?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Makgeolli is a traditional Korean rice wine reported to have anticancer, anti-inflammatory, and antioxidant effects. We developed an approach involving solvent extraction coupled with gas chromatography-mass spectrometry to determine four bioactive compounds, farnesol (FOH), squalene (SQ), and newly identified 4-vinyl guaiacol (4-VG) and 2,4-di-tert-butylphenol (DTBP), in makgeolli. The method was validated with the linearity, limit of detection, limit of quantification, intra- and inter-day precision, and accuracy. The validated method was then applied to several makgeolli, beer, and wine samples. 4-VG and DTBP were identified in all beverages, but FOH and SQ were only identified in makgeolli.
Introduction
Makgeolli is a traditional Korean rice wine brewed with nuruk, a fermentation starter that contains various microorganisms (yeast and fungi). During the fermentation process, saccharification of starches and alcohol occurs in parallel.[Citation1] The primary constituents present in standard makgeolli include water (80%), dietary fiber (10%), alcohol (6–8%), protein (2%), carbohydrates (0.8%), vitamins, and organic acids. Accumulating evidence has revealed that makgeolli has various biological effects in vitro, suggestive of anticancer, anti-inflammatory, and antioxidant properties.[Citation2] Animal studies have also demonstrated that oral administration of makgeolli elicits antitumor effects in a gastric tumor xenograft mouse model[Citation3] and antioxidant activity in rat blood plasma.[Citation4]
Various bioactive compounds such as organic acids[Citation5,Citation6] and polyphenols[Citation7] have been identified in makgeolli, as well as several volatile compounds. Wang et al. showed that ethyl acetate and butanol fractions from a methanol (MeOH) extract of makgeolli exhibit antioxidant activity and identified volatile compounds including 4-hydroxybenzaldehyde, 2-(4-hydroxyphenyl)ethanol (tyrosol), and 1H-indole-3-ethanol using nuclear magnetic resonance and mass spectrometry (MS).[Citation8] Farnesol (FOH) and squalene (SQ) have been identified and quantified in makgeolli using gas chromatography-mass spectrometry (GC-MS).[Citation9] FOH is a sesquiterpene alcohol generated by plants and microorganisms and has been reported to exhibit anticancer[Citation10–Citation12] and anti-inflammatory[Citation13–Citation15] activities. SQ is a polyunsaturated hydrocarbon found in some fish and vegetable oils, and reportedly exhibits anticancer and antioxidant effects.[Citation16]
Recently, Ha et al. developed a stir-bar sorptive extraction (SBSE) method coupled with GC-MS for the quantification of FOH and SQ in makgeolli.[Citation9,Citation17] Although SBSE is a sensitive and selective extraction method to analyze the functional ingredients present in makgeolli, specialized equipment including a thermal desorption system is required for the GC analysis. In addition, commercial coating materials available for stir-bars (polydimethylsiloxane and ethylene glycol) limit the extraction to either nonpolar or polar compounds, but not both simultaneously. Solvent extraction (SE) is a popular and straightforward extraction method used to separate compounds from solid and liquid samples using organic solvents, and can be easily performed in a laboratory without specialized analytical equipment. However, SE methods appropriate for simultaneous determination of new bioactive compounds recently identified in makgeolli have not been reported.
In the present study, we identified 22 volatile compounds using SE-GC/MS and selected two compounds, 4-vinyl guaiacol (4-VG) and 2,4-di-tert-butylphenol (DTBP), which have been reported to have specific bioactivity. We optimized the extraction solvent and validated the linearity, sensitivity, precision, and accuracy of the method for simultaneous analysis of 4-VG and DTBP and the known bioactive compounds FOH and SQ. The validated method was then applied to makgeolli, beer, and wine samples.
Materials and methods
SE procedures
Petroleum ether/chloroform/MeOH extraction
Fifty milliliters of makgeolli sample was placed in a 250 mL centrifuge bottle and 100 mL of solvent mixture (petroleum ether:chloroform:MeOH = 2:2:1, v/v) and 3% NaCl (w/v) were added. The bottle was agitated with an automatic shaker (SR-2W, Taitec, Saitama, Japan) to extract the analytes from the sample for 30 min and then centrifuged for 5 min at 10,000 rpm. The organic phase (lower phase) was dehydrated using anhydrous sodium sulfate and filtered with filter paper (Whatman No. 1), before collection in a round flask. The extraction step was performed in triplicate. The solvent was then processed with a rotary evaporator and the residue was reconstituted in 1.5 mL ethyl acetate for GC-MS analysis.
MeOH/petroleum ether extraction
Fifty milliliters of makgeolli sample was placed in a 250 mL centrifuge bottle and 100 mL of solvent mixture (petroleum ether:chloroform = 1:1, v/v) was added to the bottle. The bottle was agitated with an automatic shaker (SR-2W, Taitec, Saitama, Japan) for 30 min and centrifuged for 5 min at 10,000 rpm. The organic phase (upper phase) was dehydrated using anhydrous sodium sulfate and filtered with filter paper (Whatman No. 1), before collection in a round flask. The extraction step was performed in triplicate. The solvent was then processed in a rotary evaporator and the residue was reconstituted in 1.5 mL ethyl acetate for GC-MS analysis.
MeOH/chloroform extraction
Fifty milliliters of makgeolli sample was placed in a 250 mL separation funnel and 100 mL of solvent mixture (MeOH:chloroform = 1:1, v/v) was added to the funnel. The separation funnel was then agitated with an automatic shaker (SR-2W, Taitec, Saitama, Japan) for 30 min and left for 24 h until the separation of organic and aqueous phases occurred.
Gas chromatography-mass spectrometry
Bioactive compounds present in makgeolli were analyzed with an Agilent 7890A gas chromatography instrument coupled with a 5975c quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The separation was performed using a 5% diphenyl-95% dimethyl siloxane fused-silica capillary column (HP-5ms, 30 m, 250 μm i.d., 0.25 mm film thickness, Agilent Technologies, Middleburg, OI, USA). The carrier gas was helium at a 1.5 mL min–1 flow rate. The temperature of the GC injector was maintained at 250°C and the split ratio was set to 5:1. The GC oven temperature was programmed as follows: held at 100°C for 1 min; increased from 100°C to 220°C at 10°C min–1 for 12 min; ramped from 220°C to 290°C at 20°C min–1 for 3.5 min; increased from 290°C to 305°C at 10°C min−1; held at 305°C for 5 min. The temperatures of the MS transfer line, ion source, and quadrupole analyzer were maintained at 280°C, 230°C, and 150°C, respectively. For tentative identification of the volatiles present in makgeolli, mass spectra were recorded using the full scan mode in the mass range between m/z 35 and 350, and the mass spectra were compared with data in the Wiley and NIST08 library databases. The identification of four compounds (4-VG, DTBP, FOH, and SQ) was confirmed by comparing the retention time and the mass spectra with those of the authentic standards. For quantification of these four compounds, selected ion monitoring (SIM) mode was used. The selected ions for the four compounds for SIM mode were as follows: 4-VG (107, 135, 150 m/z); DTBP (74, 191, 206 m/z); FOH (81, 93, 107 m/z); SQ (69, 95, 137 m/z) (bolds indicate monitoring ions).
Method validation
The linearity, accuracy, sensitivity, and precision were evaluated for validation of the SE/GC-MS method. The linearity 4-VG, DTBP, FOH, and SQ was calculated as coefficient of determination (r2) values at five to six points of varying concentrations of the standards for each compound. The accuracy of the method was evaluated with a recovery test. Five or fifty micrograms of each analyte was spiked with makgeolli sample, and the fortified samples were subjected to extraction and GC-MS analysis. The recovery (%) of each analyte was calculated using the following equation:
where Cf is the concentration of fortified samples, Cu is the concentration of unfortified samples, and Ca is the concentration of analyte added.
The sensitivity of the method was determined by limit of detection (LOD) and limit of quantification (LOQ) values. The LOD and LOQ values were calculated with the equations as follows: LOD = 3.3σ/s; LOQ = 10σ/s (σ = standard deviation of response (n = 6); s = slope of calibration curve).[Citation18] The precision was evaluated by measuring intra- and inter-day repeatability. The standard solutions at three different concentrations for each analyte were injected into GC-MS analysis six times consecutively for the intra-day assay, and once daily for 3 days for the inter-day assay.
Results and discussion
Selection of extraction solvents
To select the optimal solvents for extraction of the volatile compounds present in makgeolli, we first evaluated the MeOH/dichloromethane (1:1, v/v) and MeOH/chloroform (1:1, v/v) mixtures as extraction solvents. These two solvent mixtures could not separate the aqueous and organic phases after 24 h due to extensive emulsion formation. It appears that some emulsifying factors including proteins, fatty acids, and phospholipids contribute to emulsion formation.[Citation19] To reduce the contact area between the aqueous and organic phases, we selected the more hydrophobic solvent petroleum ether, and then chloroform and dichloromethane, and evaluated the mixture of petroleum ether/MeOH (1:1, v/v) as extraction solvents. This solvent mixture clearly separated the aqueous and organic phases. The organic phase was concentrated and subjected to GC-MS analysis, and 22 compounds (14 fatty acids, 1 fatty alcohol, 3 esters, 3 phenolic compounds, and diethyl succinate) were tentatively identified (data not shown). Among these compounds, 4-VG, a 4-vinyl derivative of hydroxycinnamic acid, has been reported to exhibit antioxidant[Citation20,Citation21] and anti-inflammatory[Citation22] properties. In addition, DTBP (an alkylated phenol) has been reported to exhibit antioxidant activity in vitro and in vivo.[Citation23,Citation24] To extract FOH, SQ, 4-VG, and DTBP simultaneously, we used the chloroform/petroleum ether/MeOH (2:2:1, v/v) mixture and successfully identified all of the compounds. Notably, 4-VG and DTBP in makgeolli were the newly identified compounds in this study.
Method validation
The method for analysis of the four bioactive compounds (DTBP, 4-VG, FOH, and SQ) from makgeolli was validated for linearity, sensitivity, accuracy, and precision.
Linearity, LOD, and LOQ
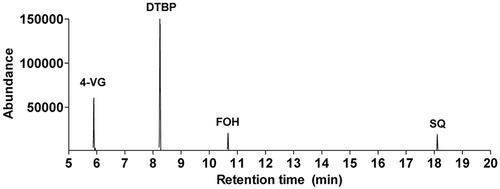
To assess the linearity of the method, different concentrations of the standard solutions for each compound were used. The chromatography results for the standard solutions are shown in . The standard solutions were injected into the GC-MS machine six times consecutively, and the linearity of each compound was calculated as the correlation coefficient (r2) by regression analysis. All compounds showed high linearity with r2 values higher than 0.99. The LOD and LOQ values for the method involving the four compounds were calculated as described in the “Materials and methods” section and ranged from 2.7 to 25.3 and 8.2 to 76.8 ng mL−1, respectively ().
Table 1. Linearity, LOD, and LOQ values for the SE/GC-MS method.
Repeatability
To evaluate the repeatability of the SE/GC-MS method, we performed an intra- and inter-day precision test. For these tests, the standard solutions of four compounds at three different concentrations were injected into GC-MS analysis six times within the same day or three times each day for three consecutive days. The intra-day precision for the four compounds ranged from 0.69% to 4.82%, and the intra-day precision ranged from 0.72% to 9.36% ().
Table 2. Inter- and intra-day precision for the SE/GC-MS method.
Accuracy
To assess the accuracy of the SE-GC/MS method, we performed a recovery test for the four compounds. Two different concentrations (100 and 1000 ng mL−1) of standard solutions for the four compounds were spiked and the recovery was calculated as described in the “Materials and methods” section. The recovery of the four compounds ranged from 88% to 114% and from 99% to 117% at 100 and 1000 ng mL−1 of the spiked concentrations, respectively ().
Table 3. Recovery test for the SE/GC-MS method.
Application to various alcoholic beverage samples
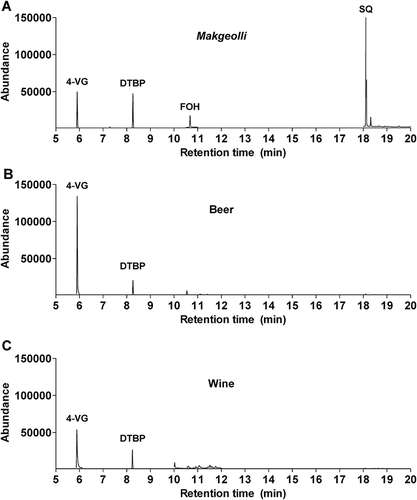
To test the applicability of the SE-GC/MS method in other alcoholic beverage samples, we determined the concentrations of 4-VG, DTBP, FOH, and SQ in makgeolli, beer, and wine samples. The chromatograms obtained by SIM are shown in . The concentration of 4-VG ranged from 72 to 1977 ng mL−1 (except for the unquantified sample 3), from 120 to 871 ng mL−1, and from 101 to 283 ng mL−1 in makgeolli, beer, and wine, respectively. The concentration of DTBP ranged from 33 to 102 ng mL−1, 38 to 50 ng mL−1, and 26 to 49 ng mL−1 in makgeolli, beer, and wine, respectively. The FOH and SQ were quantified at concentrations of 21–150 ng mL−1 and 1260–4561 ng mL−1 in makgeolli, respectively, but not detected in beer or wine (). It has been reported that 4-VG is formed from ferulic acid, which is an abundant hydroxycinnamic acid present in cereal grains, and emerges during yeast fermentation and can be determined at concentrations of 0.15–4.10 μg mL–1 in beers by HPLC analysis.[Citation25] The levels of 4-VG in wines have been reported at between 0 and 0.50 μg mL–1.[Citation26] The relatively higher concentrations of 4-VG in makgeolli and beer compared to wine are likely due to the difference in the starting materials (cereal grains vs. grapes). In addition, the volatile antioxidant DTBP has been identified in some plants including sweet potato[Citation23] and pomegranate[Citation27], and appears to be present in the raw plant materials used to produce makgeolli, beer, and wine. In previous studies, FOH and SQ were quantified at concentrations of 26–142 ng mL−1 and 513–7431 ng mL−1 using the SBSE/GC-MS method, respectively. FOH is present at concentrations below 4 ng mL−1 in beer and wine.[Citation9,Citation17] The saccharification of starches in makgeolli likely provides the precursor sugars such as glucose required for the biosynthesis of FOH and SQ.[Citation2,Citation28] Although our SE-GC/MS method did not detect FOH or SQ in beer or wine, which may be present in very low amounts, we successfully identified measurable levels of these compounds in makgeolli samples using our newly developed method.
Table 4. Comparison of concentrations of four bioactive compounds present in makgeolli, beer, and wine samples.
Conclusion
In summary, we have developed a novel SE-GC/MS approach for the analysis of the four bioactive compounds 4-VG, DTBP, FOH, and SQ in makgeolli. This analytical method showed good linearity, sensitivity, accuracy, and repeatability in validation tests. FOH and SQ were identified only in makgeolli but not in beer or wine. DTBP was quantified in all liquor samples, and 4-VG was quantified in makgeolli and beer at higher concentrations than in wine. These findings suggest that the SE-GC/MS method can be used as a simple and sensitive technique to analyze these four bioactive compounds present in makgeolli without the need for specialized analytical equipment.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Chai, C.; Lim, G. S.; Kim, Y. J.; Oh, S. W. Microbial Community Changes in Makgeolli during Brewing. Journal of the Institute of Brewing 2015, 121, 304–308. DOI: 10.1002/jib.227.
- Nile, S. H.;. The Nutritional, Biochemical and Health Effects of Makgeolli–A Traditional Korean Fermented Cereal Beverage. Journal of the Institute of Brewing 2015, 121, 457–463. DOI: 10.1002/jib.264.
- Shin, E. J.; Kim, S. H.; Kim, J. H.; Ha, J.; Hwang, J.-T. Dealcoholized Korean Rice Wine (Makgeolli), Exerts Potent Anti-Tumor Effect in AGS Human Gastric Adenocarcinoma Cells and Tumor Xenograft Mice. Journal of Microbiology and Biotechnology 2015, 25, 1485–1492. DOI: 10.4014/jmb.1503.03059.
- Wang, S.-J.; Lee, H.-J.; Cho, J.-Y.; Jang, M.-Y.; Park, K.-H.; Moon, J.-H. Inhibition Effect against the Rat Blood Plasma Oxidation of the Makgeolli (Takju) Korean Rice Wine. Korean Journal of Food Preservation 2012, 19, 116–122. DOI: 10.11002/kjfp.2012.19.1.116.
- Lee, S.-J.; Kim, J.-H.; Jung, Y.-W.; Park, S.-Y.; Shin, W.-C.; Park, C.-S.; Hong, S.-Y.; Kim, G.-W. Composition of Organic Acids and Physiological Functionality of Commercial Makgeolli. Korean Journal of Food Science and Technology 2011, 43, 206–212. DOI: 10.9721/KJFST.2011.43.2.206.
- Eom, J.-E.; Kwon, S.-C.; Moon, G.-S. Detection of 1, 4-Dihydroxy-2-Naphthoic Acid from Commercial Makgeolli Products. Preventive nutrition and food science 2012, 17, 83. DOI: 10.3746/pnf.2012.17.1.083.
- Huang, Y.; W-W, L.; Chen, B.; Wu, M.; S-G., L. Determination of 13 Phenolic Compounds in Rice Wine by High-Performance Liquid Chromatography. Food Analytical Methods 2015, 8, 825–832. DOI: 10.1007/s12161-014-9939-y.
- Wang, S.-J.; Lee, H.-J.; Cho, J.-Y.; Park, K.-H.; Moon, J.-H. Isolation and Identification of Antioxidants from Makgeolli. Korean Journal of Food Science and Technology 2012, 44, 14–20. DOI: 10.9721/KJFST.2012.44.1.014.
- Ha, J.; Wang, Y.; Jang, H.; Seog, H.; Chen, X. Determination of E, E-Farnesol in Makgeolli (Rice Wine) Using Dynamic Headspace Sampling and Stir Bar Sorptive Extraction Coupled with Gas Chromatography–Mass Spectrometry. Food chemistry 2014, 142, 79–86. DOI: 10.1016/j.foodchem.2013.07.038.
- Joo, J. H.; Jetten, A. M. Molecular Mechanisms Involved in Farnesol-Induced Apoptosis. Cancer letters 2010, 287, 123–135. DOI: 10.1016/j.canlet.2009.05.015.
- Rao, C. V.; Newmark, H. L.; Reddy, B. S. Chemopreventive Effect of Farnesol and Lanosterol on Colon Carcinogenesis. Cancer detection and prevention 2002, 26, 419–425. DOI: 10.1016/S0361-090X(02)00119-8.
- Scheper, M. A.; Shirtliff, M. E.; Meiller, T. F.; Peters, B. M.; Jabra-Rizk, M. A. Farnesol, a Fungal Quorum-Sensing Molecule Triggers Apoptosis in Human Oral Squamous Carcinoma Cells. Neoplasia 2008, 10, 954–963. DOI: 10.1593/neo.08444.
- Qamar, W.; Sultana, S. Farnesol Ameliorates Massive Inflammation, Oxidative Stress and Lung Injury Induced by Intratracheal Instillation of Cigarette Smoke Extract in Rats: An Initial Step in Lung Chemoprevention. Chemico-biological interactions 2008, 176, 79–87. DOI: 10.1016/j.cbi.2008.08.011.
- Khan, R.; Sultana, S. Farnesol Attenuates 1, 2-Dimethylhydrazine Induced Oxidative Stress, Inflammation and Apoptotic Responses in the Colon of Wistar Rats. Chemico-biological interactions 2011, 192, 193–200. DOI: 10.1016/j.cbi.2011.03.009.
- Qamar, W.; Khan, A. Q.; Khan, R.; Lateef, A.; Tahir, M.; Rehman, M. U.; Ali, F.; Sultana, S. Benzo (A) Pyrene-Induced Pulmonary Inflammation, Edema, Surfactant Dysfunction, and Injuries in Rats: Alleviation by Farnesol. Experimental lung research 2012, 38, 19–27. DOI: 10.3109/01902148.2011.632064.
- Kim, S.-K.; Karadeniz, F. Biological Importance and Applications of Squalene and Squalane. Advances in Food and Nutrition Research 2012, 65, 223–233.
- Ha, J.; Shim, Y.-S.; Cho, Y.; Seo, D.; Jang, H.; Jang, H. Analysis of E, E-Farnesol and Squalene in Makgeolli Using Stir Bar Sorptive Extraction Coupled with Gas Chromatography-Mass Spectrometry. Analytical Science and Technology 2014, 27, 60–65. DOI: 10.5806/AST.2014.27.1.60.
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chronicles of Young Scientists 2011, 2, 21. DOI: 10.4103/2229-5186.79345.
- Dalgleish, D. G.;. Food Emulsions—Their Structures and Structure-Forming Properties. Food hydrocolloids 2006, 20, 415–422. DOI: 10.1016/j.foodhyd.2005.10.009.
- Terpinc, P.; Polak, T.; Šegatin, N.; Hanzlowsky, A.; Ulrih, N. P.; Abramovič, H. Antioxidant Properties of 4-Vinyl Derivatives of Hydroxycinnamic Acids. Food chemistry 2011, 128, 62–69. DOI: 10.1016/j.foodchem.2011.02.077.
- Fujioka, K.; Shibamoto, T. Quantitation of Volatiles and Nonvolatile Acids in an Extract from Coffee Beverages: Correlation with Antioxidant Activity. Journal of agricultural and food chemistry 2006, 54, 6054–6058. DOI: 10.1021/jf060460x.
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal Stability, Antioxidant, and Anti-Inflammatory Activity of Curcumin and Its Degradation Product 4-Vinyl Guaiacol. Food & function 2015, 6, 887–893. DOI: 10.1039/C4FO00790E.
- Choi, S. J.; Kim, J. K.; Kim, H. K.; Harris, K.; Kim, C.-J.; Park, G. G.; Park, C.-S.; Shin, D.-H. 2, 4-Di-Tert-Butylphenol from Sweet Potato Protects against Oxidative Stress in PC12 Cells and in Mice. Journal of medicinal food 2013, 16, 977–983. DOI: 10.1089/jmf.2012.2739.
- Varsha, K. K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K. M. 2, 4-Di-Tert-Butyl Phenol as the Antifungal, Antioxidant Bioactive Purified from a Newly Isolated Lactococcus Sp. International journal of food microbiology 2015, 211, 44–50. DOI: 10.1016/j.ijfoodmicro.2015.06.025.
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F. R. Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces Cerevisiae. Journal of Agricultural and Food Chemistry 2004, 52, 602–608. DOI: 10.1021/jf0346556.
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of Volatile Phenols by Saccharomyces Cerevisiae in Wines. Journal of the Science of Food and Agriculture 1993, 62, 191–202. DOI: 10.1002/jsfa.2740620213.
- Choi, S. J.; Lee, J.-H.; Heo, H. J.; Cho, H. Y.; Kim, H. K.; Kim, C.-J.; Kim, M. O.; Suh, S. H.; Shin, D.-H. Punica Granatum Protects against Oxidative Stress in PC12 Cells and Oxidative Stress-Induced Alzheimer’s Symptoms in Mice. Journal of medicinal food 2011, 14, 695–701. DOI: 10.1089/jmf.2010.1452.
- Holstein, S. A.; Hohl, R. J. Isoprenoids: Remarkable Diversity of Form and Function. Lipids 2004, 39, 293–309. DOI: 10.1007/s11745-004-1233-3.