ABSTRACT
Essential oils from leaves and stems of Phlomis lurestanica at the flowering stage were extracted by hydrodistillation. The essential oils were analyzed using Gas Chromatography and Gas Chromatography-Mass Spectrometry. Twenty-eight and twenty-five compounds were identified in the stems and leaves, respectively. The main compounds of stems were α-pinene (12.40%), γ-cadinene (10.92%), and γ-elemene (6.46%). Hexadecane (8.97%), 2-dodecnenal (6.57%), and heptadecane (6.32%) were the major constituents in the essential oils of the leaves. The methanol extracts of stems and leaves were evaluated for their antioxidant properties using 2,2-diphenyl-1-picrylhydrazyl and β-carotene/linoleic acid tests. In the 2,2-diphenyl-1-picrylhydrazyl assay, the leaves (IC50 = 1168.9 µg/ml) have stronger antioxidant activity than the stems (IC50 = 1563.6 µg/ml). The extracts of the leaves and stems showed remarkable antioxidant activity in β-carotene/linoleic acid assay (96.2% and 95%, respectively). The total phenol and flavonoid contents of the species were determined using Folin-Ciocalteu and AlCl3 assays, respectively. The phenol and flavonoid contents of the leaves (301.0 µg/ml and 45.2 µg/ml, respectively) were observed more than the stems (172.3 µg/mg and 18.8 µg/mg, respectively).
Introduction
Aromatic plants are considered of great interest for their flavors and medicinal properties, along with human consumption, animal foodstuff, and ornamental uses; thus, they are specially suitable for multifunctional sustainable crop models. A large number of these aromatic species belong to the Lamiaceae family, whose center of differentiation is located in the Mediterranean area.[Citation1]
Essential oils are complex mixtures, composed of terpenoid hydrocarbons, oxygenated terpenes, and sesquiterpenes. They originate from plant secondary metabolism and are responsible for their characteristic aroma. The various applications of essential oils account for the great interest in their study. These applications may be found in the cosmetic industry, as ingredients of components of medicines and as antibacterials/antimicrobials, and in aromatherapy.[Citation2]
Oxidation is a chemical reaction that can produce free radicals, leading to chain reactions that may damage cells, for example, it may cause cancer, cardiovascular disease, cataracts, immune system decline, and brain dysfunction. An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Many natural molecules, especially those produced in the plant kingdom, have at least one benzene ring with a hydroxyl functional group in their skeleton. These compounds are collectively known as phenolic compounds and due to their hydrogen or single electron donating potentials, they usually play important roles in the antioxidant activity of the plant extracts.[Citation3]
The genus Phlomis consists of about 100 species in the world. According to the last report, this genus has 17 species in the flora of Iran, of which 10 are endemic.[Citation4] Phlomis lurestanica Jamzad (Lamiaceae) is a herbaceous plant, perennial, height 20–40 cm, and its habitat is in Southwest Iran (Lorestan province).[Citation5]
Many different glycoside compounds have been identified in Phlomis, for example, diterpenoids, iridoids, phenylpropanoids, phenylethanoids, and flavonoids. Some species of Phlomis have important activities, including antiinflammatory, immunosuppressive, antimutagenic, antinociceptive, antifibriel, free radical scavenging, antiallergic, antimalaria, and antimicrobial effects.[Citation6]
The main components in the essential oils of Phlomis olivieri were β-caryophyllene (25.7%) and germacrene-D (19.5%), while germacrene-D (17.2%) and γ-elemene (15.4%) were the major constituents in Phlomis persica.[Citation7] Chu et al.,[Citation8] reported geranial (16.5%), linalool (13.3%), cis-geraniol (7.4%), β-myrcene (6.2%), 1,8-cineol (5.3%), 4-terpineol (5.1%), and myristicin (5%) as the major compounds in the aerial parts of P. umbrosa in the flowering stage. The major compounds of P. chimera were β-caryophyllene (31.6%), α-pinene (11.0%), and germacrene D.[Citation9]
The chemical composition of the essential oils and antioxidant properties of P. lurestanica extracts have not been previously reported. Therefore, the aim of the present study was to evaluate the oil composition and antioxidant properties of its methanol extracts.
Materials and methods
Plant material
P. lurestanica were collected at the flowering stage from Kohdasht located at west of Lorestan province (Iran) and dried in the shade for a month at room temperature.
Essential oils extraction
The essential oils were extracted by hydrodistillation using Clevenger type apparatus. Distillation process was done for 3 h. The obtained essential oils were stored in the freezer at −20°C until analysis.
Analysis of essential oils
Flame ionization detector-gas chromatography (FID-GC) was performed using a Hewlett-Packard 6890 with HP-5 capillary column (phenyl methyl siloxane, 25 m × 0.25 mm i.d., 0.25 µm film thickness); carrier gas, He; split ratio, 1:25, and FID. Temperature program: 60°C (2 min) rising to 240°C at 4°C/min; injector temperature, 250°C; detector temperature, 260°C. GC-MS was performed using Hewlett-Packard 6859 with quadrupole detector, on a HP-5 column (GC), operating at 70 eV ionization energy, using the same temperature program, and carrier gas as mentioned earlier. Retention indices were calculated by using retention times of n-alkanes that were injected after the oils at the same chromatographic conditions according to Van Den Dool’s method.[Citation10] Identification of the components was done by comparing their mass spectra with those of internal Wiley Gas chromatography-Mass Spectrometry (GC-MS) spectral library, or with published mass spectra and those described by Adams.[Citation11]
Preparation of methanol extracts
The samples (10 g) were extracted separately with 100 mL of 100% methanol for 72 h at room temperature. The extracts were separated from solids by filtering using Whatman No. 1 filter paper. The remaining residue was reextracted thrice and the extracts were pooled pulled. The solvent was removed under vacuum at 45°C, using a rotary vacuum evaporator (IKA RV 06-ML1B 230V, Germany) and stored at −4°C until used for further analyses (Methanol extraction yields of leaves and stems were 22.02% and 45.38% W/W, respectively).
Antioxidant activity
DPPH assay
In this method, 50 μL of the different concentrations of the extracts was mixed with methanol solution (1 mL) containing DPPH radicals (0.004%, w/v). After 30 min, the absorbance of the specimens was measured at 517 nm using a microplate reader (Bio Tek, U.S.A). The inhibition of free radicals was measured using the following equation:
I% = (Ablank – Asample/Ablank) × 100
where Ablank is the absorbance of the control reaction (including all reagents, except the defined concentration of the given extract) and Asample is the absorbance of the test. The IC50 represents the concentration of the extracts that causes 50% inhibition of the radical.[Citation12]
β-carotene/linoleic acid assay
In this method, the antioxidative potential of the extracts was measured by plotting decolorization of the β-carotene/linoleic acid assay. To prepare the β-carotene/linoleic acid solution, 0.5 mg β-carotene was mixed with 1 mL chloroform, and then 25 μL linoleic and 200 mg Tween-40 were added. The chloroform was completely evaporated. In the next stage, 100 mL oxygen-saturated distilled water was added and the container was vigorously shaken. Then, 2500 μL reaction mixture and 350 μL of the obtained extracts (500 μg/mL) were added to the test tube. In zero time and after 2 h incubation at 50°C, the absorbance of the specimens were measured at 470 nm using a microplate reader (Bio Tek, USA). The antioxidative capacity of the extract was compared with positive tests. All the tests were carried out in triplicate. The activity was expressed as inhibition percentage using the following equation:
AA% = (1 – DRS/DRC) × 100
where AA% is the antioxidant activity, DRC and DRS are the degradation rates of β-carotene in reactant mixture without and with the sample,
DR = ln (a/b) × 1/t
where a = initial absorbance at 0 min, b = absorbance at 120 min, and t = 120.[Citation13]
Phenolic compounds
Phenolic compounds were determined according to the method of Amiri[Citation14] with some modifications. Briefly, 100 μL of the sample (2 mg/mL) was mixed with 1500 μL of Folin-Ciocalteu reagent (diluted ten-fold) and 1 mL distilled water was added; after 1 min, 1500 μL of a solution of 20% sodium carbonate was added and the mixture was kept in the dark at room temperature, then absorbance was measured at 760 nm. The same procedure was repeated for all standard Gallic acid solutions and the concentration of the phenolic compounds was calculated accordingly and the standard curve was obtained using the following equation:
Absorbance: 0.001 Gallic acid (μg/ml) + 0.111 (r2 = 0.994).
Flavonoid compounds
Here, 500 μL sample (2 mg/mL) was mixed with 1500 μL methanol, 100 μL of 10% aluminum trichloride, 100 μL of potassium acetate 1 M, and 2.8 mL of distilled water. After 10 min at room temperature, the absorbance was determined at 420 nm. The same procedure was repeated for all standard quercetin solutions and the concentration of flavonoid compounds was calculated accordingly and the standard curve was obtained using the following equation[Citation15]:
Absorbance: 0.0091 quercetin (μg/ml) + 0.0206 (r2 = 0.995).
Statistical analysis
Experimental results were represented as mean ± standard error (SE) of three parallel measurements and analyzed by the Minitab software. Differences between means were determined using Tukey’s and student’s-t-test.
Results and discussion
Chemical composition of essential oils
The results of the essential oils analysis are shown in . According to these results, hexadecane, 2-dodecnenal, and heptadecane were the main components found in the leaves and α-pinene, γ-cadinene, and γ-elemene, were the main components found in the stems. shows the major compounds in some species of Phlomis genus. Based on the results of , α-pinene as the main monoterpene hydrocarbons in P. lurestanica oil is also the major compound of essential oils in some Phlomis species such as P. herba-venti subsp. pungens (7.3%), P. anisodonta subsp. occidentalis in vegetative stage (6.8%), P. leucophracta (19.2%), P. chimera (11%), and P. bourgaei (5.65%). Other major compounds of P. lurestanica were not found in other Phlomis species as the main constituent.
Table 1. Essential oils composition of stems and leaves of P. lurestanica.
Table 2. The major compounds of essential oil of some species of Phlomis.
The volatile oil constituents in leaves and stems were divided into terpenoids and aliphatic compounds. Terpenoids are the most important components of P. lurestanica oil and have commercial uses in the pharmaceutical industry, makeup material, production of vitamins (A, D, and E), etc.
Based on previous studies, Phlomis species can be divided into four chemotypes: (1) the first chemotype is rich in sesquiterpene: in this group, germacrene-D and β-caryophyllene are the two main components; (2) the second chemotype is rich in monoterpene and sesquiterpene: the main components of this class are α-pinene, limonene, linalool, germacrene-D, and β-caryophyllene; (3) fatty acids, aliphatic compounds, and alcohol (diterpenoic alcohol, fatty acid alcohol) constitute the main components of the third chemotype: this group contains a high percentage of hexadecanoic acid, trans-phytol, and 9,12,15-octadecatrien-1-ol; (4) the last chemotype is rich in terpene, fatty acids, aliphatic compounds, and alcohol (diterpenoic alcohol, fatty acid alcohol) as its main constituents: this mixed group contains hexadecanoic acid, α-pinene, and germacrene-D as major fatty acid, monoterpene, and sesquiterpene, respectively.[Citation17] Concerning the aforementioned classification, the results of the present study showed that P. lurestanica can be placed in groups 2 and 3 according to stems and leaves oil analyses, respectively.
Antioxidant activity
Since the amount of essential oil was little, antioxidant activity was investigated just on methanolic extracts.
DPPH assay
and shows the antioxidant activity of leaves and stems in comparison with butylated hydroxytoluene (BHT) using DPPH assays. IC50 values show that all data in this method were statistically significant (p < 0.05) and the leaves have stronger antioxidant activity than the stems, but lower than the BHT (BHT> leaves> stems). In recent years, the possible toxicity of synthetic antioxidants like BHT and butylated hydroxyanisole (BHA) has been considered. Thus, the potential antioxidants of plants are suitable to protect against various diseases induced by free radicals.[Citation23]
Figure 1. Free radical scavenging of leaves and stems of Phlomis lurestanica compared with BHT by DPPH assays. All data in this method were statistically significant± standard error (n = 3) (p < 0.05).
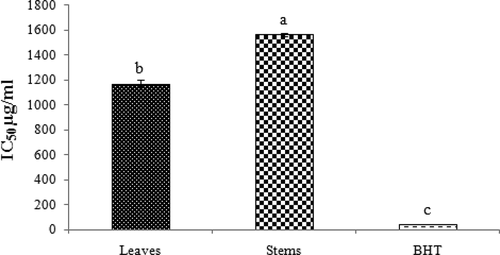
Table 3. Antioxidant activity, phenol and flavonoid contents of leaves and stems of Phlomis lurestanica.
The leaves have higher antioxidant activity when compared with the stems using DPPH method; this result showed that the type of organ can be effective on the antioxidant activity of the plant. This shows that the leaves have a stronger hydrogen donating capacity than the stems. Zhang and Wang[Citation24] reported the IC50 of P. umbrosa and P.megalantha to be 20.2 and 15.7 µg/ml, respectively; also, the IC50 in P. caucasica, P. lanceolata, and P. aucheri was reported to be 0.1 mg/mL.[Citation15]
β-carotene/linoleic acid assay
The methanolic extracts of the leaves and stems showed high antioxidant activity in β-carotene/linoleic acid assay. The inhibition percentage of β-carotene degradation of BHT, leaves, and stem were not statistically significant (p > 0.05) ( and ).
Figure 2. Antioxidant activity of leaves and stems of Phlomis lurestanica compared with BHT by β-carotene/linoleic assays. All data in this method are not statistically significant± standard error (n = 3) (p > 0.05).
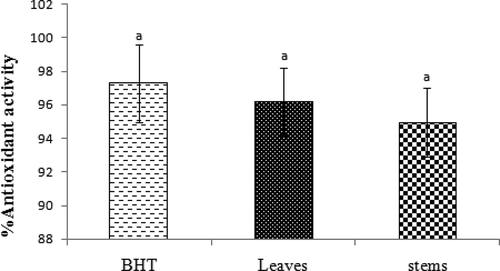
In β-carotene/linoleic acid assay, stems and leaves extracts both showed high antioxidant activity. But the percent antioxidant activity of leaves was more compared to that of stems. The antioxidant activity in P. umbrosa 88.6% and P. megalantha 87.8% have been previously reported.[Citation24] In P. bourgaei, the antioxidant activity was measured in three concentrations (0.4, 1.0, and 2.0), such that the activities were 69.28, 80.19, and 82.62%, respectively.[Citation20] Sarikurkcu et al.[Citation25] reported the antioxidant activity of P. armeniaca to be 75.63%.
Total phenolics and flavonoids
The results from phenol and flavonoid contents are as shown in and . These results showed that the leaves have more phenol and flavonoid contents in comparison with the stems and the phenol content was more than the flavonoid content. The data are statistically significant (p < 0.01).
Figure 3. Phenol and flavonoid contents of leaves and stems of Phlomis lurestanica .The leaves has more phenol and flavonoids in comparison to the stems. Data are statistically significant± standard error (n = 3) (p < 0.01).
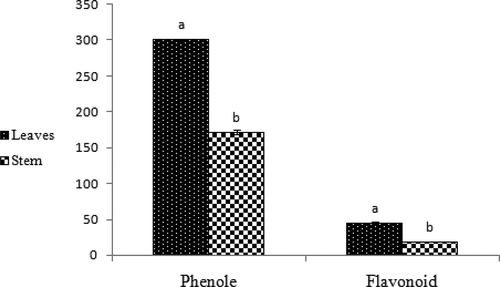
Lipid oxidation is a major cause of food quality deterioration and generation of off odors and off flavors, decreasing shelflife, altering texture and color, and decreasing the nutritional value of food.[Citation23] The results of phenol and flavonoid contents showed that leaves have more phenol and flavonoids content in comparison with the stems.
Karamian et al.,[Citation15] reported the amount of phenol in P. caucasica, P. lanceolata, and P. aucheri to be 7.65, 719, and 6.34 (mg GAE/g extract), respectively. Also, the amount of flavonoid for these three species was reported to be 4.97, 4.11, and 1.99 (mg QE/g extract), respectively. Zhang and Wang[Citation24] showed that the phenol contents of P. umbrosa and P. megalantha were 39.43 and 55.20 (mg GAE/g extract), respectively; also, the flavonoid contents were reported to be 7.12 and 35.91 (mg EE/g extract), respectively.
Naturally-occurring antioxidant compounds are flavonoids, phenolic acids, lignans, terpenes, tocopherols, phospholipids, and polyfunctional organic acids, amongst others as reported by Shahidi and Ambigaipalan.[Citation23] Phenolic and flavonoid compounds are well known for their antioxidant properties. Moreover, these compounds have some important activities, such as antiallergenic, antiartherogenic, antioxidant, anti-inflammatory, antimicrobial, antithrombotic, cardioprotective, and vasodilatory effects.[Citation26] According to the results of this research, in DPPH assays, the leaves have more antioxidant activity than the stems; this is confirmed by the high phenol and flavonoid contents in the leaves. The stronger antioxidant activities in the β-carotene/linoleic acid assay can be related to a lot of non polar compounds in the extracts.
Conclusion
Results of the present study showed that type of organ can affect the main compounds of essential oils, it can also influence the antioxidant activity, phenol and flavonoid contents. Based on the fact that P. lurestanica has medicinal and nutritional applications in West of Iran and since the results of this study showed that the antioxidant activity of the leaves are higher and better than stems therefore the outcomes can be useful in better and further application and use of this plant.
References
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. Subsp. Vulgare L. Under Different Growth Conditions. Molecules 2013, 18, 14948–14960. DOI: 10.3390/molecules181214948.
- Chamorro, E. R.; Zambón, S. N.; Morales, W. G.; Sequeira, A. F.; Velasco, G. A. Study of the Chemical Composition of Essential Oils by Gas Chromatography Lugar; Año. P: Rijeka, 2012, p. 307–324.
- Moein, S.; Farzami, B.; Khaghani, S.; Moein, M. R.; Larijani, B. Antioxidant Properties and Prevention of Cell Cytotoxicity of Phlomis persica Boiss. DARU 2007, 15, 83–88.
- Rechinger, K. H.;. Tancetum. In Flora Iranica, Compositae, No. 150; Rechinger, K. H., Hedge, I. C.; Eds.; AkademischeDruck- and Verlagsanstalt: Graz, Austria, 1982.
- Jamzad, Z. L. In Flora of Iran .Tehran, Iran. Research Institute of Forests and Rangelands. 2013. 76 P-P 272.
- Sarkhail, P.; Amin, G.; Shafiee, A. Composition of the Essential Oil of Phlomis olivieri Benth. From North of Iran. DARU 2006, 14, 71–74.
- Mohammadifar, F.; Delnavazi, M. R.; Yassa, N. Chemical Analysis and Toxicity Screening of Phlomis Olivieri Benth. And Phlomis persica Boiss. Essential Oils. Pharmaceutical Sciences 2015, 21, 12–17.
- Chu, S. S.; Liu, Q. Z.; Jiang, G. H.; Liu, Z. L. Chemical Composition and Insecticidal Activity of the Essential Oil Derived from Phlomis umbrosa against Two Grain Storage Insects. Journal Essent Oil Bear Pl 2013, 16(1), 51–58. DOI: 10.1080/0972060X.2013.764184.
- Celik, S.; Gokturk, R. S.; Flamini, G.; Cioni, P. L.; Morelli, I. Essential Oils of Phlomis leucophracta, Phlomis chimerae and Phlomis grandiflora Var. Grandiflora from Turkey. Biochemical Systematics and Ecology 2005, 33(6), 617–623. DOI: 10.1016/j.bse.2004.11.010.
- Van den Dool, H.; Kratz, P. D. Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. Journal Chromatographic A 1963, 11, 463–471. DOI: 10.1016/S0021-9673(01)80947-X.
- Adams, R. P.; Identification of Essential Oil Compounds by Gas Chromatography/Quadrupole Mass Spectroscopy Carol Stream; Allured Pub. Corp.: USA, 2001
- Tepe, B.; Sokmen, M.; Akpulat, H. A.; Sokmen, A. Screening of the Antioxidant Potentials of Six Salvia Species from Turkey. Food Chemistry 2006, 95, 200–204. DOI: 10.1016/j.foodchem.2004.12.031.
- Li, X.; Wang, Z. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil in Leaves of Salvia miltiorrhiza Bunge. Journal Essent Oil Researcher 2009, 21, 476–480. DOI: 10.1080/10412905.2009.9700222.
- Amiri, H.;. The in Vitro Antioxidative Properties of the Essential Oils and Methanol Extracts of Satureja macrosiphonia Bornm. Journal Natural Products Researcher 2011, 25(3), 232–243. DOI: 10.1080/14786410903374694.
- Karamian, R.; Azizi, A.; Asadbegy, M.; Pakzad, R. Essential Oil Composition and Antioxidant Activity of the Methanol Extracts of Three Phlomis Species from Iran. Journal BAPN 2014, 4, 343–353.
- Sarikurkcu, C.; Uren, M. C.; Kocak, M. S.; Cengiz, M.; Tepe, B. Chemical Composition, Antioxidant, and Enzyme Inhibitory Activities of the Essential Oils of Three Phlomis Species as Well as Their Fatty Acid Compositions. Food Science and Biotechnology 2016, 25, 687–693. DOI: 10.1007/s10068-016-0120-9.
- Delnavazi, M. R.; Baba-Ali, F.; Soufiabadi, S.; Sherafatmand, M.; Ghahremani, F.; Tavakoli, S.; Yassa, N. Essential Oil Composition, Antioxidant Activity and Total Phenolic Content of Some Lamiaceae Taxa Growing in Northwest of Iran. Pharmaceutical Sciences 2014, 20(1), 22–28.
- Amiri, H.; Ghiasvand, A. R. Changes in Composition and Antioxidant Activities of Essential Oils in Phlomis anisodonta (Lamiaceae) at Different Stages of Maturity. Progress Biological Sciences 2016, 6, 2, 205–212.
- Demirci, B.; Dadandı, M. Y.; Franz, G.; Başer, K. H. Chemical Composition of the Essential Oil of Phlomis linearis Boiss. & Bal., And Biological Effects on the CAM-assay: A Safety Evaluation. ZNC 2003, 58, 826–829.
- Sarikurkcu, C.; SabihOzer, M.; Cakir, A.; Eskici, M.; Mete, E. GC/MS Evaluation and in Vitro Antioxidant Activity of Essential Oil and Solvent Extracts of an Endemic Plant Used as Folk Remedy in Turkey: Phlomis bourgaei Boiss. Journal eCAM 2013, 2013, 1–7.
- Liolios, C.; Laouer, H.; Boulaacheb, N.; Gortzi, O.; Chinou, I. Chemical Composition and Antimicrobial Activity of the Essential Oil of Algerian Phlomis bovei De Noé Subsp. Bovei. Molecules 2007, 12, 772–781.
- Formisano, C.; Senatore, F.; Bruno, M.; Bellone, G. Chemical Composition and Antimicrobial Activity of the Essential Oil of Phlomis ferruginea Ten. (Lamiaceae) Growing Wild in Southern Italy. Flavour Fragrance Journal 2006, 21(5), 848–851. DOI: 10.1002/(ISSN)1099-1026.
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health effects–A Review. Journal Function Foods 2015, 18, 820–897. DOI: 10.1016/j.jff.2015.06.018.
- Zhang, Y.; Wang, Z. Z. Phenolic Composition and Antioxidant Activities of Two Phlomis Species: A Correlation Study. Comptes Rendus Biologies 2009, 332, 816–826. DOI: 10.1016/j.crvi.2009.05.006.
- Sarikurkcu, C.; Uren, M. C.; Tepe, B.; Cengiz, M.; Kocak, M. S. Phlomis armeniaca: phenolic compounds, enzyme inhibitory and antioxidant activities. Ind Crops Prod. 2015, 78: 95–101.
- Industrial Crops and Products 2015, 78, 95–101. DOI:10.1016/j.indcrop.2015.10.016.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial By-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chemistry 2006, 99(1), 191–203. DOI: 10.1016/j.foodchem.2005.07.042.
