ABSTRACT
Acid-soluble collagen (ASC) was extracted from the skin and meat of orbicular batfish (Platax orbicularis) cultured in deep-sea water (DSW) and shallow-sea water (SSW). The fish cultured in deep-sea water contained higher collagen content (42.14 mg/g) in meat than that cultured in shallow-sea water (33.67 mg/g) (p<0.05). The yield of ASC was 35.72% (dry weight basis) from the skin of DSW cultured fish being higher than that of the SSW cultured fish (25.9%). The collagen of the orbicular batfish cultured in DSW showed higher water-holding capacity and lower thermal denaturation temperature than those from SSW. Orbicular batfish collagens constituted α1-chain, α2-chain and β-chain were characterized as type I collagen. The collagen helices of DSW-cultured orbicular batfish were less stable than those of SSW-cultured orbicular batfish, due to the lower imino acid content.
Introduction
Deep-sea water (DSW) is commonly designated the water that flows 200 m below the surface of the sea. DSW possesses properties as being clean, cold and rich in essential minerals including magnesium, calcium, potassium and plentiful nutrients.[Citation1] DSW has less or free of pathogens and suspended particles in comparison to shallow sea water (SSW)[Citation2]. The DSW being low-temperature is good for culturing cold-water fish species. The DSW being nutrient-rich can be used to cultivate cold water fish in subtropical Taiwan. Although Taiwan has well-developed aquaculture technology, limited information are available in the literature regarding orbicular batfish.
Orbicular batfish are distributed from southwest Japan to northeast Australia and New Caledonia. They are also found in the Indo-Pacific, from eastern Africa to Indonesia, Red Sea, Micronesia, and Polynesia.[Citation4] Orbicular batfish has become a cultured species in southern Taiwan. Little information is known regarding the characteristics of collagens, meat texture and flavor of orbicular batfish. Therefore, the aim of this investigation was to evaluate the physicochemical properties of collagen from the skin and meat of orbicular batfish including proximate composition, viscosity, water- and oil-holding capacity, color values, amino acids and protein patterns on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In addition, the collagen obtained from orbicular batfish cultured in shallow seawater (SSW) and that from the fish cultured in deep-sea water (DSW) were compared. If noticeable difference in collagen properties are found in DSW cultured orbicular batfish, high-value collagen products can be developed from DSW cultured orbicular batfish.
Materials
Raw material and orbicular batfish preparation
Orbicular batfish (Platax orbicularis) was obtained from Taiwan Fertilizer Co., Ltd. Aquaculture Farm (Hualien, Taiwan) in October 2014. The orbicular batfish weighed 400 g to 700 g were used in this study. Deep-sea water (DSW; 24.00˚N latitude; 121.64˚N longitude) and shallow seawater (SSW) were collected from respective depths of about 662 meters and 5 meters in the Pacific Ocean.
Methods
Orbicular batfish preparation
The condition factor (K) of fish was calculated as K=100 W/L3 where; W=weight (g); L=total length (cm).[Citation5] Fish was bled, gutted, eviscerated and frozen at -35°C and stored at -20°C then sent to our laboratory at the Department of Food Science, National Taiwan Ocean University within 1 week. Upon arrival the fish was thawed at 4°C overnight and filleted. The skin was manually separated. Fillets and skin were kept in freezer until collagen extraction. All chemicals for electrophoresis were purchased from Bio Basic Inc. (Toronto, Canada).
Physicochemical analyses
The proximate composition of orbicular batfish flesh and skin were determined according to the Association of Official Analytical Chemists method.[Citation6] The moisture content of sample was conducted following AOAC procedure 984.25 by using oven drying at 105°C for 24 h. Total ash was measured by burning the sample at 530°C for 24 h. Lipid content was determined using ether extraction method.[Citation6] Crude protein (N × 6.25) was measured using Kjeldahl method 955.04. Crude carbohydrate was obtained by 100 – (moisture + crude protein + lipid content + total ash).
Extraction of orbicular batfish collagen
Collagen was extracted from orbicular batfish meat mince (5 g) and defatted skin (0.7 g) to quantify according to the method used by Nagai and Suzuki [Citation7]. All the extraction procedures were proceeded at 4°C. Fish mince and defatted skin were separately washed with 0.01 M Na2HPO4 (pH 7.4). Fish mince and defatted skin were blended with 0.5 M acetic acid containing 0.1 mg/ml pepsin (Merck 700FIP-U/g) in 210 ml and 100 ml, respectively, for 1 min (Oster 16-Speed Blender, USA). The 2 mixtures were gently stirred for 24 h. Then the mixtures were centrifuged at 1,400g for 3 min using a refrigerated centrifuge (Hitachi CR21G, Tokyo, Japan) to remove insoluble material. The concentration of soluble collagen in the supernatant was measured using the Sircol collagen assay kit (Biocolor, UK) according to the method of Archile-Contreras and Purslow.[Citation8] A 100 μl aliquot of supernatant was mixed with 1 ml of Sircol dye and shaken (100 rpm) at room temperature for 30 min then centrifuged at 13,800g for 10 min to pack the collagen-dye complex. The pellet obtained was dissolved in 750 μl acid-salt wash reagent to release the collagen-dye complex. The mixture was centrifuged at 13,800g for 10 min again and then 250 μl alkali reagent was added and mixed with the pellet. The absorbance of which (200 μl) was measured at 555 nm (MultiskanTM GO Microplate Spectrophotometer 51119300, Thermo Fisher Scientific, Vantaa, Finland). The concentration of collagen produced was determined from a standard curve prepared with the collagen standard (calf type I collagen) (Sigma-Aldrich, Inc., St. Louis, MO) provided with the assay and calculated as a percentage change relative to that from the collagen standard (0.5 mg/ml).
To prepare the collagen powder, the meat or skin (15 g) was treated with 0.1 N NaOH to remove noncollagenous proteins for 12 h. Then the meat and skin was treated with 0.3% hydrogen peroxide (1/10 w/v) to remove the pigments. After removing fat from the meat or skin, it was treated with 3% Triton-100 and washed with distilled water, and lyophilized. The lyophilized matter was extracted with 0.5 M acetic acid (1/40 w/v) for 48 h. The extract was centrifuged at 10,000g for 30 min. The supernatants were salted-out by adding NaCl to a final concentration of 0.7 M. The resultant precipitate was separated by centrifugation at 10,000g for 30 min. The residue was suspended in 0.5 M acetic acid and dialyzed against distilled water to remove salt. The dialyzed solution was freeze-dried within a bulk tray dryer (Labconco Co., Kansas City, KS). The dry matter from the freeze-dried process was ground and stored at room temperature in a desiccator.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and amino acid analysis
An SDS-PAGE was used to evaluate the cross-linking and distribution of molecular weights of orbicular batfish skin collagen. This was conducted as described by Laemmli [Citation9] with 4% stacking gel and 8% resolving gel, with a slight modification. Acid-soluble collagen (ASC) powder (450 μg) was prepared by mixing 95 μl sample buffer (0.5 M Tris–HCl, glycerol containing 10% SDS and 0.5% bromophenol). The dissolved solutions were centrifuged using a microcentrifuge at 3000g for 3 min at room temperature to remove undissolved debris. Solubilized collagen samples were mixed at a 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, glycerol containing 10% SDS and 0.5% bromophenol). Twenty micrograms of collagen samples was loaded per well. Molecular weight markers (26619, PageRulerTM Plus Prestained, Rock Ford, IL) were also loaded alongside the samples to estimate the molecular weight of the collagen samples. Electrophoretic analysis was determined using a Gene Power Supply SPS 200/400 (Pantech, Taipei, Taiwan) at a constant current of 150 mA and a temperature of 25°C. The gel was stained with 0.1% (w/v) Coomassie Blue R-250 in 20% (v/v) methanol and 7% (v/v) acetic acid. Gel was destained using a solution containing 20% methanol and 7% acetic acid.
Collagen samples (50 mg) were dissolved in 6 N HCl solution 5 ml in vacuum condition and hydrolyzed at 120°C for 24 h. After hydrolysis, samples were filtered with polytetrafluoroethylene (PTFE) 0.45 μm. The solution was diluted to a concentration of 0.1 mg/100 ml and 20 μl sample was injected into chromacol vials and taken for amino acid analysis. The solvent was analyzed with L-8900 amino acid analyzer (Hitachi High-Technologies Corporation Tokyo, Japan).
Determination of water-holding and fat-binding capacities of collagen
Water-holding capacities were performed as described by Nam et al.[Citation10] Ten mg of collagen was placed in 1 ml distilled water and held at 20 ± 1°C for 1 h. The tube was capped and vortexed for 5 s every 15 min. The mixtures were centrifuged at 4500g for 20 min. The supernatant was removed by suction and the tube containing residue was weighed. Water-holding capacity was calculated as the weight of the absorbed water per milligram of collagen sample. Fat-binding capacity was measured with the same procedure of water-holding capacity described by Balti et al.[Citation11] The 1 ml distilled water was replaced with 1 ml of soybean oil. The mixtures were centrifuged at 450g for 20 min. The centrifuge tube was tilted to a 45° angle and drained for 30 min on a filter paper.
Determination of intrinsic viscosity and denaturation temperature
Capillary viscometer (Cannon-Fenske, No 100, Ramin’ Corporation Magnolia, TX) was used to measure the passage time of solutions flowing through the capillary. The capillary viscometer was equilibrated in a water bath (Tamson TMV-40, Zoetermeer, Holland). Fifteen ml of 0.03% (w/v) collagen solution in 0.1 M acetic acid was used for viscosity measurements. The thermal denaturation curve was obtained by measuring solution viscosity at temperatures from 10°C to 40°C; the solution temperature was raised stepwise and measured every 2 min in triplicates. Time required for the fluid to drain by gravity through a viscometer tube was measured. The denaturation temperature (Td) was determined as the temperature at which the change in viscosity was half complete with an average shear gradient of 400 s−1 by the method of Nagi et al.[Citation12] Each point was the mean of triplicate determinations.
Statistical analysis
A completely randomized block design was used with three replications per treatment. Data were analyzed by analysis of variance programs using the SPSS statistic program (SPSS, version 12, 2000). Differences between means were evaluated by Duncan’s multiple range test.
Results and discussions
Proximate composition (%) of fish meat and skin
Biometric data and yields of orbicular batfish cultured in deep-sea and shallow seawater were evaluated. There were no differences in body weight, body width and skin thickness (p>0.05) between the batfish cultured in 2 types of seawater. Deep-sea water cultured orbicular batfish had longer total length (24.50±0.41 cm) and body length (20.00±0.76 cm) and lower condition factor (4.44±0.23) (p<0.05) than those cultured in shallow seawater (22.92±1.24 cm, 17.83±1.18 cm and 5.23±0.57 cm, respectively) indicating similar growth rate in weight although they were reared at lower temperature and higher salinity (34.0 ppt) and conductivity (47100.0 μS/cm) than those of shallow-sea water (18.0 ppt and 37000.0 μS/cm). Fish that were exposed to colder temperature and higher salinity of seawater spent more energy on maintaining muscle growth and metabolism.[Citation13] The condition factor of DSW cultured orbicular batfish (4.44) was lower than that of SSW cultured group (5.23) indicative of the body shape was different but showed no difference in body weight. However, the lower condition factor of DSW could also be considered as a positive fact due to the image of slimmer and leaner fish that may be favorable in the supermarket.[Citation14]
There was no significant difference on the moisture content between dorsal and ventral meat of orbicular batfish cultured in DSW or SSW. However, ventral meat had higher crude fat and lower crude protein than those of dorsal meat (). Ash, protein and carbohydrate contents of dorsal meat were higher in DSW cultured fish than those of SSW cultured fish (p<0.05). The fish skin of orbicular batfish cultured in DSW were significantly (p<0.05) higher in crude fat, ash and protein content, but lower in moisture and carbohydrate than those of SSW cultured fish (p<0.05).
Table 1. Proximate composition (%) of fish meat and skin of deep-sea water- and shallow-sea water-cultured orbicular batfish.
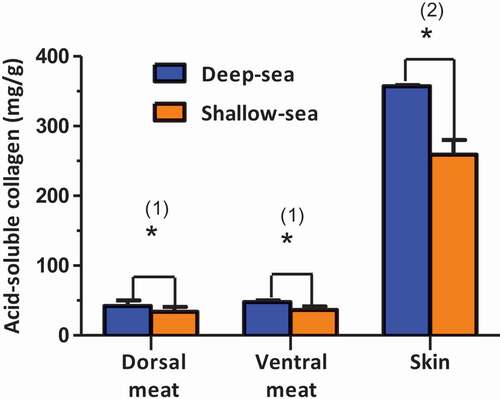
Collagen content of fish dorsal meat, ventral meat and skin of orbicular batfish cultured in DSW and SSW are shown in . Fish skin thickness of DSW (0.20 mm) and SSW (0.19 mm) cultured batfish was not significantly (p>0.05) different. However, collagen contents in dorsal meat, ventral meat and skin (42.14 mg/g, 47.72 mg/g, and 357.28 mg/g) of DSW were higher than those of SSW (33.68 mg/g, 36.62 mg/g and 259.04 mg/g, respectively) (). The micrographs of muscle interstitial material of cross-section from DSW showed more spacing than that of SSW cultured orbicular batfish (pictures not shown). Collagen fibrils in connective tissue are the determining component to maintain integrity and toughness in the structure and texture of fish meat.[Citation15] Culturing in DSW provoked a significant decrease in muscle firmness in our study. This effect was probably due to the opposite changes in collagen content. A negative correlation between the texture characteristics and muscle fat content was mentioned by Thakur et al.[Citation16] The results of this study were in agreement with them.
Figure 1. Collagen content of dorsal meat, ventral meat, and skin from deep-sea water-cultured and shallow-sea water-cultured orbicular batfish.
(1) Meat based on wet weight, (2) skin based on dry weight.
The collagen of DSW-cultured orbicular batfish skin was easily solubilized by limited pepsin and the yield was very high, reaching 52.38% on a dry weight basis comparing to that of the SSW-cultured orbicular batfish skin (33.96%), DSW-cultured dorsal meat (4.21%, wet weight basis), and SSW-cultured dorsal meat (3.37%). The yield of SSW-cultured orbicular batfish skin was similar to those of ocellate puffer (44.7%), paper nautilus (50%)[Citation17] and grass carp (46.6%).[Citation18] However, the collagen of orbicular batfish was easily extracted comparing to those fish reported in the literature (10–15%).[Citation19] DSW cultured orbicular batfish skin is a suitable source of collagen.
The higher yield of collagen could be due to higher collagen content in the skin of orbicular batfish than those of other fish species. Although the skin was not completely solubilized with 0.5 M acetic acid, the higher yield of collagen suggested a lower amount of cross-links at the telopeptide region as well as other intermolecular cross-links, leading to higher solubility of collagen in acid.[Citation18] Foegeding et al.[Citation20] proposed that the high degree of cross-linking via covalent bonds might cause a lower amount of extractable gelatin leading to the decrease in solubility of collagen. Lower collagen yields could also be due to incomplete hydrolysis of the collagen or the loss of extracted collagen through leaching during the series of washing steps in acid-treated procedure. Solvent, extraction time, and extraction temperature might affect the yield of extracted collagen. However, the extraction yield of fish collagen is still lower than the mammalian collagen.
DSW-cultured orbicular batfish skin is a suitable source of collagen since it is easily extracted at high yields at 4°C. Extraction yield of collagen could be significantly increased with addition of limited pepsin instead of extracting with 0.5 M acetic acid alone.[Citation17] The collagen of fresh salmon skin yielded increased solubility by increasing the extraction temperature to 70°C.
Protein patterns of orbicular batfish skin and meat collagens
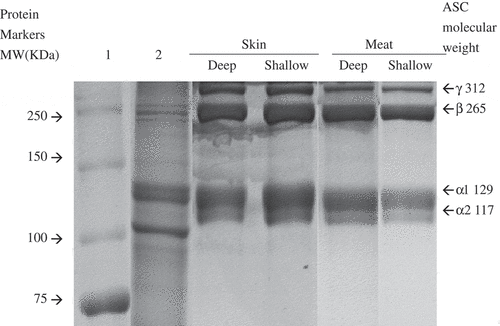
SDS-PAGE patterns of collagen from orbicular batfish skin and meat under reducing conditions are shown in . Commercial calf skin collagen type I was used as a reference for α1-, α2-, and β-chains. Orbicular batfish collagens had α1-chain (129 kDa), α2-chain (117 kDa), and β-chain (265 kDa; dimer of α1-chain) as the major constituents and were characterized to be type I. The band intensity of α1-chain was twofold higher than that of α2-chain. Apart from α-chain and β-chain, the higher molecular weight proteins of γ-chain (312 kDa) were observed in all orbicular batfish meat and skin collagens. Proteins with molecular weight lower than 117 kDa were not observed in these samples. They were present in commercial bovine type I collagen, which might be produced by endogenous proteinases or the thermal process during collagen extraction and purification (). Endogenous metallo- and serine proteinases of the skin of P. macracanthus caused autolysis of the skin at 50–60°C.[Citation21] The band intensity of β-chains was considerably higher in the orbicular batfish collagens, suggesting that the extraction conditions were more conductive to extract intact, covalently linked dimer of α1-chain. No significant differences in protein bands were observed among meat and skin collagen of orbicular batfish. The positions of α2-chain of orbicular batfish skin and meat collagens differed from those of bovine type I collagen. Collagen from the skin of ocellate puffer fish with high proportions of α- and β-chains has also been reported.[Citation17]
Figure 2. SDS-PAGE of meat and skin collagen from deep-sea water-cultured and shallow-sea water-cultured orbicular batfish.
Lane 1: protein marker. Lane 2: bovine type I collagen.
Amino acid composition of collagen from deep-sea water and shallow-sea water cultured orbicular batfish, expressed as residues per 1000 total residues, was very similar as shown in . Glycine was the most abundant amino acid in orbicular batfish meat collagen and accounted for 30.53% and 32.94%, respectively, from DSW and SSW cultured orbicular batfish. Asghar and Henrickson[Citation22] proposed 50-60% of α-chains consist of tripeptides having the general formula Gly-X-Y, where X is generally proline and Y is mainly hydroxyproline. There were relatively high contents of glutamic acid, alanine and proline, each of which accounted about 10% of amino acids in decreasing order (), similar to those obtained from skin, scale and bone collagen of carp.[Citation23] No tryptophan nor cysteine were found in both collagens. No cysteine indicating there was no disulfide bond in acid-soluble collagen (ASC) in both collagens.
The total content of imino acid (proline and hydroxyl proline was 133 and 164 residues per 1000 residues (), which were lower than those of carp skin collagen (190 residues) and calf skin collagen (215 residues). Collagens of lower quantities of hydroxylproline and proline, leading to less cross-linking and lower stability.[Citation24] Therefore, calf skin collagen might have greater stability than the orbicular batfish meat collagen. It has been suggested that the total content of proline and hydroxyproline of marine organism collagen to be lower than those of land animals.[Citation18] The total contents of imino acid (proline and hydroxyproline) of deep-sea redfish, the collagen from skin, scale, and bone were significantly lower than those of collagens from temperate and tropical fish species.[Citation25] Fish collagens have lower imino acid contents than mammalian collagens.[Citation20] The higher the imino acid contents, the more stable are the helices, because the molecular structure of collagen is maintained mainly by restrictions on changes in the secondary structure of the polypeptide chain, imposed by the pyrrolidine ring of proline and hydroxyproline, and also maintained partially by the hydrogen bonds through the hydroxyl group of hydroxyproline.[Citation26] Therefore, the collagen helices of DSW-cultured orbicular batfish might be less stable than those of SSW-cultured orbicular batfish, due to the lower imino acid content ().
Table 2. Amino acid composition of acid - soluble collagen of Orbicular Batfish cultured in deep-sea water and shallow-sea water.
Determination of water-holding and fat-binding capacities of collagen
shows the water holding capacities of the acid-solubilized collagens extracted from the meat of orbicular batfish cultured in DSW and SSW. Water holding capacity of that from the DSW fish acid-solubilized collagen was significantly higher (25.22 μl/mg) than that of SSW cultured orbicular batfish (20.94 μl/mg). But they are significantly lower than those of inner and outer squid skin collagens (99.7 μl/mg and 98.5 μl/mg) and bovine tendon collagen (51.0 μl/mg). Berisio et al.[Citation27] proposed that a hydration network of collagen triple-helix polypeptide chains was suggested by the formation of inter- and/or intrachain H-bonds of polypeptide C = O and NH groups through mediating water molecules. This suggested that orbicular batfish meat collagen polypeptides seemed to have less available C = O and NH groups holding water molecules than did squid skin collagen and bovine tendon collagen. Therefore, the structural flexibility of orbicular batfish meat collagens could be less than the above collagens. It implies that orbicular batfish meat collagens have little potential to be used as stabilizers and bulking agents in food, cosmetic, and medical industry. Collagen triple helix is stabilized by intra- and interchain water-mediated hydrogen bonding and direct interchain hydrogen bonding.[Citation28] Water-binding capacity depends on the degree of exposure of the hydrophilic residues inside of collagen.
Figure 3. (A) Water-holding capacity and (B) oil-binding capacity of meat acid-solubilized collagen extracted from deep-sea water cultured and shallow-sea water cultured Orbicular Batfish. All data are shown as mean ± S.D. (n=3). Data with star are significantly (p < 0.05) different analyzed with t-test.
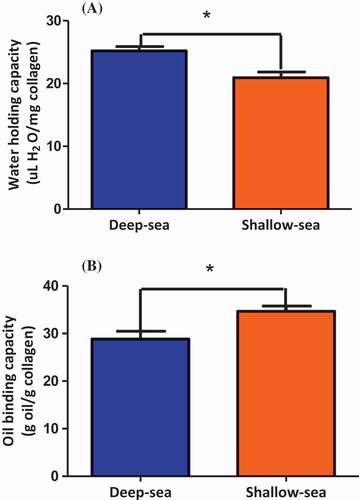
Fat-binding capacity is also a functional property which related to texture contributed by the interaction between oil and other components. shows the acid-solubilized collagen extracted from the meat of orbicular batfish cultured in DSW had lower fat-binding capacity (28.85 g oil/g collagen) than that of the SSW acid-solubilized collagen (34.67 g oil/g collagen). Fat-binding capacity depends on the degree of exposure of the hydrophobic residues inside of collagen.
Viscosity of collagen solution
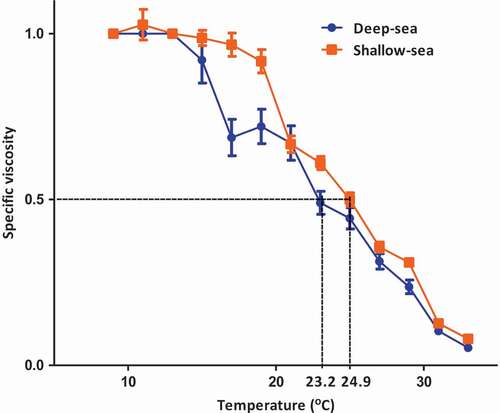
High viscosity is one of the physicochemical properties of collagen, which is due to the stronger electrostatic repulsion among the molecular chains even at low concentrations of collagen solution.[Citation29] The triplex helix structure of collagen stabilized by hydrogen bonds is irreversibly destroyed and converted into a random coil configuration. Upon heating the viscosity of collagen will decrease.[Citation30] The continuous decrease in viscosity of acid-soluble collagen was recorded when the temperature increased from 10 to 40°C (). Rearrangements of α-chain, β-chain and γ-chain might occur and formed a less compact configuration which could lead to the decrease in viscosity. The denaturation temperature (Td) of ASC was estimated to be 23.2°C and 24.9°C for deep-sea and shallow seawater cultured orbicular batfish meat collagens, respectively (). They were about 5°C and 14°C lower than those of ocellate puffer fish skin collagen (28°C) and porcine skin collagen (37°C).[Citation17] The high viscosity could be due to high proportion of higher molecular weight β-chain and γ-chain in the solution at specific temperature.[Citation31] Collagen structure stabilized by hydrogen bonds was dissociated when it underwent heat treatment at high temperature.[Citation32]
Figure 4. Thermal denaturation curve of acid-solubilized collagen from orbicular batfish meat. All data are shown as mean±SD (n = 3).
The denaturation temperatures of collagen from marine organisms such as cuttlefish outer skin (27.0°C)[Citation12], purple sea urchin (28.0°C)[Citation33], rhizostomous jellyfish mesoglea (28.8°C)[Citation34], Japanese sea bass fin (29.1°C), yellow sea bream and horse mackerel (29.5°C), skipjack, and ayu (29.7°C)[Citation35], bone of Japanese sea bass (30.0°C), bullhead shark (25.0°C), chub mackerel (25.6°C), skin of Japanese sea bass (26.5°C)[Citation36], body wall of starfish (23.0°C)[Citation37], chum salmon (19.4°C), Saury (23.0°C), common mackerel (26.1°C), eel (29.3°C), skins of carp (31.7°C), muscles of carp (32.5°C)[Citation24], swim bladder (18.4°C), and Alaska Pollack skin (16.8°C)[Citation38] are all lower than those of land animals.[Citation39] Rigby suggested the denaturation temperature is correlated with body temperatures and their environment.[Citation39]
Conclusion
Orbicular batfish cultured in deep-sea water (DSW) were significantly (p<0.05) lower in condition factor, meat lipid content while the content of protein in meat were significantly (p<0.05) higher than those fish cultured in the shallow-sea water (SSW). A DSW cultured orbicular batfish had 25.1% collagen content (p<0.05), a water holding capacity of 20.4% (p<0.05) both of which were higher than those of SSW. The thermal denaturation temperature of the DSW cultured orbicular batfish meat collagen was 1.7°C lower than that of SSW collagen. The results also demonstrated that the oil binding capacity of SSW cultured orbicular batfish meat collagen was better than that of DSW cultured fish meat collagen. The imino acids content of DSW cultured orbicular batfish meat collagen was lower than that of SSW cultured orbicular batfish meat collagen. Although collagens extracted from the orbicular batfish cultured in DSW and SSW had different functional characteristics and amino acid compositions, all orbicular batfish collagens had α1-chain, α2-chain, and β-chain as the major constituents and were characterized to be type I collagen. Acid pre-treatment on orbicular batfish meat and skin facilitated extraction of collagen for specific functional properties. The results have demonstrated that orbicular batfish meat and skin collagen could replace commercial calf skin collagen and be used as water holding and oil binding agents in food industry. Further investigations are ongoing to observe the texture and flavor properties of DSW and SSW cultured orbicular batfish.
Additional information
Funding
References
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-Sea Water Improves Cardiovascular Hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) Rabbits. Biological and pharmaceutical Bulletin 2008, 31, 38–44. DOI: 10.1248/bpb.31.38.
- Watanabe, M.; Ohtsu, J.; Otsuki, A. Daily Variations in Nutrient Concentrations of Seawater at 321 M Depth in Toyama Bay, Japan Sea. Journal of Oceanography 2000, 56, 553–558. DOI: 10.1023/A:1011153111688.
- Kirke, B. Enhancing Fish Stocks with Wave-Powered Artificial Upwelling. Ocean and Coastal Management. 2003, 46, 901–915. DOI: 10.1016/S0964-5691(03)00067-X.
- Allen, G.; Steene, R.; Humann, P.; Deloach, N. Reef Fish Identification; Odyssey Publications: Jacksonville, Florida and Tropical Pacific New World Publication Inc: EI Cajon, CA, 2003.
- Olurin, K. B.; Aderibigbe, O. A. Length-Weight Relationship and Condition Factor of Pond Reared Juvenile Oreochromis niloticus. World Journal of Zoology. 2006, 1, 82–85.
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000.
- Nagai, T.; Suzuki, N. Preparation and Partial Characterization of Collagen from Paper Nautilus (Argonauta argo, Linnaeus) Outer Skin. Food Chemistry 2001, 76, 149–153. DOI: 10.1016/S0308-8146(01)00255-2.
- Archile-Contreras, A. C.; Purslow, P. P. Oxidative Stress May Affect Meat Quality by Interfering with Collagen Turnover by Muscle Fibroblasts. Food Research International 2011, 44, 582–588. DOI: 10.1016/j.foodres.2010.12.002.
- Laemmli, U. K. Cleavage of Structural Proteins during Assembly Head of Bacteriophage T4. Nature 1970, 277, 680–685. DOI: 10.1038/227680a0.
- Nam, K.; Kimura, T.; Kishida, A. Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvent. Macromolecular Bioscience 2007, 8, 32–37. DOI: 10.1002/mabi.200700206.
- Balti, R.; Jridi, M.; Sila, A.; Souissi, N.; Nedjar-Arroume, N.; Guillochon, D.; Nasri, M. Extraction and Functional Properties of Gelatin from the Skin of Cuttlefish (Spia officinalis) Using Smooth Hound Crude Acid Protease-Aided Process. Food Hydrocolloids 2011, 25, 943–950. DOI: 10.1016/j.foodhyd.2010.09.005.
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Kanamori, N.; Suzuki, N. Isolation and Characterization of Collagen from the Outer Skin Waste Material of Cuttlefish (Sepia lycidas). Food Chemistry 2001, 72, 425–429. DOI: 10.1016/S0308-8146(00)00249-1.
- Imsland, A. K.; Gústavsson, A.; Gunnarsson, S.; Foss, A.; Árnason, J.; Árnarson, I.; Thorarensen, H. Effects of Reduced Salinities on Growth, Feed Conversion Efficiency and Blood Physiology of Juvenile Atlantic Halibut (Hippoglossus hippoglossus L.). Aquaculture 2008, 274, 254–259. DOI: 10.1016/j.aquaculture.2007.11.021.
- Einen, O.; Waagan, B.; Thomassen, M. S. Starvation Prior to Slaughter in Atlantic Salmon, Salmo salar I. Effects on Weight Loss, Body Shape, Slaughter and Fillet-Yield, Proximate and Fatty Acid Composition. Acquaculture 1998, 166, 85–104. DOI: 10.1016/S0044-8486(98)00279-8.
- Bremner, H. A.; Hallett, I. C. Degradation of Muscle Fiber Connective Tissue Junction in the Spotted Trevalla, Seriolella punctata Examined by Electron Microscopy. Journal of the Science of Food and Agriculture 1986, 37, 1011–1018. DOI: 10.1002/jsfa.2740371009.
- Thakur, D. P.; Morioka, K.; Itoh, Y.; Obatake, A. Lipid Composition and Deposition of Cultured Yellowtail, Seriola quinqueradiata Muscle at Different Anatomical Locations in Relation to Meat Texture. Fisheries Science 2003, 69, 487–494. DOI: 10.1046/j.1444-2906.2003.00649.x.
- Nagai, T.; Arakib, Y.; Suzuki, N. Collagen of the Skin of Ocellate Puffer Fish. Food Chemistry 2002, 78, 173–177. DOI: 10.1016/S0308-8146(01)00396-X.
- Zhang, Y.; Liu, W. T.; Li, G. Y.; Shi, B.; Miao, Y. Q.; Wu, X. H. Isolation and Partial Characterization of Pepsin-Soluble Collagen from the Skin of Grass Carp (Ctenopharyngodon idella). Food Chemistry 2007, 103, 906–912. DOI: 10.1016/j.foodchem.2006.09.053.
- Nakai, S.; Li Chan, E. Hydrophobic interaction in food systems. CPC Press Inc.: Boca Raton, 1988, 63–128.
- Foegeding, E. A.; Lanier, T. C.; Hultin, H. O. Collagen. In Food Chemistry, 3rd ed.; Fennema, O. P., Ed.; Marcel Dekker: New York, 1996; 902–906.
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M.; Howell, N. K. Autolysis Study of Bigeye Snapper (Priacanthus macracanthus) Skin and Its Effect on Gelatin. Food Hydrocolloids 2007, 21, 537–544. DOI: 10.1016/j.foodhyd.2006.05.012.
- Asghar, A.; Henrickson, R. L. Chemical, Biochemical Functional, and Nutritional Characteristics of Collagen in Food Systems. In Advances in Food Research; Chichester, C. O., Mark, E. M., Steward, G. E., Eds.; Academic Press: London, 1982; Vol. 28, 232–372.
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of Collagen from Skin, Scale and Bone of Carp (Cyprinus Carpio). Food Chemistry 2009, 112, 702–706. DOI: 10.1016/j.foodchem.2008.06.020.
- Kimura, S.; Zhu, X. P.; Matsui, R.; Shijoh, M.; Takamizawa, S. Characterization of Fish Muscle Type I Collagen. Journal of Food Science 1988, 53, 1315–1318. DOI: 10.1111/j.1365-2621.1988.tb09266.x.
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and Characterization of Collagens from the Skin, Scale and Bone of Deep-Sea Redfish (Sebastes Mentella). Food Chemistry 2008, 108, 616–623. DOI: 10.1016/j.foodchem.2007.11.017.
- Piez, K. A.; Gross, J. The Amino Acid Composition of Some Fish Collagens: The Relation between Composition and Structure. The Journal of Biological Chemistry 1960, 235, 995–998.
- Berisio, R.; Vitagliano, L.; Mazzarella, L.; Zagari, A. Crystal Structure Determination of the Collagen-Like Polypeptide with Repeating Sequence Pro-Hyp-Gly: Implications for Hydration. Biopolymers 2001, 56, 8–13. DOI: 10.1002/1097-0282(2000)56:1<8::AID-BIP1037>3.0.CO;2-W.
- Brodsky, B.; Persikov, A. V. Molecular Structure of Collagen Triple Helix. Advances in Protein Chemistry 2005, 70, 301–339.
- Zhang, M.; Liu, W.; Li, G. Isolation and Characterisation of Collagens from the Skin of Largefin Longbarbel Catfish (Mystus macropterus). Food Chemistry 2009, 115, 826–831. DOI: 10.1016/j.foodchem.2009.01.006.
- Gurdak, E.; Booth, J.; Roberts, C. J.; Rouxhet, P. G.; Dupont-Gillain, C. C. Influence of Collagen Denaturation on the Nanoscale Organization of Adsorbed Layers. Journal of Colloid and Interface Science 2006, 302, 475–484. DOI: 10.1016/j.jcis.2006.06.064.
- Ogawa, M.; Portier, J. R.; Moody, M. W.; Bell, J.; Schexnayder, M. A.; Losso, J. N. Biochemical Properties of Bone and Scale Collagens Isolated from the Subtropical Fish Black Drum (Pogonia cromis) and Sheepshead Sea Bream (Archosargus probatocephalus). Food Chemistry 2004, 88, 495–501. DOI: 10.1016/j.foodchem.2004.02.006.
- Wong, D. W. S.; Mechanism and Theory in Food Chemistry; Van Nostrand Reinhold Company Inc: New York, 1989.
- Nagai, T.; Suzuki, N. Partial characterization of collagen from purple sea urchin. (Anthocidaris crassispina) test. International Journal of Food Science & Technology 2000, 35, 497–501.
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Naka, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chemistry 2000, 70, 205–208.
- Nagai, T.; Suzuki, N. Isolation of Collagen from Fish Waste Material-Skin, Bone and Fins. Food Chemistry 2000, 68, 277–281. DOI: 10.1016/S0308-8146(99)00188-0.
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of Edible Jellyfish Exumbrella. Journal of the Science of Food and Agriculture 1999, 79, 855–858. DOI: 10.1002/(ISSN)1097-0010.
- Kimura, S.; Omura, Y.; Ishida, M.; Shirai, H. Molecular Characterization of Fibrillar Collagen from the Body Wass of Starfish Asterias amurensis. Comparative Biochemistry and Physiology 1993, 104B, 663–668.
- Kimura, S.; Ohno, Y.; Miyauchi, Y.; Uchida, N. Fish Skin Type I Collagen: Wide Distribution of an α3 Subunit in Teleosts. Comparative Biochemistry and Physiology 1987, 88B, 27–34.
- Rigby, B. J. Amino-Acid Composition and Thermal Stability of the Skin Collagen of the Antarctic Ice-Fish. Nature 1968, 219, 166–167. DOI: 10.1038/219166a0.
