 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study reports a comparative screening of four species of Origanum in Turkey, based on their essential oil composition, total phenolic content, antioxidant and antibiofilm activities. The major components of essential oils were p-cymene, linalool, and thymol. The total phenolic contents differed from 3.81 to 47.54 mg of GAE/g of extract. The concentrations of flavonoids varied from 12.74 to 58.39 mg of Ru/g of extract. Antioxidant activity was determined in vitro using DPPH reagent and expressed as concentration of each extract required to inhibit radical by 50% (IC50) values that ranged from 16.03 to 48.94 μg/ml. Our results indicated that chloroform extracts of species O. majorana and O. onites, with a total content of polyphenols (47.54 mg of GAE/g and 45.17 mg of GAE/g, respectively) and an IC50 of 16.03 μg/ml and 16.89 μg/ml, respectively were more antioxidant. Among the essential oil concentrations tested, maximum antibiofilm activity was found as 92.24% against M. luteus NRRL-B 1013 by O. majorana essential oil at 50 mg/ml.
Introduction
Many medicinal plants are known to comprise large amounts of antioxidants which can play an important role in scavenging free radicals and reactive oxygen species. During the time of stress created by drugs, toxic substances, or diseases, the production of active oxygen species increases, which has the potential to cause oxidative damage.[Citation1] Antioxidants have crucial functions in preventing oxidative mechanisms that lead to degenerative diseases.[Citation2] Today, free radicals exhibit important functions in the etiology of cardiovascular diseases, such as cancer, Alzheimer, and Parkinson.[Citation3] Evidence shows that vegetable and fruits consumptions and intakes of certain nonnutrients which can be found in foods decrease the risk of several pathological events, such as cancer [Citation4–Citation7] and cardio- and cerebro-vascular diseases.[Citation8] Moreover, plants or their crude extracts have been used in the prevention and/or treatment of some diseases in various different communities around the world.[Citation1]
Origanum is a member of Lamiaceae family and is a herbaceous plant native to the Mediterranean, Euro-Siberian, and Irano-Turanian regions. Many of Origanum species are found in the Eastern Mediterranean subregion.[Citation9] As chemicals and aromatic characteristics are variable, Origanum plants are extensively used in agriculture and pharmaceutical, and cosmetic industries as a culinary herb, flavouring substances of food products, alcoholic beverages, and perfumery for their spicy fragrance.[Citation10–Citation14] These plants have potential health-promoting benefits and antioxidant properties from phenolic contents.[Citation15] Furthermore, Origanum plants have been reported to exhibit multiple biological activities such as antioxidant, antimicrobial, antiviral, antihepatotoxic, and antiulcerogenic activities which are assigned to an array of biologically active plant chemicals including triterpenes, proteins and steroids.[Citation16] O. hypericifolium and O. sipyleum are narrowly distributed endemic species classified as “Least Concern (LC)” category of IUCN (International Union for Conservation of Nature) and taxonomically belongs to section Anatolicon.[Citation17] O. onites is a perennial species with woody stem and is commonly known as “Turkish oregano” and O. majorana is a culinary herb and is called as “sweet marjoram” because of its citrus flavours. Additionally, Turkey has become a major supplier of Origanum herb and its oil to world markets due to its high quality. Turkey dominates the global support of oregano oil, supplying around 70% of global needs.[Citation18]
Pathogenic bacteria growing in slime-enclosed aggregates known as biofilms are associated with many persistent and chronic bacterial infections such as cystic fibrosis, chronic wounds, otitis media, dental plaque, and infections related to the use of medical devices, such as catheters and implants.[Citation19] These bacteria are especially resistant to antibiotics. Herewith, it is very difficult to combat these diseases. Although most research have focused on antimicrobial effects of essential oils and/or active constituents of plants, information about antibiofilm effects of plants is lacking.[Citation20] This is first study undertook the antibiofilm effects of O. onites, O. hypericifolium, O. majorana, and O. sipyleum. The present study aimed to examine the distribution of phenolic compounds and antibiofilm acitivity of essential oils in four species of Origanum from southeastern Turkey.
Material and methods
Plant materials
Origanum majorana, O. onites, O. hypericifolium, and O. sipyleum at the flowering stage were collected from certain localities in southeast Turkey (). The taxonomic identification of plant materials was confirmed by Dr. Gürkan SEMİZ, in Department of Biology, Pamukkale University, Denizli-Turkey. The voucher specimens have been deposited at the Laboratory of Chemical Ecology of Pamukkale University (Denizli, Turkey).
Table 1. Collection sites, dates, voucher specimens, and yields of Origanum L. species studied.
Preparation of the extracts
Plant samples were dried in a dry and dark place at ambient temperature. The dried flowering shoots and leaves were cut off from the stem and ground in a grinder with a 2 mm diameter mesh. The collected plant material, air-dried and fine powdered (10 g), was extracted with 50 ml of solvent (water, methanol, and chloroform), respectively for 6 h at 50°C in a temperature controlled shaker (Memmert, SV 1422). The resulting extracts were filtered through Whatman No. 1 paper and residues were re-extracted with equal volume of solvents. Methanol and chloroform extracts were evaporated under vacuum at 37°C using rotary evaporator (IKA, RV10). The water extracts were concentrated to dryness under a vacuum on a freeze dry system (Labconco FreeZone) at −105°C. Same lyophilization procedure was applied to methanol and chloroform extracts to remove moisture from extracts. The obtained extracts were kept in dark and stored at 4°C.
Extraction of the essential oil
Air-dried parts of the Origanum species were subjected to steam distillation for 4 h using a Clevenger apparatus to obtain essential oil. The essential oil was dried in anhydrous sodium sulphate and after filtration stored in a sealed dark vial at 4°C until analysis. The yields of the essential oils were calculated by the formula:
GC/MS analysis
Chemical analyses of the essential oil were performed on gas chromatography-mass spectrometry (Hewlett Packard GC type 7820A, MSD 5975; Hewlett Packard, Wilmington, DE, USA) using a 30-m long HP-5MS (ID 0.25 mm, film thickness 0.25 mm, Hewlett Packard) capillary column. The chromatographic conditions were as follows: helium was used as the carrier gas at 1.2 ml min−1; the temperature program for terpenes ranged from 50°C to 250°C; the heating rate was 5 °C min−1; SCAN technique (mass numbers from m/z 30 to 350 were recorded; signal ions in monitoring; 93, 133, 136, 161, and 204 m/z) was used; the samples of 1 μl were injected automatically and in the splitless mode. Mass spectra were taken at 70 eV. The individual peaks were identified by comparison of their retention indices (relative to C8-C25 n-alkanes for HP-5MS) as well as by comparing their mass spectra with Wiley 7 MS library (Wiley, New York, NY, USA) and NIST02 (Gaithersburg, MD, USA) mass spectral database. A series of n-alkanes was also injected under same analytical conditions with that of essential oil for the calculation of Retention Indices (RI). The percentages of the samples were calculated from the GC peak areas with the normalization method. The relative amount of compounds was calculated as mean values from duplicate GC and GC/MS analyses.
Total phenolic content
Total phenolic content was estimated spectrophotometrically according to the Folin-Ciocalteu colorimetric method.[Citation21] The reaction mixture was prepared by mixing 0.5 ml of methanolic solution (1 mg/ml) of extract, 2.5 ml of 10% Folin-Ciocalteu’s reagent dissolved in water and 2.5 ml 7.5% NaHCO3. The samples were incubated at 45°C for 15 min. The absorbance was determined at λmax = 765 nm. It was calibrated against gallic acid standards and expressed the results as mg gallic acid equivalents (GAE)/g extract. Data presented are average values of three measurements for each sample.
Total flavonoid content
Flavonoid content was measured according to aluminum chloride colorimetric method. [Citation22] The sample contained 1 ml of methanolic solution of the extract in the concentration of 1 mg/ml and 1 ml of 2% AlCl3 solution dissolved in methanol. The samples were incubated for an hour at the room temperature. The absorbance was determined at λmax = 415 nm.[Citation23] A calibration curve was prepared with rutin and the results were expressed in terms of rutin equivalent (mg of Ru/g of extract). Data presented are average values of three measurements for each sample.
Free radical scavenging activity using DPPH
The DPPH free radical scavenging activity of each sample was determined following the method described by Stankovic et al.[Citation23] The stock solution (1 mg/ml) of the plant extract was prepared in methanol. Dilutions were made to obtain concentrations of 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90, 1.99, and 0.97 μg/ml. Diluted solutions (1 ml each) were mixed with 1 ml of DPPH (2,2-diphenyl-1-picrylhydrazyl) methanolic solution (0.2 mM). After 30 min in darkness at room temperature, the absorbance was recorded at 517 nm against a blank (methanol solution). The control samples contained all the reagents except the extract. The DPPH radicals scavenging activity was calculated using the following equation:
IC50 was obtained from sigmoidal graph by using non-linear regression analysis. The experiment was performed in triplicate. Synthetic antioxidants [butylated hydroxytoluene (BHT) and quercetin] were used as positive controls.
Microorganisms and medium
Six gram positive (Staphylococcus aureus ATCC 33862, S. aureus ATCC 29213, Enterococcus faecalis M10 (clinic isolate), E. faecalis M18 (clinic isolate), E. faecalis ATCC 19433, Micrococcus luteus NRRL-B 1013) and three gram negative (Salmonella tphymurium ATCC 14028, Enterobacter cloacea ATCC 28355, Pseudomonas fluorescens ATCC 55241) bacteria were used as test microorganisms. The bacterial strains were collected from Bacteriology Laboratory of Pamukkale University. Bacterial cultures were inoculated in growth media Tryptic Soy Broth (TSB) which consisted of peptone from casein (17.0 g/L), peptone from soymeal (3.0 g/L), glucose (2.5 g/L), NaCl (5.0 g/L), and K2HPO4 (2.5 g/L). The culture was aerobically incubated and the growth was followed by measuring the optical density (OD) at 600 nm.
Antibiofilm activity
Crystal violet assay was used to test antibiofilm activity of the essential oils against the bacteria on 96-well polystyrene plates.[Citation24] The bacterial cultures were grown in TSB at 37°C and 30°C under aerobic conditions for 24 h. Then, bacterial suspension at 0.5 McFarland turbidity standard was dispensed into each well of 96-well plates in the presence of TSB supplemented with glucose, containing essential oils. The essential oils were prepared at 50 mg/ml, 25 mg/ml, and 12.5 mg/ml ratios. The plates were then incubated for 48 h at 37°C (30°C for Pseudomonas florescens ATCC 55241 and Micrococcus luteus NRRL-B 1013). Following incubation, crystal violet staining assay was applied. Negative control (cells+TSB) was used as growth control. Each experiment was performed in duplicate. And the biofilm inhibition percentage was calculated by using the following formula:
Statistical analysis
All measurements were performed in triplicate and results were expressed as mean ± standard deviation (SD) of each triplicate test. The data were subjected to analysis of variance, and appreciate mean separation was conducted using Tukey’s multiple range test in SPSS 15.0 software.[Citation25]
Results and discussion
Chemical composition of the essential oils
The composition of essential oils obtained from Origanum species is shown in . Overall, 34 compounds representing >97% of the oils were identified by GC and GC/MS. The essential oil yield for the collected samples, ranging from 1.10% (v/w) in O. sipyleum to 2.02% (v/w) in O. onites based on the dry weight of the samples. Although the total contents of terpene profile are quite similar, there are differences in the content of individual components. p-Cymene, linalool, and thymol are the most abundant terpenoids in Origanum species sampled. Earlier studies similarly reported the essential oil compositions of the same Origanum species from different regions and found that carvacrol, p-cymene, terpinen-4-ol, and thymol were the major components but their percentages varied from the results of the current study.[Citation26–Citation30] The differences in the chemical composition of Origanum essential oils among studies may be related to different environmental and climatic conditions, sampling time, genetic origins of plants, vegetative plant phases, and extraction and quantification methods. Carvacrol, thymol, and linalool are known to possess strong antioxidant properties [Citation31–Citation33] and carvacrol and thymol also exhibit antibacterial activity against several bacteria.[Citation10,Citation34]
Table 2. Chemical composition (%) of essential oils of Origanum species growing in southeastern Turkey.
Total phenolic and flavonoid content
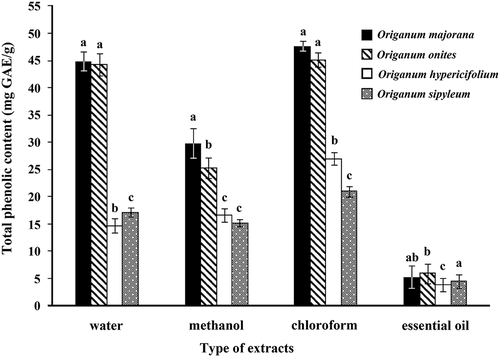
The total phenolic contents of the extracts and essential oil ranged between 3.81 to 47.54 mg of GAE/g of extract (). O. majorana and O. onites chloroform extracts had the highest total phenolic content (47.54 mg GAE/g and 45.17 mg GAE/g, respectively). Water extracts of O. majorana and O. onites also contain high levels of phenolics (44.81 mg GAE/g and 44.26 mg GAE/g, respectively), while phenolic contents in methanol and essential oil extracts were relatively lower. Varying solubility of the phenolic compounds due to different solvents could be explained by the solvent polarity.[Citation35] Moreover, phenolics may also be associated with other plant metabolites (e.g. carbohydrates and proteins). These results suggest that methanol and ethanol seem to be more efficient in extracting lower molecular weight polyphenols while the higher molecular weight flavanols are better extracted with aqueous acetone. Additionally, ethanol is also safe for human consumption.[Citation36]
Figure 1. Total phenolic contents in the extracts of O. majorana, O. onites, O. hypericifolium, and O. sipyleum expressed in terms of gallic acid equivalent (mg of GAE/g of extract). results are presented as the mean from three independent experiments and expressed as relative mean ± standard deviation. Those with the same letter are not significantly different at the P < 0.05 level.
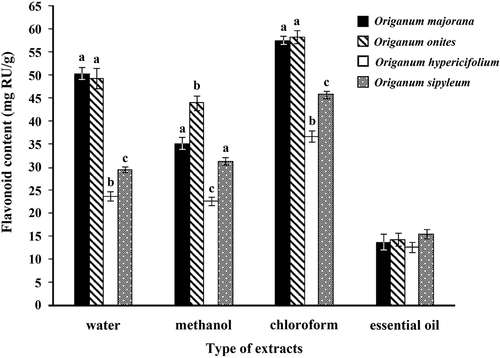
The concentrations of flavonoids varied from 12.74 to 58.39 mg of Ru/g of extract (). Chloroform extracts of O. majorana and O. onites have been found to be rich in flavonoids with a value of 57.50 mg of Ru/g and 58.39 mg of Ru/g, respectively, than other extracts examined and essential oils. Similar to the phenolic contents, the water extracts of O. majorana and O. onites also contain high levels of flavonoid (50.24 mg of Ru/g and 49.35 mg of Ru/g, respectively). Based on these results, chloroform seem to be the best solvent for extracting flavonoids and phenolics from Origanum species.
Figure 2. Flavonoid contents in the extracts of O. majorana, O. onites, O. hypericifolium, and O. sipyleum expressed in terms of rutin equivalent (mg of Ru/g of extract). results are presented as the mean from three independent experiments and expressed as relative mean ± standard deviation. Those with the same letter are not significantly different at the P < 0.05 level.
Free radical scavenging activity using DPPH
DPPH free radical scavenging activities of species O. majorana, O. onites, O. hypericifolium, and O. sipyleum extracts and essential oil are shown in . Antioxidant activity values that are determined in vitro using DPPH reagent and expressed as concentration of each extract and essential oil required to inhibit radical by 50% (IC50) ranged from 16.03 to 48.94 μg/ml. A higher DPPH radical scavenging activity is associated with a lower IC50 values. When compared to the activity of reference antioxidant rutin (IC50 = 9.28 μg/ml) [Citation23], DPPH scavenging activity of chloroform extract of species O. majorana and O. onites is high (IC50 = 16.03 μg/ml and 16.89 μg/ml, respectively). Water extract of species O. majorana and O. onites also showed considerable DPPH scavenging activity with IC50 value of 18.02 μg/ml and 18.32 μg/ml, respectively). These results show that extracts from O. majorana and O. onites have antioxidant power and ability to scavenge free radicals. There is enough evidence to support that the antioxidant activity of plant materials is well correlated with the content of phenolic compounds.[Citation23,Citation37,Citation38] In the last decade, polyphenol-rich foods and herbs have received specific focus because of their biological effects including antioxidant activity.[Citation39] Various researches on the antioxidant activity of plant extracts have shown a high linear correlation between the values of phenolic content and antioxidant activity.[Citation40–Citation44] It is well known that phenolic substances such as flavonoids, phenolic acids, and tannins contribute directly to the antioxidant capacity of plants.[Citation45,Citation46] Flavonoids have been shown to be highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various free radicals [Citation47] implicated in several diseases. Epidemiological studies suggest that the consumption of flavonoid-rich foods protects against human diseases associated with oxidative stress.[Citation3] Generally, plant materials rich in phenolics are increasingly being used in food industry because they retard oxidative degradation of lipids and improve the quality and nutritional value of food.[Citation41,Citation48]
Table 3. DPPH scavenging activity of extracts from O. majorana, O. onites, O. hypericifolium, and O. sipyleum presented as IC50 values.
Our results show that O. majorana and O. onites can be a source of polyphenols and flavonoids and can provide considerable benefits when used as food ingredients and for human consumption. Earlier studies reported that Origanum has important biological activities and acts against different types of diseases.[Citation20,Citation49,Citation50] However, safety and toxicity issues of these extracts and essential oil should be evaluated beforehand.
Microbial tests
Biofilm is more resistant to antimicrobials compared to planktonic cells. Biofilm-related infections threaten the human life and cause recurrent infections in hospitals. Therefore, discovery of alternative drugs for curing biofilm infections has become one of the attractive areas for researchers. Origanum is well known for its antimicrobial activity against such bacteria like Escherichia coli, Klebsiella pneumonia, Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans, Pseudomonas aeruginosa, Vibrio splendidus, Sarcina lutea, and Salmonella typhimurium.[Citation51–Citation55] However, there is no adequate information regarding the antibiofilm properties of Origanum species. In this study, the effects of four essential oils of Origanum species on biofilm formation of six gram positive and three gram negative bacteria was determined (). Essential oils consistently showed an inhibitory effect on biofilm formation of pathogens with a dose dependent manner and as the essential oil concentrations increased the biofilm inhibition percentage also increased. The maximum biofilm inhibition activity was observed at 50 mg/ml. Maximum biofilm inhibition rates for O. onites was 58.73% against S. aureus ATCC 29213, O. hypericifolium and O. majorana was 87.40% and 92.24% against M. luteus NRRL-B 1013, respectively. Also, maximum biofilm inhibition rate of O. sipyleum was 85.71% against S. tphymurium ATCC 14028. In addition, the biofilm biomass of P. fluorescens ATCC 55241 and S. tphymurium ATCC 14028 were inhibited at 56.58% and 56.69% by the O. hypericifolium and O. majorana essential oil at 25 mg/ml concentration, in consecutive order.
Table 4. The biofilm inhibition effect of essential oils of Origanum species, expressed as percentage inhibition.
On the other hand, 12.5 mg/ml concentration of essential oils exhibited lower antibiofilm activity against bacteria species tested. Biofilm inhibition percentage of O. onites ranged from 13.58% to 27.43%, O. hypericifolium ranged from 10.00% to 44.28%, O. majorana ranged from 16.02% to 48.25%, O. sipyleum ranged from 12.55% to 32.45% at 12.5 mg/ml of essential oils (). Essential oil of O. onites prevented the biofilm formation of S. aureus ATCC 29213, E. faecalis ATCC 19433 and P. fluorescens ATCC 55241 over 50% concentration of 50 mg/ml. O. hypericifolium exhibited higher inhibition activity against bacteria. Over the 60% biofilm inhibition percentages were determined on S. aureus ATCC 29213, M. luteus NRRL-B 1013, E. faecalis ATCC 19433, and P. fluorescens ATCC 55241 by O. hypericifolium. O. majorana had the biofilm inhibition percentage of 92.24% against the M. luteus NRRL-B 1013 at 50 mg/ml oil concentration. Also, O. majorana showed good antibiofilm activity at 25 mg/ml and 12.5 mg/ml essential oil concentrations. Additionally, S. tphymurium ATCC 14028 and P. fluorescens ATCC 55241 were more susceptible to O. sipyleum essential oil than other strains. In an earlier study, essential oils from six different populations of Origanum vulgare subsp. hirtum were investigated for their antibiofilm properties and it was found that one sample showed the highest effect against all bacterial strains tested at 50 mg/ml and its inhibition percentages rangedfrom 30% to 52%.[Citation56] Similarly, the essential oils of O. vulgare subsp. viride have different rates of antibiofilm activity against Staphylococcus aureus, S. epidermidis, Pseudomonas aeruginosa, P. fluorescens, and the yeast Candida albicans.[Citation57]
Conclusion
In this study, free radical scavenging and antibiofilm activity, total phenolic and flavonoid content of three extracts and essential oils of four Origanum species, were determined. Extracts of both O. majorana and O. onites had a certain level of radical scavenging effect, depending on proportion of their total phenolic content. In addition, the chloroform extracts had a stronger radical scavenging effect and the water extracts had a higher total antioxidant capacity. As a result, O. majorana and O. onites can be used in pharmaceutical products as a source of natural antioxidants. In present study, the extracts of Origanum were highly efficient antibiofilm agent. These data have verified our hypothesis that these essential oils have variable antibiofilm effects on pathogens tested. The results suggest that essential oils of Origanum species may be of great value to industries that experience problems related to biofilms.
Our study should be broadened for future phytochemical and pharmacological studies for other species of Origanum. This may have the potential to be of a vital importance and to pave the way for new therapeutic products. What is more, as the extracts of the genus Origanum and its essential oils can function as dietary supplements or for medicinal purposes, it is important to monitor them in order to secure authenticity and product quality as toxic adulterants may be life threatening. Nevertheless, further studies are necessary to evaluate the cost and efficacy of these extracts on industrial applications.
Acknowledgements
The authors declare that there is no conflict of interests regarding the publication of this article. These authors contributed equally to this work.
Additional information
Funding
References
- Frei, B.;. Natural Antioxidants in Human Health and Disease. Free Radical Biology & Medicine 1996, 20, 157–159.
- Cardador-Martinez, A.; Loacra-Pina, G.; Oomah, B. D. Antioxidant Activity in Common Beans (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry 2002, 50(24), 6975–6980.
- Enujiugha, V. N.; Talabi, J. Y.; Malomo, S. A.; Olagunju, A. L. DPPH Radical Scavenging Capacity of Phenolic Extracts from African Yam Bean (Sphenostylis stenocarpa). Food and Nutrition Sciences 2012, 3, 7–13.
- Bostancıoğlu, R. B.; Kürkçüoğlu, M.; Başer, K. H. C.; Koparal, A. T. Assessment of Anti-Angiogenic and Anti-Tumoral Potentials of Origanum onites L. Essential Oil. Food and Chemical Toxicology 2012, 50, 2002–2008.
- Goodwin, J. S.; Brodwick, M. Diet, Aging and Cancer. Clinics in Geriatric Medicine 1995, 11, 577–589.
- Kogiannou, D. A. A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M. G.; Kaliora, A. C. Herbal Infusions; Their Phenolic Profile, Antioxidant and Anti-Inflammatory Effects in HT29 and PC3 Cells. Food and Chemical Toxicology 2013, 61, 152–159.
- Steinmetz, K. A.; Potter, J. D. Vegetables, Fruit and Cancer Prevention: A Review. Journal of The American Dietetic Association 1996, 96, 1027–1039.
- Rimm, E. B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M. J.; Willett, W. C. Vegetable, Fruit and Cereal Fiber Intake and Risk of Coronary Heart Disease among Men. Journal of the American Medical Association 1996, 275, 447–451.
- Ietswaart, J. H.; A taxonomic revision of the genus Origanum (Labiatae). Ph.D Thesis. Leiden Botanical Series 4. Leiden University Press, The Hague, 1980.
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I. B. Composition and Antimicrobial Activity of the Essential Oils of Two Origanum Species. Journal of Agricultural and Food Chemistry 2001, 49, 4168–4170.
- Novak, J.; Christina, B.; Langbehn, B.; Pank, F.; Skoula, M.; Gotsiou, Y.; Franz, C. M. Ratios of Cis- and Trans-Sabinene Hydrate in Origanum majorana L. And Origanum midrophyllum (Bentham) Vogel. Biochemical Systematics and Ecology 2000, 28, 697–704.
- Oke, F.; Aslim, B. Biological Potentials and Cytotoxicity of Various Extracts from Endemic Origanum minutiflorum O. Schwarz & P.H. Davis. Food and Chemical Toxicology 2010, 48, 1728–1733.
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oil. Journal of Agricultural and Food Chemistry 1996, 44, 1202–1205.
- Vera, R. R.; Chane-Ming, J. Chemical Composition of the Essential Oil of Marjoram (Origanum majorana L.) From Reunion Island. Food Chemistry 1999, 6, 143–145.
- Benchikha, N.; Lanez, T.; Menaceur, M.; Barhi, Z. Extraction and Antioxidant Activities of Two Species Origanum Plant Containing Phenolic and Flavonoid Compounds. Journal of Fundamental and Applied Sciences 2013, 5(1), 120–128.
- Grover, J. K.; Yadav, S. P. Pharmacological Actions and Potential Uses of Momordica charantia: A Review. Journal of Ethnopharmacology 2004, 93, 123–132.
- Ekim, T.; Koyuncu, M.; Vural, M.; Duman, H.; Aytaç, Z.; Adıgüzel, N. Red Data Book of Turkish Plants (Pteridophyta and Spermatophyta). TTKD-Van Yüzüncü Yıl University: Barışcan Ofset, Ankara, 2000.
- ITC. Market Insider: Essential Oils and Oleoresins. International Trade Center, Switzerland, September 2015, pp 10. Report.
- Lewis, K.;. Riddle of Biofilm Resistance. Antimicrobial Agents and Chemotherapy 2001, 45, 999–1007.
- Chishti, S.; Kaloo, Z. A.; Sultan, P. Medicinal Importance of Genus Origanum: A Review. Journal of Pharmacognosy and Phytochemistry 2013, 5(10), 170–177.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol 1999, 299, 152–178.
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J. C.; Bailleul, F.; Trotin, F. Phenolic Compounds and Antioxidant Activities of Buckwheat (Fagopyrum esculentum moench) Hulls and Flour. Journal of Ethnopharmacology 2000, 72(1–2), 35–42.
- Stankovic, M. S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant Activity, Total Phenolic Content and Flavonoid Concentrations of Different Plant Parts of Teucrium polium L. Subsp. Acta Societatis Botanicorum Poloniae 2012, 81(2), 117–122.
- Merritt, J. H.; Kadouri, D. E.; O’Toole, G. A. Growing and Analyzing Static Biofilms. Current Protocols in Microbiology 2005, 0(1), 1B.1.1–1B.1.17.
- SPSS for Windows Release 15.0 (Computer Software). SPSS Inc.: Chicago, IL, 2006.
- Baser, K. H. C.; Ermin, N.; Kürkçüoglu, M.; Tümen, G. Essential Oil of Origanum hypericifolium O. Schwarz Et P.H. Davis. Journal of Essential Oil Research 1994, 6, 631–633.
- Baser, K. H. C.; Tumen, G. Composition of the Essential Oil of Origanum sipyleum of Turkish Origin. Journal of Essential Oil Research 1992, 4, 139–142.
- Jiang, Z. T.; Li, R.; Wang, Y.; Chen, S. H.; Guan, W. Q. Volatile Oil Composition of Natural Spice, Origanum majorana L Grown in China. Journal of Essential Oil Bearing Plants 2011, 14, 458–462.
- Sevki, C.; Girisgin, O.; Kurkcuoglu, M.; Malyer, H.; Girisgin, A. O.; Kinmer, N.; Baser, K. H. C. Acaricidal Efficacy of Origanum onites L. Essential Oil against Rhipicephalus turanicus (Ixodidae). Parasitology Research 2008, 103(2), 259–261.
- Vági, E.; Simándi, B.; Suhajda, Á.; Héthelyi, É. Essential Oil Composition and Antimicrobial Activity of Origanum majorana L Extracts Obtained with Ethyl Alcohol and Supercritical Carbon Dioxide. Food Research International 2005, 38, 51–57.
- Ruberto, G.; Baratta, M. T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chemistry 2000, 69, 167–174.
- Safaei-Ghomi, J.; Ebrahimabadi, A. H.; Djafari-Bidgoli, Z.; Batooli, H. GC/MS Analysis and in Vitro Antioxidant Activity of Essential Oil and Methanol Extracts of Thymus caramanicus Jalas and Its Main Constituent Carvacrol. Food Chemistry 2009, 115, 1524–1528.
- Yanishlieva, N. V.; Marinova, E. M.; Gordon, M. H.; Raneva, V. G. Antioxidant Activity and Mechanism of Action of Thymol and Carvacrol in Two Lipid Systems. Food Chemistry 1999, 64, 59–66.
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G. J.; Haroutounian, S. A. Essential Oils of Satureja, Origanum, and Thymus Species: Chemical Composition and Antibacterial Activities against Foodborne Pathogens. Journal of Agricultural and Food Chemistry 2004, 52, 8261–8267.
- Marinova, E. M.; Yanishlieva, N. V. Antioxidative Activity of Extracts from Selected Species of the Family Lamiaceae in Sunflower Oil. Food Chemistry 1997, 58(3), 245–248.
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of Polyphenolics from Plant Material for Functional Foods-Engineering and Technology. Food Research International 2005, 21(1), 139–166.
- Moein, S.; Moein, R. M. Relationship between Antioxidant Properties and Phenolics in Zhumeria majdae. Journal of Medicinal Plants Research 2010, 4(7), 517–521.
- Zorzetto, C.; Sánchez-Mateo, C. C.; Rabanal, R. M.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Iannarelli, R.; Papac, F.; Maggi, F. Antioxidant Activity and Cytotoxicity on Tumour Cells of the Essential Oil from Cedronella canariensis Var. Canariensis (L.) Webb & Berthel. (Lamiaceae). Natural Product Research 2015, 29(17), 1641–1649.
- Zhang, X. L.; Guo, Y. S.; Wang, C. H.; Li, G. Q.; Xu, J. J.; Chung, H. Y.; Ye, W. C.; Li, Y. L.; Wang, G. C. Phenolic Compounds from Origanum vulgare and Their Antioxidant and Antiviral Activities. Food Chemistry 2014, 152, 300–306.
- Alimpic, A.; Oaldje, M.; Matevski, V.; Marin, P. D.; Duletic-Lausevic, S. Antioxidant Activity and Total Phenolic and Flavonoid Contents of Salvia amplexicaulis Lam. Extracts. Archives of Biological Sciences Belgrade 2014, 66(1), 307–316.
- Chedia, A.; Ghazghazi, H.; Brahim, H.; Abderrazak, M. Secondary Metabolite, Antioxidant and Antibacterial Activities of Teucrium polium L. Methanolic Extract. IJAPP 2013, 4(8), 1790–1797.
- Leccese, A.; Viti, R.; Bartolini, S. The Effect of Solvent Extraction on Antioxidant Properties of Apricot Fruit. Central European Journal of Biology 2011, 6(2), 199–204.
- Riahi, L.; Chograni, H.; Elferchichi, M.; Zaouali, Y.; Zoghlami, N.; Mlik, A. Variations in Tunusian Wormwood Essential Oil Profiles and Phenolic Contents between Leaves and Flowers and Their Effects on Antioxidant Activities. Industrial Crops and Products 2013, 46, 290–296.
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total Phenolic Content, Antioxidant and Antimicrobial Activities of Some Medicinal Plants. Pakistan Journal of Pharmaceutical Sciences 2009, 22(1), 102–106.
- Rice-Evans, C. A.; Miller, N. J.; Bolwell, P. G.; Bramley, P. M.; Pridha, J. B. The Relative Antioxidant Activities of Plant Derived Polyphenolic Flavonoids. Free Radical Research. 1995, 22(4), 375–383.
- Hayase, F.; Kato, H. Antioxidative Compounds of Sweet Potatoes. Journal of Nutritional Science and Vitaminology 1984, 30(1), 37–46.
- Bravo, L.;. Polyphenols: Chemistry, Dietary Sources, Metabolism and Nutritional Significance. Nutrition Research 1998, 56(11), 317–333.
- Kahkonen, M. P.; Hopia, A. I.; Vuorela, H. J.; Rauha, J. P.; Pihlaja, K.; Kujala, T. S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. Journal of Agricultural and Food Chemistry. 1999, 47(10), 3954–3962.
- Cervato, G.; Carabelli, M.; Gervasio, S.; Cittera, A.; Cazzola, R.; Cestaro, B. Antioxidant Properties of Oregano (Origanum vulgare) Leaf Extracts. Journal of Food Biochemistry 2000, 24, 453–465.
- Şahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological Activities of the Essential Oils and Methanol Extracts of Origanum vulgare Ssp vulgare in the Eastern Anatolia Region of Turkey. Food Control. 2004, 15, 549–557.
- Busatta, C.; Vidal, R. S.; Popiolski, A. S.; Mossi, A. J.; Dariva, C.; Rodrigues, M. R. A.; Corazza, F. C.; Corazza, M. L.; Vladimir-Oliveira, J.; Cansian, R. L. Application of Origanum majorana L Essential Oil as an Antimicrobial Agent in Sausage. Food Microbiology 2008, 25, 207–211.
- Gendy, A. N.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V. V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical Composition and Antimicrobial Activity of Essential Oil of Wild and Cultivated Origanum syriacum Plants Grown in Sinai, Egypt. Industrial Crops and Products 2015, 67, 201–207.
- Marques, J. L.; Volcão, L. M.; Funck, G. D.; Kroning, I. S.; Silva, W. P.; Fiorentinia, A. ̂. M.; Ribeiro, G. A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. And Origanum majorana L. Against Staphylococcus aureus Isolated from Poultry Meat. Industrial Crops and Products 2015, 77, 444–450.
- Stefanakis, M. K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H. E.; Makridis, P. Antibacterial Activity of Essential Oils from Plants of the Genus Origanum. Food Control. 2013, 34, 539–546.
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Industrial Crops and Products 2015, 70, 178–184.
- Schillaci, D.; Napoli, E. M.; Cusimano, M. G.; Vitale, M.; Ruberto, A. Origanum vulgare Subsp. Hirtum Essential Oil Prevented Biofilm Formation and Showed Antibacterial Activity against Planktonic and Sessile Bacterial Cells. Journal of Food Protection 2013, 76(10), 1747–1752.
- Ceylan, O.; Sarac, N.; Ugur, A.; Sahin, M. D. The Antimicrobial and Antibiofilm Activities of Origanum vulgare Ssp. Viride Essential Oils, Endemic in Turkey. Journal of Selcuk University National Applied Science 2014, 3(2), 28–34.
