stress, even when consumed in small amounts. It is also possible to suggest that the cranberry polyphenols act both as free radical scavengers (when present in larger quantities) and influence gene expression, culminating in a heightened endogenous antioxidant capacity, reduced oxidative stress and, consequently, balanced cellular redox. Nevertheless, scientific evidence to support this is still insufficient.
Anti-inflammatory property of cranberry
Recent research shows that chronic inflammatory process lies at the origin of several diseases, including cancer, cardiovascular disease, diabetes, insulin resistance, and rheumatoid arthritis.[Citation53–Citation55] During a single inflammatory event, a cascade of biochemical events which are kick-started involve the local vascular system, immune system, and various inflammatory site cells. Nuclear factor kappa B (NF-ĸB), which plays a crucial role in the inflammatory process, is the main transcription factor responsible for stimulating and encoding several genes, including those responsible for the production of cytokines, chemokines, immunoreactors, adhesion molecules, and the acute phase.[Citation56]
Considering the deleterious effects of chronic inflammation on human health, several fruits and fruit-derived products - juices, extracts, and nutraceutical supplements - are being studied at present, with the specific goal of determining their anti-inflammatory effects.[Citation48] Because of its rich polyphenolic content, cranberry offers great anti-inflammatory potential. It has been observed in vitro that even under pronounced pro-inflammatory stimulus, the cranberry bioactive compounds are capable of suppressing the macrophage and T cell activation.[Citation57] Polyphenols such as resveratrol, also found in cranberry, have been associated with the suppression of the inflammatory genes by their action on the transcription factors NF-ĸB and Janus Kinase/signal transducer and activator of transcription (JAK/STAT) in cell cultures.[Citation7]
When the effects of the cranberry fractions were investigated on caco-2/15 intestinal cells, Denis et al. (2014) observed that, even under LPS-induced inflammation, cells cultured in the presence of cranberry had a lowered NF-ĸB activation rate and a reduced pro-inflammatory mediator tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).[Citation58] A lower expression was also noted of cyclooxygenase 2 (COX2), an enzyme responsible for raising the prostaglandin E2 (PGE2) levels, both associated with inflammatory processes. A similar finding was observed in experimental studies performed.[Citation16,Citation46] Male rats supplemented with cranberry extract also produced a decline in the rate of expression of NF-ĸB.[Citation46] Concurrently, other studies have reported that cranberry encouraged a drop in the levels of interleukin-4 (IL-4) and interleukin-1 beta (IL-1β), as well as an increase in the anti-inflammatory cytokine interleukin-10 (IL-10)[Citation6,Citation16] (). Contrary to the evidence, a few studies that also aimed at demonstrating the anti-inflammatory effects of cranberry did not obtain positive results on the inflammatory markers.[Citation2,Citation42] Simão et al. (2013) did not observe any change in the NF-ĸB expression, even after 60 days of intervention with cranberry juice.[Citation2] Also, subjects with metabolic syndrome showed no changes in the C-reactive protein (CRP) and IL-6 levels after consuming 480 mL/day of cranberry juice for 8 weeks[Citation42]
Among the studies that identified an anti-inflammatory effect related to cranberry intake, the main action mechanism recorded was the ability of the polyphenols to reduce the NF-ĸB expression and consequently all the inflammatory cytokines it stimulated. A similar mechanism was observed in other studies that evaluated the anti-inflammatory effect of the bioactive compounds like resveratrol, curcumin, and anthocyanins received from other dietary sources.[Citation59–Citation61]
Despite the central role played by the NF-ĸB in inflammation associated with gene expression, this transcription factor requires assistance from other specific transcription factors, including Mitogen-activated Protein Kinase (MAPK).[Citation62,Citation63] These belong to the Serine/Threonine kinases family, which regulates the vital cellular processes of cell growth, proliferation, death, and differentiation, by modulating the genetic transcription in response to cellular environmental changes and constitute the upstream regulators of the transcription factors.[Citation58]
The action of the polyphenols on the cell signaling processes is clearly evident, mainly from their potential action on the inflammatory pathways.[Citation59–Citation61] A few of these actions are secondary to the ability of the polyphenols to modify the cellular redox state, while others are directly obtained. Among their direct actions on cell signaling are the blocking or downregulation of the receptors and transcription factors, leading to a reduced expression of the proinflammatory genes, including interleukins and Toll-like receptors (TLR) −4, NF-ĸB, activator protein (AP-1), and JNK or action as a natural ligand for peroxisome proliferator-activated receptor-gamma (PPAR-γ).[Citation48,Citation59–Citation61,Citation64] Polyphenols may also induce the production of anti-inflammatory markers such as IL-4, IL-10, interleukin-13 (IL-13) and adiponectin. In general, polyphenols potentially interfere with human health through several mechanisms, notably through their ability to modulate cellular events and promote the balance of the inflammatory state.[Citation48]
Other molecular mechanisms purported to be involved in the anti-inflammatory activity of polyphenols include the inhibition of the pro-inflammatory enzymes such as COX2, lipoxygenase (LOX) and induction of nitric oxide synthesis by the activation of PPARγ; inhibition of phosphoinositide 3-kinase (PI3-kinase) and tyrosine kinase; activation of phase II detoxifying enzymes, MAPK, protein kinase c (PKc), serine/threonine protein kinase akt/PKB and modulation of several genes involved in the cell cycle.[Citation65–Citation68]
Prevention of mitochondrial damage
Mitochondria are extremely essential in the supply of cellular energy in the form of ATP, antioxidant defense, fat oxidation, intermediate processes of metabolism, and cell death.[Citation69,Citation70] It is well established that mitochondrial functions are suppressed when living cells or organisms are exposed to potentially toxic agents, including alcohol, high fat diets, smoking, certain medications, or some pathophysiological conditions that induce increased oxidative stress. Under conditions of high oxidative stress, the cellular macromolecules may undergo different oxidative modifications, leading to their rupture and impairment of physiological functions.[Citation71]
As they are the chief producers of ROS in the mammalian cells, the mitochondria naturally become predisposed to oxidative damage due to their proximity to free radicals.[Citation69,Citation70] In the mitochondrial matrix, the respiratory chain is present, which is a stage in the cellular respiration process, responsible for generating the largest quantity of ROS within the cell. This continuous output of ROS facilitates the direct interaction of these radicals with the cell macromolecules. When they occur in excess, the free radicals produced during cellular respiration impair the mitochondrial functions. To protect themselves against oxidative damage, the mitochondria utilise multiple antioxidant defense systems that include antioxidant enzymes and other molecules, particularly glutathione.[Citation69,Citation72]
Several studies have reported the beneficial effects of antioxidants on enhancing the mitochondrial function during pathological processes.[Citation73–Citation75] Recently, bioactive compounds have been observed to directly affect the mitochondrial functions by inhibiting specific enzymes, or indirectly by modulating the signals to or from the mitochondria.[Citation70,Citation76–Citation78] One of the mechanisms is the improvement of the mitochondrial function by inducing the expression of the genes responsible for oxidative phosphorylation and mitochondrial biogenesis by the acetylation of the peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC1-α), a principal regulator of mitochondrial biogenesis. Thus, the functional activity of the mitochondria would subsequently increase, evaluated mainly through the ATP production.[Citation70] For instance, a study evaluating the cytoprotective effects of the apple polyphenols on indomethacin-treated Caco-2 cells, Carrasco-Pozo et al. (2010) reported a direct effect on mitochondrial oxidative damage, increase in ATP concentrations, maintenance of GSH/GSSG balance, rise in the mitochondrial membrane potential and elimination of the ROS produced both inside and outside the mitochondria.[Citation79]
A few studies are available which have evaluated the cranberry polyphenols in the prevention of mitochondrial damage. Denis et al. (2015) observed that the cells preincubated in neutral medium to which iron/ascorbate (Fe/Asc) was added, produced six times less ATP than those cultured under the same stimulus but with added fractions of cranberry containing 35% flavonols and 65% procyanidins in the culture medium. In this study, the prevention of oxidative stress-induced mitochondrial damage was attributed to the cranberry phytochemicals (). Another study, evaluating the protective effect of potential antioxidants on acute or chronic carbon tetrachloride (CCl4)-induced mitochondrial damage, found that the most effective antioxidant complex in preventing the damage was the one containing the melatonin, succinate and flavonoids of cranberry at a single dose of 7 mg/kg/day.[Citation80] However, the paucity of research regarding the effects of cranberry on mitochondrial damage limits this discussion. To date, the direct action of these polyphenols on free radicals and interference in gene expression have been reported, both of which possess the potential to improve mitochondrial function.
Conclusion
Cranberry is one of the main dietary sources of phytochemicals. Dehydrated cranberry, juices, extracts, and other products derived from this fruit have been shown in studies performed in vitro, experimentally and on humans to possess a range of biochemical and physiological effects, mediated by their phytochemical constituents. Most studies on cranberry have been focused on the antioxidant capacity, as the etiology of most chronic diseases are associated with redox imbalance. The critical evaluation of the articles presented in this review emphasised that the cranberry polyphenols are potent antioxidants, capable of eliminating free radicals and reducing cellular damage. Among the mechanisms presented is the direct action of the polyphenols which can neutralise the reactive oxygen species, and an indirect action, where the cranberry polyphenols were able to interfere in the cell signaling pathways. Thus, they would be capable of modulating the expression of those genes critical in the process of response to stress, inducing a rise in the endogenous antioxidant response (). Among the studies that evaluated the anti-inflammatory capacity of the cranberry polyphenols, the majority reported a reduction in the inflammatory markers like cytokines, adhesion molecules, and acute phase proteins. Besides, the potential of the polyphenols in reducing the expression of NF-κB, a transcription factor critical in the inflammatory process, has also been identified as the main anti-inflammatory action mechanism of the polyphenols. There continues to be very little evidence to support the effects of cranberry on mitochondrial damage. However, the studies available have reported the potential of the polyphenols in preventing mitochondrial damage, as well as boosting the ATP production. These effects were due to both the modulation of the genomic expression by the polyphenols and their antioxidant effect. However, the paucity of studies is a limiting factor, necessitating more study on the effects of polyphenols on mitochondria. Despite the benefits of using cranberry for improving human health, no consensus has been reached regarding the dose or form of supplementation. Therefore, the present review emphasises the need to make recommendations regarding cranberry intake to experience its beneficial effects. Further, more studies are required to clarify the action mechanisms of the polyphenols and enhance the health benefits they can confer.
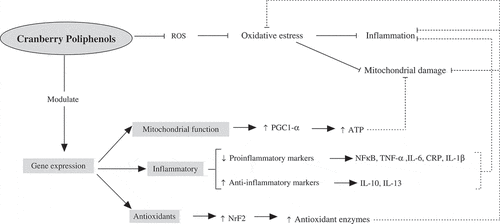
Figure 1. Effect of cranberry polyphenols on oxidative stress, inflammation, and mitochondrial damage. Cranberry polyphenols act as free radical neutralizers, impacting directly on antioxidant status. Hence, after free radicals reduction occur reduction of the stimulus for inflammation and mitochondrial damage. In addition, cranberry polyphenols are also able to modulate the expression of key genes related to mitochondrial function, inflammation, and antioxidant response.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Notes on contributors
Ana Paula Silva Caldas
All authors participated in the different steps of elaboration this present article, including selection, reading, and critical analysis of all articles used as reference. Furthermore, all authors contributed greatly to the writing of this article.
References
- Vinson, J. A.; Bose, P.; Proch, J.; Al Kharrat, H.; Samman, N. Cranberries and Cranberry Products: Powerful in Vitro, Ex Vivo, and in Vivo Sources of Antioxidants. Journal of Agricultural and Food Chemistry 2008, 56(14), 5884–5891. DOI: 10.1021/jf073309b.
- Simão, T. N. C.;Lozovoy, M. A. B.; Simão, A. N. C.; Oliveira, S. R.; Venturini, D.; Morimoto, H. K.; Miglioranza, L. H. S.; Dichi, I. Reduced-Energy Cranberry Juice Increases Folic Acid and Adiponectin and Reduces Homocysteine and Oxidative Stress in Patients with the Metabolic Syndrome. The British Journal of Nutrition 2013, 110, 1885–1894. DOI: 10.1017/S0007114513001207.
- Blumberg JB, Camesano T a, Cassidy A, Kris-Etherton P, Howell A, Manach C, et al. Cranberries and Their Bioactive Constituents in Human Health. Advances in Nutrition 2013, 4(6), 618–632. DOI: 10.3945/an.113.004473.
- McKay, D. L.; Chen, C. Y. O.; Zampariello, C. A.; Blumberg, J. B. Flavonoids and Phenolic Acids from Cranberry Juice Are Bioavailable and Bioactive in Healthy Older Adults. Food Chemistry 2015, 168, 233–240. DOI: 10.1016/j.foodchem.2014.07.062.
- Neto, C. C.;. Cranberry and Blueberry: Evidence for Protective Effects against Cancer and Vascular Diseases. Molecular Nutrition and Food Research 2007, 51, 652–664. DOI: 10.1002/mnfr.200600279.
- Mathison, B. D.; Kimble, L. L.; Kaspar, K. L.; Khoo, C.; Chew, B. P. Consumption of Cranberry Beverage Improved Endogenous Antioxidant Status and Protected against Bacteria Adhesion in Healthy Humans: A Randomized Controlled Trial. Nutrition Research 2014, 34(5), 420–427. DOI: 10.1016/j.nutres.2014.03.006.
- McKay, D. L.; Blumberg, J. B. Cranberries (Vaccinium Macrocarpon) and Cardiovascular Disease Risk Factors. Nutrition Reviews 2007, 65(11), 490–502. DOI: 10.1301/nr.2007.nov.490-502.
- Pappas, E.; Schaich, K. M. Phytochemicals of Cranberries and Cranberry Products: Characterization, Potential Health Effects, and Processing Stability. Critical Reviews in Food Science and Nutrition Oct 19, 2009, 49(9), 741–781. DOI: 10.1080/10408390802145377.
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive Compounds in Cranberries and Their Biological Properties. Critical Reviews in Food Science and Nutrition 2010, 50(7), 666–679. DOI: 10.1080/10408390903044107.
- Del Rio, D.; Rodriguez-Mateo, A.; Spencer, J. P. E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid Redox Signal 2012, 18(14), 1818–1892. DOI: 10.1089/ars.2012.4581.
- Feliciano, R. P.; Krueger, C. G.; Reed, J. D. Methods to Determine Effects of Cranberry Proanthocyanidins on Extraintestinal Infections: Relevance for Urinary Tract Health. Molecular Nutrition & Food Research 2015, 59(7), 1292–1306. DOI: 10.1002/mnfr.201500108.
- Howell, A. B.;. Bioactive Compounds in Cranberries and Their Role in Prevention of Urinary Tract Infections. Molecular Nutrition & Food Research 2007, 51(6), 732–737. DOI: 10.1002/mnfr.200700038.
- Shmuely, H.; Yahav, J.; Samra, Z.; Chodick, G.; Koren, R.; Niv, Y.; Ofek, I. Effect of Cranberry Juice on Eradication of Helicobacter Pylori in Patients Treated with Antibiotics and a Proton Pump Inhibitor. Molecular Nutrition and Food Research 2007, 51(6), 746–751. DOI: 10.1002/mnfr.200600281.
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mantian, M.; Tianru, J.; Wenhua, L. Purified Anthocyanin Supplementation Improves Endothelial Function via NO-cGMP Activation in Hypercholesterolemic Individuals. Clinical Chemistry 2011, 57(11), 1524–1533. DOI: 10.1373/clinchem.2011.167361.
- Kim, M. J.; Chung, J.; Kim, J. H.; Kwak, H. Effects of Cranberry Powder on Biomarkers of Oxidative Stress and Glucose Control in Db/Db Mice. Nutrition Research and Practice 2013, 7(6), 430–438. DOI: 10.4162/nrp.2013.7.6.430.
- Kim, M. J.; Ohn, J.; Kim, J. H.; Kwak, H. K. Effects of Freeze-Dried Cranberry Powder on Serum Lipids and Inflammatory Markers in Lipopolysaccharide Treated Rats Fed an Atherogenic Diet. Nutrition Research and Practice 2011, 5(5), 404–411. DOI: 10.4162/nrp.2011.5.5.404.
- Lee, I. T.; Chan, Y. C.; Lin, C. W.; Lee, W. J.; Sheu, W. H. H. Effect of Cranberry Extracts on Lipid Profiles in Subjects with Type 2 Diabetes. Diabetic Medicine 2008, 25(12), 1473–1477. DOI: 10.1111/j.1464-5491.2008.02588.x.
- Ruel, G.; Pomerleau, S.; Couture, P.; Lamarche, B.; Couillard, C. Changes in Plasma Antioxidant Capacity and Oxidized Low-Density Lipoprotein Levels in Men after Short-Term Cranberry Juice Consumption. Metabolism: Clinical and Experimental 2005, 54(7), 856–861. DOI: 10.1016/j.metabol.2005.01.031.
- Rosa, C. O. B.; Santos, C. A.; Leite, J. I. A.; Caldas, A. P. S.; Bressan, J. Impact of Nutrients and Food Components on Dyslipidemias: What Is the Evidence? Advances in Nutrition 2015, 6(6). DOI: 10.3945/an.115.009480.
- Dohadwala, M. M.; Holbrook, M.; Hamburg, N. M.; Shenouda, S. M.; Chung, W. B.; Titas, M.; Matthew, A. K.; Wang, N. A.; Palmisano, J.; Milbury, P. E.; et al. Effects of Cranberry Juice Consumption on Vascular Function in Patients with Coronary Artery Disease. The American Journal of Clinical Nutrition 2011, 93(5), 934–940. DOI: 10.3945/ajcn.110.004242.
- Flammer AJ, Martin E a., Gössl M, Widmer RJ, Lennon RJ, Sexton J a, et al. Polyphenol-Rich Cranberry Juice Has a Neutral Effect on Endothelial Function but Decreases the Fraction of Osteocalcin-Expressing Endothelial Progenitor Cells. European Journal of Nutrition 2013, 52(1), 289–296. DOI: 10.1007/s00394-012-0334-4.
- Duthie, S. J.; Jenkinson, A. M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.;Yap, L. S.; Christen, P.; Duthie, G. G. The Effects of Cranberry Juice Consumption on Antioxidant Status and Biomarkers Relating to Heart Disease and Cancer in Healthy Human Volunteers. European Journal of Nutrition 2006, 45(2), 113–122. DOI: 10.1007/s00394-005-0572-9.
- Rull, A.; Camps, J.; Alonso-Villaverde, C.; Joven, J. Insulin Resistance, Inflammation, and Obesity: Role of Monocyte Chemoattractant Protein-1 (Orccl2) in the Regulation of Metabolism. Mediators of Inflammation 2010(Figure 1), 1–11. DOI: 10.1155/2010/326580.
- Geronikaki, A. A.; Gavalas, A. M. Antioxidants and Inflammatory Disease: Synthetic and Natural Antioxidants with Anti-Inflammatory Activity. Combinatorial Chemistry & High Throughput Screening 2006, 9(6), 425–442. DOI: 10.2174/138620706777698481.
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, Oxidative Stress and Cell Death. Apoptosis 2007, 12(5), 913–922. DOI: 10.1007/s10495-007-0756-2.
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M. D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez, M. F. Effects of Flavonoids and Other Polyphenols on Inflammation. Critical Reviews in Food Science and N utrition 2011, 51(4), 331–362. DOI: 10.1080/10408390903584094.
- Rahman, I.; Biswas, S. K.; Kirkham, P. A. Regulation of Inflammation and Redox Signaling by Dietary Polyphenols. Biochemical Pharmacology Nov 2006, 72(11), 1439–1452. DOI: 10.1016/j.bcp.2006.07.004.
- Lin, M. T.; Beal, M. F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443(7113), 787–795. DOI: 10.1038/nature05292.
- Rasouli, H.; Farzei, M. H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. International Journal of Food Properties Aug 2017, 4(August), 2912. 10942912.2017.1354017.
- Barbosa, K. B. F.; Costa, N. M. B.; De Cássia Gonçalves Alfenas, R.; De Paula, S. O.; Minim, V. P. R.; Bressan, J. Estresse Oxidativo: Conceito, Implicações E Fatores Modulatórios. Revista de Nutricao 2010, 23, 629–643. DOI: 10.1590/S1415-52732010000400013.
- Mayne, S. T.;. Antioxidant Nutrients and Chronic Disease: Use of Biomarkers of Exposure and Oxidative Stress Status in Epidemiologic Research. The Journal of nutrition 2003, 133(Suppl(2)), 933S–940S. DOI: 10.1093/jn/133.3.933S.
- Hd, K.; Karki, K.; Pande, D.; Negi, R. R. K. Microinflammation Inflammation, Free Radical Damage. Oxidative Stress and Cancer 2014, 1(1), 1–5.
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Patents on Inflammation & Allergy Drug Discovery 2009, 3(1), 73–80. DOI: 10.2174/187221309787158371.
- Huang D Prior R L OB. The Chemistry behind Antioxidant Capacity Assays. J Agric Food Chem 2005, 53, 1841–1856. DOI: 10.1021/jf030723c.
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Analyzing Cranberry Bioactive Compounds. Critical Reviews in Food Science and Nutrition 2010, 50(9), 872–888. DOI: 10.1080/10408390903042069.
- Kaliora, A. C.; Dedoussis, G. V. Z.; Schmidt, H. Dietary Antioxidants in Preventing Atherogenesis. Atherosclerosis 2006, 187, 1–17. DOI: 10.1016/j.atherosclerosis.2005.11.001.
- Schneider, C. D.; De Oliveira, A. R. Radicais Livres De Oxigênio E Exercício: Mecanismos De Formação E Adaptação Ao Treinamento Físico. Revista Brasileira de Medicina do Esporte 2004, 10(4), 308–318. DOI: 10.1590/S1517-86922004000400008.
- Ford, E. S.; Mokdad, A. H.; Giles, W. H.; Brown, D. W. The Metabolic Syndrome and Antioxidant Concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes Sep 1, 2003, 52(9), 2346–2352. DOI: 10.2337/diabetes.52.9.2346.
- Pandey, K. B.; Rizvi, S. I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity 2009, 2(5), 270. DOI: 10.4161/oxim.2.5.9498.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. The American Journal of Clinical Nutrition 2005, 81, 243S–255S. DOI: 10.1093/ajcn/81.1.230S.
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Low-Calorie Cranberry Juice Supplementation Reduces Plasma Oxidized LDL and Cell Adhesion Molecule Concentrations in Men. The British Journal of Nutrition 2008, 99(2), 352–359. DOI: 10.1017/S0007114507811986.
- Basu, A.; Betts, N. M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T. J. Low-Energy Cranberry Juice Decreases Lipid Oxidation and Increases Plasma Antioxidant Capacity in Women with Metabolic Syndrome. Nutrition Research 2011, 31(3), 190–196. DOI: 10.1016/j.nutres.2011.02.003.
- Valentová K, Stejskal D, Bednář P, Vostálová J, Číhalík Č, Večeřová R, et al. Biosafety, Antioxidant Status, and Metabolites in Urine after Consumption of Dried Cranberry Juice in Healthy Women: A Pilot Double-Blind Placebo-Controlled Trial. Journal of Agricultural and Food Chemistry 2007, 55(8), 3217–3224. DOI: 10.1021/jf0636014.
- Roberts, C. K.; Sindhu, K. K. Oxidative Stress and Metabolic Syndrome. Life Sciences 2009, 84(21–22), 705–712. DOI: 10.1016/j.lfs.2009.02.026.
- Kim, M. J.; Kim, J. H.; Kwak, H. Antioxidant Effects of Cranberry Powder in Lipopolysaccharide Treated Hypercholesterolemic Rats. Prevention Nutrition Food Science 2014, 19(2), 75–81. DOI: 10.3746/pnf.2014.19.2.075.
- Anhê, F. F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T. V.;Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia Spp. Population in the Gut Microbiota of Mice. Gut 2014, 64(6), 872–883. DOI: 10.1136/gutjnl-2014-307142.
- Martín, M. A.; Ramos, S.; Mateos, R.; Marais, J. P. J.; Bravo-Clemente, L.; Khoo, C.; Luis G. Chemical Characterization and Chemo-Protective Activity of Cranberry Phenolic Powders in a Model Cell Culture. Response of the Antioxidant Defenses and Regulation of Signaling Pathways. Food Research International 2015, 71, 68–82. DOI: 10.1016/j.
- Joseph, S. V.; Edirisinghe, I.; Burton-Freeman, B. M. Fruit Polyphenols: A Review of Anti-Inflammatory Effects in Humans. Critical Reviews in Food Science & Nutrition 2016, 56(3), 419–444. DOI: 10.1080/10408398.2013.767221.
- Milbury, P. E.; Vita, J. A.; Blumberg, J. B. Anthocyanins are Bioavailable in Humans following an Acute Dose of Cranberry Juice. The Journal of Nutrition 2010, 140(6), 1099–1104. DOI: 10.3945/jn.109.117168.
- Martín, M. Á.; Serrano, A. B. G.; Ramos, S.; Pulido, M. I.; Bravo, L.; Goya, L. Cocoa Flavonoids Up-Regulate Antioxidant Enzyme Activity via the ERK1/2 Pathway to Protect against Oxidative Stress-Induced Apoptosis in HepG2 Cells. Journal of Nutritional Biochemistry 2010, 21(3), 196–205. DOI: 10.1016/j.jnutbio.2008.10.009.
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel Mechanisms of Natural Antioxidant Compounds in Biological Systems: Involvement of Glutathione and Glutathione-Related Enzymes. Journal of Nutritional Biochemistry 2005, 16(10), 577–586. DOI: 10.1016/j.jnutbio.2005.05.013.
- Ramos, S.;. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Molecular Nutrition and Food Research 2008, 52(5), 507–526. DOI: 10.1002/mnfr.200700326.
- Libby, P.;. Inflammation and Cardiovascular Disease Mechanisms. The American Journal of Clinical Nutrition Feb 2006, 83(2), 456S–460S. DOI: 10.1093/ajcn/83.2.456S.
- Moss, S. F.; Blaser, M. J. Mechanisms of Disease: Inflammation and the Origins of Cancer. Nature Clinical Practice Oncology 2, 2005, Feb(2), 90–97. DOI: 10.1038/ncponc0081.
- Shoelson, S. E.; Lee, J.; Goldfine, A. B. Review Series Inflammation and Insulin Resistance. The Journal of Clinical Investigation 2006, 116(7), 1793–1801. DOI: 10.1172/JCI29069.
- Pahl, H. L.;. Activators and Target Genes of Rel/NF-kappaB Transcription Factors. Oncogene 1999, 18(49), 6853–6866. DOI: 10.1038/sj.onc.1203239.
- Bodet, C.; Chandad, F.; Grenier, D. Anti-Inflammatory Activity of a High-Molecular-Weight Cranberry Fraction on Macrophages Stimulated by Lipopolysaccharides from Periodontopathogens. Journal of Dental Research 2006, 85(3), 235–239. DOI: 10.1177/154405910608500306.
- Denis, M.; Desjardins, Y.; Furtos, A.; Marcil, V.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Delvin, E.; Marette, A.; Levy E. Prevention of Oxidative Stress, Inflammation and Mitochondrial Dysfunction in the Intestine by Different Cranberry Phenolic Fractions. Clinical Science 2015, 128(3), 197–212. DOI: 10.1042/CS20140210.
- Kunnumakkara, A. B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B. B. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear factor-κB-regulated Gene Products. Cancer Research 2007, 67(8), 3853–3861. DOI: 10.1158/0008-5472.CAN-06-4257.
- Collett, G. P.; Campbell, F. C. Curcumin Induces C-Jun N-Terminal Kinase-Dependent Apoptosis in HCT116 Human Colon Cancer Cells. Carcinogenesis 2004, 25(11), 2183–2189. DOI: 10.1093/carcin/bgh233.
- Choi, J.-S.; Choi, Y.-J.; Park, S.-H.; Kang, J.-S.; Kang, Y.-H. Flavones Mitigate Tumor Necrosis Factor-Alpha-Induced Adhesion Molecule Upregulation in Cultured Human Endothelial Cells: Role of Nuclear Factor-Kappa B. The Journal of Nutrition 2004, 134(5), 1013–1019. DOI: 10.1093/jn/134.5.1013.
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate. Cancer Research 2006, 66(5), 2500–2505. DOI: 10.1158/0008-5472.CAN-05-3636.
- Karin, M.;. The Regulation of AP-1 Activity by Mitogen-Activated Protein Kinases. Journal of Biological Chemistry Jul 14, 1995, 270(28), 16483–16486. cited. DOI: 10.1074/jbc.270.28.16483.
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Benedetto R. D.; Filesi, C.; Masella, R. Polyphenols, Intracellular Signalling and Inflammation. Ann Ist Super Sanita 2007;43(4), 394–405.
- Williams, R. J.; Spencer, J. P. E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radical Biology and Medicine 2004, 36(7), 838–849. DOI: 10.1016/j.freeradbiomed.2004.01.001.
- Yoon, J. H.; Baek, S. J. Molecular Targets of Dietary Polyphenols with Anti-Inflammatory Properties. Yonsei Medical Journal 2005, 46(5), 585–596. DOI: 10.3349/ymj.2005.46.5.585.
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular Targets of Tea Polyphenols in the Cardiovascular System. Cardiovascular Research 2007, 73(2), 348–358. DOI: 10.1016/j.cardiores.2006.08.022.
- Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. Journal of Pharmacological Sciences 2004, 96(3), 229–245. DOI: 10.1254/jphs.CRJ04003X.
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. Journal of Signal Transduction 2012, 2012, 1–13. DOI: 10.1155/2012/646354.
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evidence-Based Complementary and Alternative Medicine 2015, 2015, 1–13. DOI: 10.1155/2015/527209.
- Song B-J, Akbar M, Abdelmegeed M a., Byun K, Lee B, Yoon SK, et al. Mitochondrial Dysfunction and Tissue Injury by Alcohol, High Fat, Nonalcoholic Substances and Pathological Conditions through Post-Translational Protein Modifications. Redox Biology 2014, 3, 1–15. DOI: 10.1016/j.redox.2014.10.004.
- Arnér, E. S. J.; Holmgren, A. Physiological Functions of Thioredoxin and Thioredoxin Reductase. European Journal of Biochemistry 2000, 267(20), 6102–6109. DOI: 10.1046/j.1432-1327.2000.01701.x.
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and Cytochrome C are Molecular Targets of Flavonoids that Inhibit Hydrogen Peroxide Production by Mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics Dec 2011, 1807(12), 1562–1572. DOI: 10.1016/j.bbabio.2011.09.022.
- Lapshina, E. A.; Zamaraeva, M.; Cheshchevik, V. T.; Olchowik-Grabarek, E.; Sekowski, S.; Zukowska, I.;Golovach, N. G.; Burd, V. N.; Zavodnik, I. B. Cranberry Flavonoids Prevent Toxic Rat Liver Mitochondrial Damage in Vivo and Scavenge Free Radicals in Vitro. Cell Biochemistry and Function Jun 2015, 33(4), 202–210. DOI: 10.1002/cbf.3104.
- Vladimirov, Y. a.; Proskurnina, E. V.; Demin, E. M.; Matveeva, N. S.; Lubitskiy, O. B.; Novikov, A. A.; Izmailov, D. Y. U.; Osipov, A. N.; Tikhonov, V. P.; Kagan, V. E. l. Dihydroquercetin (Taxifolin) and Other Flavonoids as Inhibitors of Free Radical Formation at Key Stages of Apoptosis. Biochemistry Biokhimiia 2009, 74(3), 301–307. DOI: 10.1134/S0006297909030092.
- Zheng, J.; Ramirez, V. D. Inhibition of Mitochondrial Proton F0F1-ATPase/ATP Synthase by Polyphenolic Phytochemicals. British Journal of Pharmacology 2000, 130(5), 1115–1123. DOI: 10.1038/sj.bjp.0703397.
- Sekiya, M.; Hisasaka, R.; Iwamoto-Kihara, A.; Futai, M.; Nakanishi-Matsui, M. A Unique Mechanism of Curcumin Inhibition on F1 ATPase. Biochemical and Biophysical Research Communications 2014, 452(4), 940–944. DOI: 10.1016/j.bbrc.2014.09.027.
- Kipp, J. L.; Ramirez, V. D. Effect of Estradiol, Diethylstilbestrol, and Resveratrol on F0F1-ATPase Activity from Mitochondrial Preparations of Rat Heart, Liver, and Brain. Endocrine 2001, 15(2), 165–175. DOI: 10.1385/ENDO:15:2:165.
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Protection by Apple Peel Polyphenols against Indometacin-Induced Oxidative Stress, Mitochondrial Damage and Cytotoxicity in Caco-2 Cells. The Journal of Pharmacy and Pharmacology Jul 2010, 62(7), 943–950. DOI: 10.1111/(ISSN)2042-7158.
- Cheshchevik, V. T.; Lapshina, E. a.; Dremza, I. K.; Zabrodskaya, S. V.; Reiter, R. J.; Prokopchik, N. I.; Zavodnik, I. B. Rat Liver Mitochondrial Damage under Acute or Chronic Carbon Tetrachloride-Induced Intoxication: Protection by Melatonin and Cranberry Flavonoids. Toxicology and Applied Pharmacology 2012, 261(3), 271–279. DOI: 10.1016/j.taap.2012.04.007.
