ABSTRACT
An investigation into the efficient use of water as a solvent and the influence of extraction temperature, extraction time, water to leaf powder ratio, particle size, and extraction cycle on the nutraceutical and antioxidant profile of aqueous mulberry leaf extract were conducted using a single-factor experiment approach. All the assessed extracting parameters showed a significant effect on the nutraceutical compounds and antioxidant properties. The optimum extraction conditions were as follows: extraction temperature of 70°C, extraction time of 40 min, water to leaf powder ratio of 40:1 ml/g, particle size of 25 µm, and two extraction cycles. Based on these optimal conditions, chlorogenic acid (62.10 mg/g), caffeic acid (32.21 mg/g), kaempferol-7-O-glucoside (19.30 mg/g), quercetin-3-rutinose (15.69 mg/g), quercetin-3-O-glucoside (32.38 mg/g), kaempferol-3-(6-rhamnosylglucoside) (42.52 mg/g), quercetin-3-(6-malonylglucoside) (65.19 mg/g), kaempferol-3-glucoside (66.27 mg/g), kaempferol-3-(6-malonylglucoside) (50.18 mg/g), 1-deoxynojirimycin (15.58 mg/g), and gamma-aminobutyric acid (5.05 mg/g) were obtained. The optimal aqueous extract had high antioxidant properties of 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (39.98 mM/g), cupric ion reducing capacity (58.93 mM/g), 1,1-diphenyl-2-picrylhydrazyl (101.33 mM/g), and ferric reducing antioxidant power capacity (233.77 mM/g) of dried mulberry leaf extract.
Introduction
Mulberry leaf is a rich source of valuable bioactive compounds.[Citation1] In vitro, in vivo, and epidemiological studies have proven that mulberry leaf nutraceuticals exhibit biological functions toward some human diseases.[Citation2] Mulberry leaf extract (MLE) phenolics, especially flavonol glycosides, have been recognized to possess potent hypolipidemic properties[Citation3], while 1-deoxynojirimycin (DNJ) has been demonstrated to be the most efficient α-glycosidase inhibitors.[Citation2–Citation4] Moreover, the antihypertensive capacity of MLE has been attributed to the presence of gamma-aminobutyric acid (GABA).[Citation5] As a result, in recent years there is a growing interest in the extraction of nutraceutical compounds from mulberry leaf to be used in food and pharmaceutical products.[Citation4–Citation6]
Food and pharmaceutics industries require standardization of herbal extracts. Thus, extraction procedure is a crucial step which determines the nutraceutical quality and yield of the individual biological compounds.[Citation7] Although several novel extraction techniques such as microwave-assisted extraction, subcritical fluid extraction, ultrasonic extraction, and pressurized liquid extraction have been promoted during the last decade[Citation8,Citation9], solid–liquid extraction is still predominant in the food and pharmaceutical industries.[Citation10,Citation11]
Nutraceuticals components extracted from plant matrices and their associated antiradical activities are highly reliant on the extraction parameters.[Citation11–Citation14] The most significant parameters that impact on the extraction efficiency in terms of yield and quality include temperature, time, particle size, and extraction cycle.[Citation11–Citation16] Up till now, solvents such as ethanol, methanol, acetone, water and their combination have been used for extracting nutraceutical compounds from the herbal plant.[Citation17,Citation18] Despite the fact that aqueous organic solvents have been reported to be more efficient than water for bioactive compounds extraction from plant materials[Citation17,Citation18], water remains the most solvent used in the food and pharmaceutical industries owing to its inexpensive, non-toxic and environmentally friendliness.[Citation13–Citation20] Moreover, Zhang et al.[Citation21] reported better water extraction efficiency of nutraceutical compounds from mulberry leaf than aqueous methanol extraction. However, to date, there are few methodical inquiries on the optimized utilization of water as a solvent in isolating and characterizing of nutraceutical components from medicinal herbs. Even though aqueous MLE has been extensively studied in literature,[Citation5–Citation24] there are no reports on the impact of aqueous extraction parameters on the recovery of its nutraceutical compounds. To the best of the author’s knowledge, no report on the optimized conditions of water extraction of mulberry leaf nutraceuticals has been established.
Therefore, this study aimed to investigate the impact of five extraction parameters (temperature, time, water to leaf powder ratio, particle size, and extraction cycle) on the yield of mulberry leaf nutraceutical compounds and antioxidants properties by means of a single factor experiments approach.
Materials and methods
Plant material and chemicals
Mulberry (Morus alba) leaf was obtained from Zhenjiang mulberry variety nursery base (China). Fresh young leaf was freeze-dried (at −60°C, 48 h, at 0.02 mbar, FD-1A-50, Boyikang Laboratory Instruments, Beijing, China) and powdered using a jet miller (0101S Jet-O-Mizer Milling, Fluid Energy Processing and Equipment Company, Telford, USA). The mulberry leaf powder (MLP) with a mean diameter of 270 µm was passed through apertures (EFL 2000 stainless steel sieves, Endecotts, England) and sized into 150, 48, 25, 18, and 13 µm. Then, the MLPs were packed in a vacuum-sealed aluminum bag and stored at −20°c prior to the aqueous extraction.
The reagents and chemical used in this study were of analytical grade and purchased from Sigma-Aldrich (St. Louis, USA).
Experimental design
The effect of five extraction parameters as well as their optimal conditions were determined by assessing in chronological order: the extraction temperature, the extraction time, the water to powder ratio, the particle size, and the extraction cycle.
Firstly, to investigate the impact of the extraction temperature, 1 g of MLP (270 µm) was extracted with 100 ml of purified water at different temperatures (5, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100°C) for 30 min using a rotary water bath isothermal shaker (300 rpm, DK-600B, Jianqiao Testing Equipment, Guangdong, China). Secondly, to assess the impact of the extraction time, 1 g of MLP (270 µm) in 100 ml of purified water was extracted at the optimal extraction temperature (70°C) at several periods (5, 10, 15, 20, 25, 30, 40, 50, 60, 90, and 120 min). Thirdly, the effect of the water to leaf powder ratio was determined by extracting in purified water at different weights (10:1, 20:1, 30:1, 40:1, 50;1, 60:1, 70:1, 80:1, 90:1 and 100:1 ml/g) of MLP (270 µm) under the optimum extraction temperature (70°C) and extraction time (40 min). Fourthly, to investigate the effect of the particle size, the optimal extraction temperature (70°C), extraction time (40 min) and water to leaf powder ratio (40 ml/g) were employed to extract MLP of various particle size (270, 150, 48, 25, 18, and 13 µm). Finally, the effect of the extraction cycle (number of time the same sample is extracted) was evaluated by extracting (1, 2, and 3 time) in purified water MLP using the optimum extraction temperature (70°C), extraction time (40 min), water to leaf powder ratio (40 ml/g), and particle size (25 µm).
After the extraction, the mixture was immediately cooled in ice. Then, the infusion was centrifuged (at 15,000 rpm for 15 min at 4°C, Anke GL-20B, Shanghai Anting Scientific Instrument Factory, Shanghai, China), filtered (Whatman N°1, Lomb Scientific, Taren Point, Australia), and the MLE was freeze-dried (at −60°C, 72 h, at 0.02 mbar, FD-1A-50, Boyikang Laboratory Instruments, Beijing, China). The dried MLE were stored at −20°C in aluminum seal bag until used.
Prior to analysis, each dried extract (500 mg) was dissolved in purified water (50 ml) and vortex (IKA Vortex Genius 3, Ika, Germany) for 10 min.
Nutraceutical determinations
The nutraceutical analyses were performed with an HPLC system made up of an LC-20AB pump, a SIL 20AC autosampler, a DGU-20A5R degasser, an SPD-M20A photodiode array detector, CTO-20AC column oven, SCL-10A system controller, and an LC Solution software (Shimadzu Corporation, Kyoto, Japan). A ZORBAX-SB C18 (250 mm × 4.6 mm, 5-μm) column (Agilent, Santa Clara, USA) was used for the chromatographic separation of nutraceutical compounds. Each dried MLE was filtered through 0.45 µm syringe filter before injecting into the HPLC system.
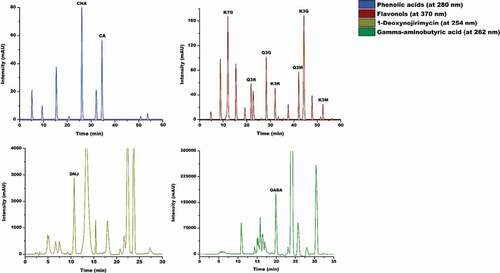
The validation of the HPLC assays was assessed by means of correlation coefficient, limit of detection, and limit of quantification which were calculated according to Callejón et al.[Citation25] The performance parameters () revealed that the HPLC methods used in this study were highly sensitive, reliable, and reproductive. Therefore, these methods were considered as accurate and good enough to allow adequate quantification of all the individual nutraceutical compounds identified in the MLE. The nutraceutical compounds were quantified in milligram per gram of dried MLE using the standard curves with HPLC peak areas as a function of the concentration. A typical HPLC profile of the aqueous MLE, representing the peaks of the individual phenolics, DNJ, and GABA are shown in .
Table 1. Performance parameters of the HPLC-DAD methods.
Figure 1. Representative HPLC chromatograms of nutraceuticals extract in aqueous mulberry leaf extract. This sample of aqueous mulberry leaf extract was obtained under the optimal extraction conditions: extraction temperature of 70°C, extraction time of 40 min, water to leaf powder ratio of 40:1 ml/g, particle size of 25 µm, and two extraction cycles. Peaks Identification, as ascertained with standards: chlorogenic acid (CHA), caffeic acid (CA), kaempferol-7-O-glucoside (K7G), quercetin-3-rutinose (Q3R), quercetin-3-O-glucoside (Q3G), kaempferol 3-(6-rhamnosylglucoside) (K3R), quercetin 3-(6-malonylglucoside) (Q3M), kaempferol-3-glucoside (K3G), kaempferol 3-(6-malonylglucoside) (K3M), 1-deoxynojirimycin (DNJ), and gamma aminobutyric acid (GABA).
Phenolics condition
Chlorogenic acid (CHA), caffeic acid (CA), kaempferol-7-O-glucoside (K7G), quercetin-3-rutinose (Q3R), quercetin-3-O-glucoside (Q3G), kaempferol-3-(6-rhamnosylglucoside) (K3R), quercetin-3-(6-malonylglucoside) (Q3M), kaempferol-3-glucoside (K3G), and kaempferol-3-(6-malonylglucoside) (K3M) were measured as described by Sugiyama et al.[Citation4] with slight modification. Briefly, an aliquot of reconstituted MLE (10 µl) was eluted at 40°C with mobile phases made up of eluent (A) acetonitrile and eluent (B) 0.1% formic acid (in water). The gradient was as follows: 80% B isocratic (1–60 min) with a flow rate of 1 ml/min. The data were recorded at 280 nm for phenolic acids and 370 nm for flavonols. The standard flavonols of K3R, Q3M, and K3M were purified from the mulberry leaf as reported by Sugiyama et al.[Citation4]
1-deoxynojirimycin condition
DNJ was determined as described by Kim et al.[Citation26] with slight modification. Briefly, 500 µl of reconstituted MLE was mixed with 50 µl of potassium borate buffer (0.4 M, pH 8.5) and 100 µl of 9-fluorenylmethyl chloroformate (5 mM in acetonitrile). The mixture was held for 20 min at 20°C before adding 50 µl of glycine. Afterwards, the volume was brought up to 5 ml with 4.3 ml of acetic acid (17.5 mM), then, 10 µl was injected into the HPLC. The mobile phases were made up of eluent (A) acetonitrile and eluent (B) 0.1% acetic acid (in water). The gradient was as follows: 50% B isocratic (1–30 min) with a flow rate of 1 ml/min. The analysis was conducted at 25°C and data were recorded at 254 nm.
Gamma-aminobutyric acid condition
GABA was determined as described by Horanni et al.[Citation27] with slight modification. Briefly, 400 µl of reconstituted MLE was mixed with 100 µl of potassium borate buffer (0.5 M, pH 8.5) and 500 µl of 9-fluorenylmethyl chloroformate (3 mM in acetonitrile). The mixture was held for 10 min at room temperature before adding 100 µl of acetic acid (1 M). Then, 20 µl was injected into the HPLC. The mobile phase was made up of eluent (A) sodium acetate buffer (0.1 M, pH 5.8, containing 0.05% of triethylamine) and eluent (B) 80% acetonitrile (in water). The gradient was as follows: 20 to 50% B (0–5 min), 50 to 100% B (5–10 min), 100% B isocratic (10–20 min), 100 to 20% B (20–25 min), and 20% B isocratic (25–35 min) with a flow rate of 0.5 ml/min. The analysis was conducted at 40°C and data were recorded at 262 nm.
Antioxidant determinations
The antioxidant analyses were performed according to Tchabo et al.[Citation28] with slight modification. The 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), cupric ion reducing capacity (CUPRAC), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and ferric reducing antioxidant power capacity (FRAP) were expressed in millimolar of Trolox per gram of dried MLE using a relevant linear calibration curve of the Trolox.
ABTS determination
Reconstituted MLE (125 µl) was mixed with 5000 µl of an ABTS solution (2.45 mM of ABTS in ammonium persulfate incubated for 16 h in darkness). The mixture was held for 15 min at room temperature and the absorbance was read at 734 nm.
CUPRAC determination
Reconstituted MLE (100 µl) was mixed with 4 ml of a solution comprising copper(II) chloride (10 mM), neocuproine (7.5 mM), ammonium acetate (1M), and purified water (1:1:1:1). The mixture was held for 60 min at room temperature and the absorbance was read at 450 nm.
DPPH determination
Reconstituted MLE (1000 µl) was mixed with 6000 µl of a DPPH solution (60 mM of DPPH in methanol). The mixture was held for 30 min in darkness at room temperature and the absorbance was read at 517 nm.
FRAP determination
Reconstituted MLE (200 µl) was mixed with purified water (600 µl) and 6 ml of a solution comprising iron(III) chloride (20 mM), acetate buffer (300 mM, pH 3.6), TPTZ (10 mM in 40 mM of HCl) (10:1:1). The mixture was held for 30 min at 37°C and the absorbance was read at 593 nm.
Statistical analysis
All extractions and assays were performed in triplicate. The data were presented as mean values and pooled standard deviation. Analysis of the variance, followed by Tukey’s test (at a 5% significance level) was carried out to assess the effect of different extraction conditions on the nutraceutical and antioxidant profile of the aqueous MLE. The statistical analysis was performed using Minitab version 17 (Minitab Inc., Pennsylvania, USA).
Results and discussion
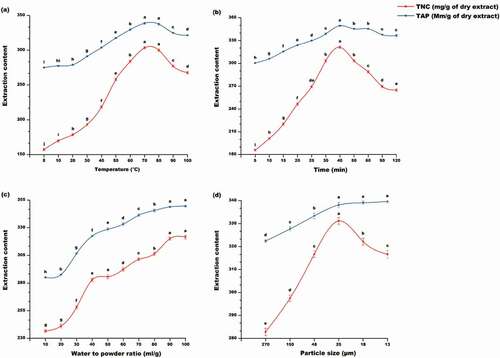
Effect of extraction temperature on nutraceutical yield and antioxidant properties
Theoretically, solid–liquid extraction at high extraction temperature leads to an upsurge of nutraceutical yield due to its effect on diffusion coefficient (mass transfer), solubility (equilibrium), and stability of bioactive compounds.[Citation29] As reported by Mokrani et al.,[Citation30] heating might soften the herbal tissue and weaken the phenol-polysaccharide and phenol interactions, thus promoting the migration of nutraceutical into the solvent, chiefly flavonols which are commonly found as glycosides.[Citation31] However, high temperature may promote epimerization, oxidation, and degradation reaction of nutraceutical compounds.[Citation32] Hence, high extraction temperature might not be suitable for all types of nutraceutical compounds.
The results tabulated in revealed that the yield of each of the nutraceutical compounds increased as the extraction temperature increased. However, the highest extraction yield occurred at 60°C for Q3R; 70°C for CHA, CA, K3R, and DNJ; 80°C for Q3G K3G, and K3M; 90°C for K7G and Q3M; and 100°C for GABA. It is interesting to note that temperature did not have a negative effect on GABA extraction. Furthermore, as reported by previous authors,[Citation30–Citation33] elevated temperatures result in an increase in antioxidant properties of the extract. However, increasing extraction temperature above a certain threshold may have an adverse effect on antioxidant properties due to degradation of residual biocompounds in the herbal matrix or even the decomposition of nutraceuticals which were previously extracted at lower temperature.[Citation30] From the results (), ABTS, DPPH, and FRAP increased with increasing temperature up to 70°C, while CUPRAC increased up to 90°C. A similar trend has been reported by Chiang et al.[Citation33] This was confirmed by the high significant Pearson correlation (r = 0.997) found between the total nutraceutical compounds (TNC, as the sum of the individual nutraceutical compounds) and the total antioxidant properties (TAP, as the sum of individual antioxidant properties) under the impact of extraction temperature. This finding is in line with that of Thoo et al.,[Citation34] thereby suggesting that nutraceutical compounds present in mulberry leaf are thermally stable.
Table 2. Effect of extraction temperature on the nutraceutical compounds and antioxidant properties of aqueous mulberry leaf extract.
Moreover, as shown in , a curvilinear relationship was found between extraction temperature and recovery of the TNC and the TAP, which increased as temperature increased from 5°C to 70°C where they reached a plateau before decreasing at 90°C. Similar optimal extraction temperature was reported for water extraction of papaya leaf[Citation35] and Thai rice by-products[Citation36] phenolic compounds. Hence, the optimum extraction temperature was selected to be 70°C and used for the subsequent assessment of the impact of the other extraction parameters on the nutraceutical yield and antioxidant properties.
Effect of extraction time on nutraceutical yield and antioxidant properties
Extraction time is a critical parameter during solid–liquid extraction owing its influence on solubility and mass transfer of biocompounds which are related to their structure and molecular weight.[Citation37,Citation38] However, extended extraction time increases the probability of oxidation, epimerization, and degradation of bioactive compounds.[Citation37] Therefore, prolonged extraction time may not be appropriate for all kinds of nutraceutical compounds.
The results () highlighted that extraction time significantly altered the extraction yield of individual nutraceutical compounds. The difference observed in the optimal extraction time for each component (30 min for K7G; 40 min for CHA, CA, Q3R, and K3R; 50 min for K3G, K3M, and DNJ; 60 min for Q3G and Q3M; and 120 min for GABA) could be attributed to their different degree of solubility, polymerization, epimerization, and interaction with other MLP components resulting in difference time to reach the equilibrium before their corresponding degradations.[Citation34] Besides, it was also found that the optimal extraction time for antioxidant property varies with the antioxidant assay (40 min for ABTS, DPPH, and FRAP; and 60 min for CUPRAC). This discrepancy may be due to the fact that radical scavenging properties are not entirely depending on a particular group of antioxidant component but on the capacity of any biocompounds to scavenge-free radicals.[Citation39] This assertion was supported by the strong significant Pearson correlation (r = 0.973) found between TNC and TAP under the impact of extraction time.
Table 3. Effect of extraction time on the nutraceutical compounds and antioxidant properties of aqueous mulberry leaf extract.
Furthermore, as shown in , the TAP increased with an increase in extraction time up to 40 min, then decreased slightly before stabilizing at 90 min. This suggests that the final equilibrium in antioxidant components in MLE may be attained at 90 min. This finding is in agreement with Fick’s second law of diffusion as previously reported by Thoo et al.[Citation34] Besides, contrary to the TAP, increase in extraction time above 40 min resulted in a sharp decrease in TNC. Similar trends have been reported by Zhang et al.[Citation21] in which an increase in extraction time beyond 50 min led to a drastic decrease in bioactive components of MLE. As reported by Mokrani et al.,[Citation30] prolonged extraction time resulted in decomposition of nutraceuticals and also in solvent loss through evaporation, thereby affecting mass transfer loss during extraction.[Citation38–Citation40] Therefore, taking into consideration that extraction time is crucial in economizing extraction process cost, the optimal extraction time was selected to be 40 min, and used for the subsequent assessment of the effect of the other extraction parameters on the nutraceutical yield and antioxidant properties.
Effect of water to leaf powder ratio on nutraceutical yield and antioxidant properties
In theory, during liquid–solid extraction, water to powder ratio affect significantly the extraction kinetics of bioactive compounds due to its effect on the concentration gradient between the solute in the powder and the solvent at the surface of the raw material.[Citation12]
As presented in , the yield of individual nutraceutical compounds as well as antioxidant properties increased as the water to leaf powder ratio increased. In accordance with mass transfer principle, the higher the water to powder ratio, the higher the driving force to extract antioxidant components trapped inside the powder.[Citation35] In line with previous authors,[Citation10–Citation35] a strong significant Pearson correlation (r = 0.991) was found between TNC and TAP under the impact of water to leaf powder ratio.
Table 4. Effect of water to leaf powder ratio on the nutraceutical compounds and antioxidant properties of aqueous mulberry leaf extract.
Moreover, as depicted in , the yield in TNC and TAP was not significantly affected when the water to leaf powder ratio increased from 10:1 to 20:1 ml/g. Thereafter, yields sharply increased significantly and reached a plateau when the water to leaf powder ratio was 90:1 ml/g. However, taking into consideration the major criteria such as production cost, environmental friendliness, and extraction yield for the selection of extraction process[Citation41], the water to leaf powder ratio of 40:1 ml/g was found to give the best results. This ratio was ascertained to require 2.25 less amount of water to achieve 88.37% and 92.28% extraction efficiency of TNC and TAP, respectively. A similar optimum water to leaf powder ratio was reported for the aqueous extraction of bitter melon phenolic compounds.[Citation10] Therefore, the optimal water to leaf powder ratio was chosen to be 40:1 ml/g and used for subsequent assessment of the impact of particle size on the nutraceutical yield and antioxidant properties.
Effect of particle size on nutraceutical yield and antioxidant properties
In theory, reduction in particle size results in an increment in solubility and diffusion due to increase in surface area available for mass transfer between the particle and solvent,[Citation42] thereby enhancing the extraction rate.[Citation43] However, a large surface area may also lead to more exposition of bioactive compounds to physical and chemical stress, thus resulting in their degradation.[Citation44]
The results presented in show that the rate of extraction of the individual nutraceuticals significantly increased as the particle size decreased. This might be due to the increase in surface contact between solvent and particulate.[Citation42] A similar finding was reported on the effect of particle size in phenolic extraction from plants byproducts.[Citation45–Citation47] The highest extraction yield for CHA and CA were obtained using a particle size of 25 µm; 18 µm for Q3G, Q3M, and K3M; and 13 µm for K7G, Q3R, K3R, K3G, DNJ, and GABA. As reported by Zaiter et al.[Citation48], particle size has a significant effect on antioxidant properties of plant extract. From the results (), reduction in particle size led to an increase in antioxidant properties of the extract. Hence, the highest antioxidant properties were recorded at 13 µm for ABTS, CUPRAC DPPH, and FRAP. In agreement with earlier reports,[Citation43–Citation48] a high significant Pearson correlation (r = 0.931) was found between TNC and TAP under the impact of particle size.
Table 5. Effect of particle size on the nutraceutical compounds and antioxidant properties of aqueous mulberry leaf extract.
Furthermore, as illustrated in , the TAP significantly increased from 270 to 25 µm where it reached a plateau. As reported by Xiao et al.,[Citation47] reduction in particle size leads to an increase in equilibrium yields of antioxidant compounds. Nonetheless, reducing particle size more than 25 µm resulted in a reduction in TNC. This could be attributed to the increase in the contact surface between particles and oxygen which might have resulted in oxidation of nutraceutical compounds, especially phenolics, thus resulting in their degradation.[Citation43] Consequently, the MLP of 25 µm particle size was chosen for the assessment of the impact of extraction cycle on the nutraceutical yield and antioxidant properties.
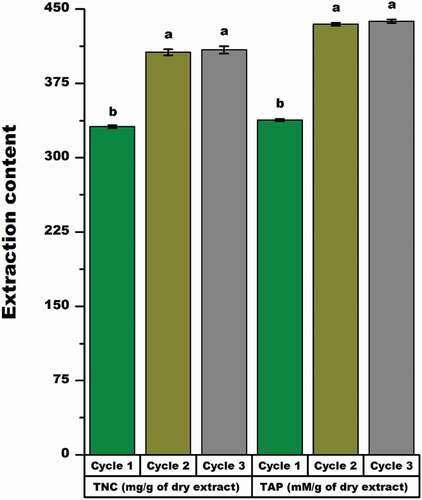
Effect of extraction cycle on nutraceutical yield and antioxidant properties
To assess the influence of multiple extraction cycles on the nutraceutical compounds and antioxidant property all the above optimal extraction parameters were used to determine the effect of a single, double, and triple extraction cycles on the TNC and the TAP.
As illustrated in , a higher yield of nutraceutical and antioxidant property was obtained when MLP was extracted using multiple extraction cycles as compared with a single extraction. Significant percentage of increases in the TNC (22.74%) and the TAP (28.57%) were found between the single and the double extraction cycles. However, there was no significant difference in TAP and TNC between the double and triple extraction cycles. Therefore, the maximum extraction cycles for MLP was two extraction cycles owing to the optimal water efficiency, for lower extraction cost. This finding is in agreement with that of Vuong et al.[Citation37] for the aqueous extraction of catechin from green tea and that of Tan et al.[Citation12] for aqueous extraction of flavonoids from bitter melon.
Conclusion
In the current research, single factor experiment approach was employed to determine the optimal cost-effective condition of the extraction process of mulberry leaf nutraceuticals, investigating some influential parameters such as extraction temperature, extraction time, water to leaf powder ratio, particle size, and extraction cycle. The outcomes revealed that individual nutraceuticals and antioxidant properties were significantly altered by all the investigated parameters. A high significant Pearson correlation was found between the sum of the individual of nutraceutical compounds and the sum of individual antioxidant properties as influenced by the influence extraction temperature (r = 0.997), extraction time (r = 0.973), water to leaf powder ratio (r = 0.991), and particle size (r = 0.931). The optimal extraction efficiency with respect to the yield of nutraceuticals (chlorogenic acid, caffeic acid, kaempferol-7-O-glucoside, quercetin-3-rutinose, quercetin-3-O-glucoside, kaempferol-3-(6-rhamnosylglucoside), quercetin-3-(6-malonylglucoside), kaempferol-3-glucoside, and kaempferol-3-(6-malonylglucoside) and the antioxidants properties (ABTS, CUPRAC, DPPH, and FRAP) was achieved with aqueous extraction at 70°C for 40 min, with 25 µm particle size and a water to leaf powder ratio of 40:1 (ml/g). In terms of cost-effectiveness and efficient use of water, the best extraction cycle was revealed to be two.
This research serves as the basis for an in-depth investigation on the optimization of extraction procedure of nutraceuticals and antioxidant activity from mulberry leaf using response surface methodology approach.
References
- Chan, E. W. C.; Lye, P. Y.; Wong, S. K. Phytochemistry, Pharmacology, and Clinical Trials of Morus Alba. Chinese Journal of Natural Medicines 2016, 14, 17–30.
- Gryn Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New Potential Phytotherapeutics Obtained from White Mulberry (Morus Alba L.). leaves. Biomedicine & Pharmacotherapy 2016, 84, 628–636. DOI: 10.1016/j.biopha.2016.09.081.
- Jeszka‐Skowron, M.; Flaczyk, E.; Podgórski, T. In Vitro and In Vivo Analyses of Morus Alba Polish Var. Wielkolistna Zolwinska Leaf Ethanol–Water Extract—Antioxidant and Hypocholesterolemic Activities in Hyperlipideamic Rats. European Journal of Lipid Science and Technology 2017, 119. DOI: 10.1002/ejlt.201600514.
- Sugiyama, M.; Katsube, T.; Koyama, A.; Itamura, H. Varietal Differences in the Flavonol Content of Mulberry (Morus Spp.) Leaves and Genetic Analysis of Quercetin 3-(6-Malonylglucoside) for Component Breeding. Journal of Agricultural and Food Chemistry 2013, 61, 9140–9147. DOI: 10.1021/jf403136w.
- Yang, N. C.; Jhou, K. Y.; Tseng, C. Y. Antihypertensive Effect of Mulberry Leaf Aqueous Extract Containing γ-aminobutyric Acid in Spontaneously Hypertensive Rats. Food Chemistry 2012, 132, 1796–1801. DOI: 10.1016/j.foodchem.2011.11.143.
- Kim, J. Y.; Kwon, H. J.; Jung, J. Y.; Kwon, H. Y.; Baek, J. G.; Kim, Y.-S.; Kwon, O. Comparison of Absorption of 1-Deoxynojirimycin from Mulberry Water Extract in Rats. Journal of Agricultural and Food Chemistry 2010, 58, 6666–6671. DOI: 10.1021/jf100322y.
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. Journal of Food Engineering 2013, 117, 426–436. DOI: 10.1016/j.jfoodeng.2013.01.014.
- Wang, L.; Weller, C. L. Recent Advances in Extraction of Nutraceuticals from Plants. Trends in Food Science & Technology 2006, 17, 300–312. DOI: 10.1016/j.tifs.2005.12.004.
- Kadam, S. U.; Tiwari, B. K.; O’Donnell, C. P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. Journal of Agricultural and Food Chemistry 2013, 61, 4667–4675. DOI: 10.1021/jf400819p.
- Tan, S. P.; Stathopoulos, C.; Parks, S.; Roach, P. An Optimised Aqueous Extract of Phenolic Compounds from Bitter Melon with High Antioxidant Capacity. Antioxidants 2014, 3, 814–829. DOI: 10.3390/antiox3040814.
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of Solid–Liquid Extraction Kinetics of Total Polyphenols from Grape Seeds. Journal of Food Engineering 2007, 81, 236–242. DOI: 10.1016/j.jfoodeng.2006.10.027.
- Tan, S. P.; Parks, S. E.; Stathopoulos, C. E.; Roach, P. D. Extraction of Flavonoids from Bitter Melon. Food and Nutrition Sciences 2014, 5, 458. DOI: 10.4236/fns.2014.55054.
- Qu, W.; Pan, Z.; Ma, H. Extraction Modeling and Activities of Antioxidants from Pomegranate Marc. Journal of Food Engineering 2010, 99, 16–23. DOI: 10.1016/j.jfoodeng.2010.01.020.
- Tušek, A. J.; Benković, M.; Cvitanović, A. B.; Valinger, D.; Jurina, T.; Kljusurić, J. G. Kinetics and Thermodynamics of the Solid-Liquid Extraction Process of Total Polyphenols, Antioxidants and Extraction Yield from Asteraceae Plants. Industrial Crops and Products 2016, 91, 205–214. DOI: 10.1016/j.indcrop.2016.07.015.
- Hinneburg, I.; Neubert, R. H. Influence of Extraction Parameters on the Phytochemical Characteristics of Extracts from Buckwheat (Fagopyrum Esculentum) Herb. Journal of Agricultural and Food Chemistry 2005, 53, 3–7. DOI: 10.1021/jf049118f.
- Ignat, I.; Volf, I.; Popa, V. I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chemistry 2011, 126, 1821–1835. DOI: 10.1016/j.foodchem.2010.12.026.
- Metrouh-Amir, H.; Duarte, C. M. M.; Maiza, F. Solvent Effect on Total Phenolic Contents, Antioxidant, and Antibacterial Activities of Matricaria Pubescens. Industrial Crops and Products 2015, 67, 249–256. DOI: 10.1016/j.indcrop.2015.01.049.
- Boeing, J. S.; Barizão, É. O.; E Silva, B. C.; Montanher, P. F.; De Cinque Almeida, V.; Visentainer, J. V. Evaluation of Solvent Effect on the Extraction of Phenolic Compounds and Antioxidant Capacities from the Berries: Application of Principal Component Analysis. Chemistry Central Journal 2014, 8, 48. DOI: 10.1186/s13065-014-0048-1.
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of Air-Drying Temperature on Antioxidant Capacity and Stability of Polyphenolic Compounds in Mulberry (Morus Alba L.). leaves. Food Chemistry 2009, 113, 964–969. DOI: 10.1016/j.foodchem.2008.08.041.
- Tan, S. P.; Vuong, Q. V.; Stathopoulos, C. E.; Parks, S. E.; Roach, P. D. Optimized Aqueous Extraction of Saponins from Bitter Melon for Production of a Saponin‐Enriched Bitter Melon Powder. Food Chemistry 2014, 79. DOI: 10.1111/1750-3841.12514.
- Zhang, L. L.; Bai, Y. L.; Shu, S. L.; Qian, D. W.; Ou Yang, Z.; Liu, L.; Duan, J. A., Simultaneous Quantitation of Nucleosides, Nucleobases, Amino Acids, and Alkaloids in Mulberry Leaf by Ultra High Performance Liquid Chromatography with Triple Quadrupole Tandem Mass Spectrometry. Journal of Separation Science 2014, 37, 1265–1275. DOI: 10.1002/jssc.201301267.
- Chan, K. C.; Ho, H. H.; Lin, M. C.; Wu, C. H.; Huang, C. N.; Chang, W. C.; Wang, C. J. Mulberry Water Extracts Inhibit Rabbit Atherosclerosis through Stimulation of Vascular Smooth Muscle Cell Apoptosis via Activating P53 and Regulating Both Intrinsic and Extrinsic Pathways. Journal of Agricultural and Food Chemistry 2014, 62, 5092–5101. DOI: 10.1021/jf501466t.
- Peng, C. H.; Liu, L. K.; Chuang, C. M.; Chyau, C. C.; Huang, C. N.; Wang, C. J. Mulberry Water Extracts Possess an Anti-Obesity Effect and Ability to Inhibit Hepatic Lipogenesis and Promote Lipolysis. Journal of Agricultural and Food Chemistry 2011, 59, 2663–2671. DOI: 10.1021/jf1043508.
- Tang, C. C.; Huang, H. P.; Lee, Y. J.; Tang, Y. H.; Wang, C. J. Hepatoprotective Effect of Mulberry Water Extracts on Ethanol-Induced Liver Injury via Anti-Inflammation and Inhibition of Lipogenesis in C57BL/6J Mice. Food and Chemical Toxicology 2013, 62, 786–796. DOI: 10.1016/j.fct.2013.10.011.
- Callejón, R.; González, A.; Troncoso, A.; Morales, M. Optimization and Validation of Headspace Sorptive Extraction for the Analysis of Volatile Compounds in Wine Vinegars. Journal of Chromatography 2008, 1204, 93–103. DOI: 10.1016/j.chroma.2008.07.064.
- Kim, J. W.; Kim, S. U.; Lee, H. S.; Kim, I.; Ahn, M. Y.; Ryu, K. S. Determination of 1-Deoxynojirimycin in Morus Alba L. Leaves by Derivatization with 9-Fluorenylmethyl Chloroformate Followed by Reversed-Phase High-Performance Liquid Chromatography. Journal of Chromatography 2003, 1002, 93–99. DOI: 10.1016/S0021-9673(03)00728-3.
- Horanni, R.; Engelhardt, U. H. Determination of Amino Acids in White, Green, Black, Oolong, Pu-Erh Teas and Tea Products. Journal of Food Composition and Analysis 2013, 31, 94–100. DOI: 10.1016/j.jfca.2013.03.005.
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Li, X.; Afoakwah, N. A. Effects of Ultrasound, High Pressure, and Manosonication Processes on Phenolic Profile and Antioxidant Properties of a Sulfur Dioxide-Free Mulberry (Morus Nigra) Wine. Food and Bioprocess Technology 2017, 10, 1210–1223. DOI: 10.1007/s11947-017-1892-5.
- Casazza, A. A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-Pressure High-Temperature Extraction of Phenolic Compounds from Grape Skins. International Journal of Food Science & Technology 2012, 47, 399–405. DOI: 10.1111/j.1365-2621.2011.02853.x.
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus Persica L.). Fruit. Separation and Purification Technology 2016, 162, 68–76. DOI: 10.1016/j.seppur.2016.01.043.
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. DOI: 10.3390/molecules15128813.
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C. G. Ultrasound Assisted Extraction of Phenolic Compounds from Grapes. Analytica chimica acta 2012, 732, 100–104. DOI: 10.1016/j.aca.2011.11.032.
- Chiang, P.-S.; Lee, D.-J.; Whiteley, C. G.; Huang, C.-Y. Extracting Antioxidant Phenolic Compounds from Compressional-Puffing Pretreated Pinus Morrisonicola: Effects of Operational Parameters, Kinetics and Characterization. Journal of the Taiwan Institute of Chemical Engineers 2017, 75, 70–76. DOI: 10.1016/j.jtice.2017.03.041.
- Thoo, Y. Y.; Ho, S. K.; Liang, J. Y.; Ho, C. W.; Tan, C. P. Effects of Binary Solvent Extraction System, Extraction Time and Extraction Temperature on Phenolic Antioxidants and Antioxidant Capacity from Mengkudu (Morinda Citrifolia). Food Chemistry 2010, 120, 290–295. DOI: 10.1016/j.foodchem.2009.09.064.
- Vuong, Q. V.; Hirun, S.; Roach, P. D.; Bowyer, M. C.; Phillips, P. A.; Scarlett, C. J. Effect of Extraction Conditions on Total Phenolic Compounds and Antioxidant Activities of Carica Papaya Leaf Aqueous Extracts. Journal of Herbal Medicine 2013, 3, 104–111. DOI: 10.1016/j.hermed.2013.04.004.
- Wanyo, P.; Kaewseejan, N.; Meeso, N.; Siriamornpun, S. Bioactive Compounds and Antioxidant Properties of Different Solvent Extracts Derived from Thai Rice By-Products. Applied Biological Chemistry 2016, 59, 373–384. DOI: 10.1007/s13765-016-0173-8.
- Vuong, Q. V.; Golding, J. B.; Stathopoulos, C. E.; Nguyen, M. H.; Roach, P. D. Optimizing Conditions for the Extraction of Catechins from Green Tea Using Hot Water. Journal of Separation Science 2011, 34, 3099–3106. DOI: 10.1002/jssc.v34.21.
- Belwal, T.; Dhyani, P.; Bhatt, I. D.; Rawal, R. S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits Using Response Surface Methodology (RSM). Food chemistry 2016, 207, 115–124. DOI: 10.1016/j.foodchem.2016.03.081.
- Spigno, G.; Tramelli, L.; De Faveri, D. M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. Journal of Food Engineering 2007, 81, 200–208. DOI: 10.1016/j.jfoodeng.2006.10.021.
- Tan, M.; Tan, C.; Ho, C. Effects of Extraction Solvent System, Time and Temperature on Total Phenolic Content of Henna (Lawsonia Inermis) Stems. International Food Research Journal 2013, 20, 3117–3123.
- Tchabo, W.; Ma, Y.; Engmann, F. N.; Zhang, H. Ultrasound-Assisted Enzymatic Extraction (UAEE) of Phytochemical Compounds from Mulberry (Morus Nigra) Must and Optimization Study Using Response Surface Methodology. Industrial Crops and Products 2015, 63, 214–225. DOI: 10.1016/j.indcrop.2014.09.053.
- Pinelo, M.; Del Fabbro, P.; Manzocco, L.; Nuñez, M. J.; Nicoli, M. C. Optimization of Continuous Phenol Extraction from Vitis Vinifera Byproducts. Food Chemistry 2005, 92, 109–117. DOI: 10.1016/j.foodchem.2004.07.015.
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of Superfine Grinding on the Physicochemical Properties and Antioxidant Activity of Red Grape Pomace Powders. Powder Technology 2015, 286, 838–844. DOI: 10.1016/j.powtec.2015.09.025.
- Hu, J.; Chen, Y.; Ni, D. Effect of Superfine Grinding on Quality and Antioxidant Property of Fine Green Tea Powders. LWT-Food Science and Technology 2012, 45, 8–12. DOI: 10.1016/j.lwt.2011.08.002.
- Ramachandraiah, K.; Chin, K. B. Evaluation of Ball-Milling Time on the Physicochemical and Antioxidant Properties of Persimmon By-Products Powder. Innovative Food Science & Emerging Technologies 2016, 37, 115–124. DOI: 10.1016/j.ifset.2016.08.005.
- Zhong, C.; Zu, Y.; Zhao, X.; Li, Y.; Ge, Y.; Wu, W.; Zhang, Y.; Li, Y.; Guo, D. Effect of Superfine Grinding on Physicochemical and Antioxidant Properties of Pomegranate Peel. International Journal of Food Science & Technology 2016, 51, 212–221. DOI: 10.1111/ijfs.12982.
- Xiao, W.; Zhang, Y.; Fan, C.; Han, L. A Method for Producing Superfine Black Tea Powder with Enhanced Infusion and Dispersion Property. Food Chemistry 2017, 214, 242–247. DOI: 10.1016/j.foodchem.2016.07.096.
- Zaiter, A.; Becker, L.; Karam, M. C.; Dicko, A. Effect of Particle Size on Antioxidant Activity and Catechin Content of Green Tea Powders. Journal of Food Science and Technology 2016, 53, 2025–2032. DOI: 10.1007/s13197-016-2201-4.