ABSTRACT
The activation of the apoptosis pathway in tilapia muscle during postmortem storage was studied. Changes in caspase-3 activity, ATP content, cytochrome c levels, and ratio of Bcl-2/Bax levels of tilapia muscle were observed during postmortem storage at 20°C. Caspase-3 activity was found to be significantly increased at first, followed by a decrease (P < 0.05); the highest caspase-3 activity was observed at 1 h. The ATP content decreased significantly (P < 0.05), and almost exhausted after 10 h storage. The cytochrome c level in the cytosol showed a significant increase after 5 h of storage (P < 0.05), while the mitochondrial cytochrome c levels showed a decrease. The Bcl-2/Bax ratio was stable from 0–5 h, followed by a rapid decreased at 10–20 h and a significant increased after 20 h (P < 0.05), suggesting that the apoptosis process occurred until 20 h of postmortem storage. Thus, we concluded that the availability of ATP and the increase in cytosolic cytochrome c levels are essential for the activation of caspase-3, and that the former partly limits caspase-3 activity.
Introduction
Meat quality during postmortem storage is attributed to the degradation of certain major myofibrillar proteins[Citation1], which are responsible for maintaining the integrity of the myofibrillar structure.[Citation2,Citation3] Previous studies have reported that endogenous enzymes in the skeletal muscles, such as calpains, proteasomes, lysosomal cathepsins, and caspases, are responsible for the degradation of myofibrillar proteins.[Citation4–Citation6] Calpains are the most important of these proteins, and have been performed to many in-depth studies. However, the mechanism underlying the changes in quality that occur during postmortem storage has not been elucidated. For example, the calpains system cannot completely explain the degradation of major cytoskeleton proteins during the whole postmortem storage. Even, myofibrils were found to be cleaved in the presence of calpain inhibitors.[Citation7] In addition, the presence or absence of the calpains seems to have no effects on the degradation of some cytoskeletal proteins during postmortem storage. It has been suggested that calpains system is not the sole proteolytic determinant of meat quality.[Citation6] In contrast, meat quality is affected by the collective effects of multi-enzyme systems. A recent study suggested that caspases are also associated with changes in meat quality during postmortem storage, and their effects cannot be ignored.[Citation8]
Caspases belong to the family of cysteine proteases. So far, 14 caspases have been identified, which are present in either the original form or a zymogen, before being activated and inducing apoptosis.[Citation9] They play important roles in signal transduction and apoptosis.[Citation8] Study has demonstrated that the intrinsic mitochondrial pathway is the most possibly apoptosis pathway to be induced by oxygen deprivation.[Citation10] After animal slaughter, the blood circulation is terminated, resulting in oxygen deprivation, which initiates apoptosis.[Citation11,Citation12] Caspase-3, a key downstream executive molecule for apoptosis, plays a role in many pathways of apoptotic signaling and can cleave many target proteins, such as cytoskeletal proteins, leading to the typical morphological changes which are associated with apoptosis.[Citation13] Therefore, caspase-3 is considered to be an important indicator of apoptosis.[Citation12] Study has confirmed that caspase-3 can cleave cytoskeletal proteins such as actin, myosin, troponin I, troponin I, α-actinin, and desmin.[Citation14] Moreover, caspase-3 can even cleave calpastatin, a specific endogenous inhibitor of calpain.[Citation15,Citation16] In addition, N-Ac-Asp-Glu-Val-Asp-CHO (DEVD-CHO), a selective inhibitor of caspase-3, was used to inhibited caspase-3, and the results showed that DEVD-CHO have less effect on calpain, but significantly inhibit the degradation of myofibrillar proteins such as desmin, titin, and nebulin.[Citation17] Therefore, caspase-3 is also potential contributor to the postmortem tenderization of meat. However, caspase-3 cannot be activated automatically. Therefore, this study mainly investigates the activation pathway of caspase-3, as well as its related apoptosis factors, aiming to explore the apoptotic pathways in tilapia muscles during postmortem storage.
Materials and methods
Fish and samples
Tilapias were purchased from the local supermarket (Guangzhou, China). Twenty-four fish (731 ± 57 g) were killed by knocking on the head and stored separately at 20 ± 1°C. Samples (20 g) of muscle tissues were sampled at pre-determined points of time within 36 h postmortem (0, 1, 5, 10, 15, 20, 24, and 36 h) and immediately stored at −80°C, until analyses for caspase-3 activity, ATP content, expression levels of mitochondrial and cytosolic cytochrome c, and Bcl-2 and Bax proteins levels.
Caspase-3 activity
A caspase-3 assay kit (Beyotime Co., Ltd, Shanghai, China) was used to determine the caspase-3 activity in the samples. Briefly, 10 mg of each sample was lysed in 100 μL lysate. A total of 100 μL reaction system, containing 40 μL detection buffer, 50 μL muscle lysate, and 10 μL reaction substrate (2 mmol/L), was incubated at 37°C in water for 120 min. The samples were measured using a Synergy H1 Microplate reader (BioTek, USA) at 405 nm, and the results were expressed as percentage of enzyme activity compared with controls.
ATP content
The ATP content was measured using high performance liquid chromatography (HPLC), with a slightly modified version of the extracted method previously described by Zhang and others.[Citation18] One gram of minced muscle was homogenized for 3 min in 2 ml pre-cooled 100 g kg−1 perchloric acid. The homogenate was centrifuged (10,000 × g, 6 min, 4°C) and separated the supernatant into another centrifuge tube. The sediment was re-suspended in 2 ml 50 g kg−1 perchloric acid and centrifuged again. This step was repeated twice, and all the supernatants were pooled together. The pH of this supernatant mixture was adjusted to 6.5–6.8 using10 mol/L KOH and 1 mol/L KOH, and the resulting solution was diluted with water to a final volume of 10 ml. The samples were filtered into the HPLC-dedicated bottle using a 0.22-μm water micro-porous membrane. The entire process was carried out at 4°C. HPLC-grade ATP was obtained from Adamas-beta (Adamas Reagent Co.,Ltd, China).
Extraction of tissue protein and isolation of mitochondrial and cytosolic protein fractions
Muscle samples (50 mg) were homogenized on ice with 0.5 ml RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris [pH 7.4], 1 mmol/L EDTA, 1 g kg−1 SDS, 10 g kg−1 sodium deoxycholate, and 100 g kg−1 Triton X-100). The mixture was then centrifuged (10,000 × g, 20 min, 4°C) and the supernatant was retained as the tissue protein.
Mitochondrial and cytosolic protein fractions were isolated using a modified version of the method devised by Quadrilatero and Rush.[Citation19] One gram of minced muscle was added to the pre-cooling mitochondrial extraction buffer (20 mmol/L sucrose, 20 mmol/L HEPES, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L DTT, 1 mmol/L EGTA, and 1 mmol/L EDTA[pH 7.4]) at a ratio of 1:3 (w/v) and then ground using a Potter-Elvehjem homogenizer at a low temperature. The homogenate was centrifuged at a low speed (800 × g, 10 min), and the supernatant (S1) was retained and centrifuged again (1600 × g, 20 min); the second supernatant (S2) and sediment (S2) were subjected to the next step. The resultant supernatant (S2) was centrifuged a third time (1600 × g, 20 min) to remove the residual mitochondria, and the obtained supernatant (S3) was the cytosolic protein fraction. The sediment (S2) was dissolved in the mitochondrial extraction buffer and centrifuged again (1600 × g, 20 min); this step was repeated twice. The resulting sediment was dissolved in the mitochondrial extraction buffer and freeze-thawed thrice to obtain the mitochondrial fraction.
The protein concentrations in the tissue protein, mitochondrial fraction, and cytosolic fraction were measured using a BCA protein assay kit (Jiancheng Co., Ltd, Nanjing, China).All the protein fractions were mixed with 5x loading buffer and heated in boiled water for 5 min before being stored at −20°C for western blot analysis.
SDS-PAGE and western blotting
The expression levels of the mitochondrial and cytosolic cytochrome c and those of Bcl-2 and Bax were determined by western blot analysis, as described previously, with some modifications.[Citation20] Twenty micrograms of each sample was loaded on 12.5% SDS-PAGE, and electrophoresis was performed at a constant 100 V for 90 min. The gels were then transferred onto polyvinylidene difluoride membranes (Millipore, USA) and electrophoresed at a constant 200 mA for 90 min at 4°C. The primary antibodies were diluted to 1:1000 for Bax (Proteintech, China), 1:2000 for Bcl-2 (Proteintech, China), 1:500 for cytochrome c (Proteintech, China), and 1:4000 for GAPDH (Proteintech, China) in 50 g· kg−1 milk TBS-T; the appropriate HRP-conjugated secondary antibodies (Proteintech, China) were at a dilution of 1:4000. The blot was developed using BeyoECL Plus (Beyotime Co., Ltd, Shanghai, China). The western blot data were analyzed using Quantity One Quantitation Software (Bio-Rad, USA).
Statistical analyses
Statistical analyses were performed with one-way ANOVA, using the SPSS 17.0 software. Differences were considered significant at P < 0.05. Pearson correlation was performed to analyze the correlations between ATP content, caspase-3 activity, and the expression levels of the different apoptosis factors.
Results and discussion
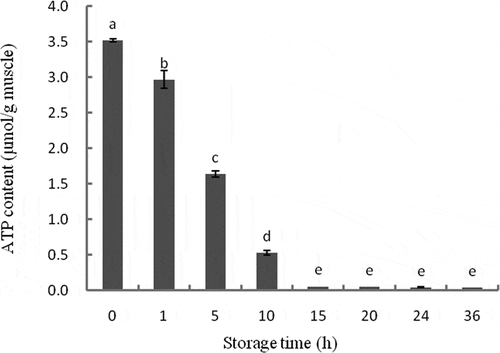
Changes in ATP content of tilapia muscle
The results of ATP content measurement in tilapia muscle during storage are presented in . The ATP content decreased significantly during storage (P < 0.05), after 10 h, the ATP content was almost exhausted. Upon slaughter, all cells and tissues of the animal are deprived of oxygen and nutrients, leading to rapid death of cells.[Citation21] When there is sufficient intracellular ATP to provide energy for apoptosis, the caspases are activated, initiating the apoptosis procedure. Once the intracellular ATP is insufficient, cells die through necrosis.[Citation22] Insufficient oxygen in muscles as a result of stagnant blood circulation occurs after animal slaughter, causing a shift in energy metabolism and resulting in a rapid decrease in ATP content, which synthesizes from ADP and creatine phosphate.[Citation3,Citation23] Consistent with these observations, the ATP content was observed to decrease immediately after slaughter in 80 pigs through four different experiments.[Citation24] Green[Citation25] believed that it only takes around 10 min for the activation of caspases after first mitochondrial changes. Therefore, cells undergo apoptosis immediately after tilapia slaughter.
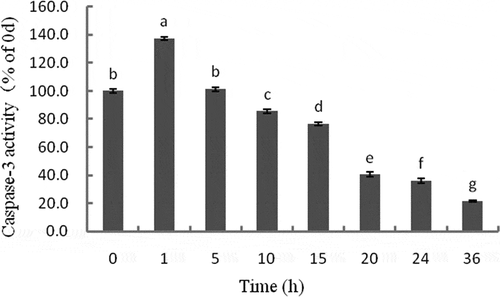
Changes in caspase-3 activity of tilapia muscle
Caspase-3 is one of the most important apoptotic performers in the caspases family and is considered the only way for the cascade of apoptotic proteases. Caspase-3 activity significantly increased initially, and then decreased during storage (P < 0.05). The highest activity was observed at 1 h (). A positive correlation was observed between caspase-3 activity and ATP content (r = 0.802, P < 0.05) (). The changes in caspase-3 activity observed in the present study suggest which was activated during postmortem storage. Caspase-3, as the executioner or effector caspases, will target and cleave specific substrates after it is activated by upstream initiator caspases such as caspase-9.[Citation26] Caspase-3 activity was found to increase in 1 h postmortem, after which it started decreasing. The positive correlation between caspase-3 activity and ATP content suggests that ATP is a key factor in the activation of the apoptosis pathway, which may responsible for the decrease in caspase-3 activity. Eguchiand others[Citation27] have reported both upstream and downstream of caspase-3 activation were ATP-dependent steps. The changes in caspase-3 activity during postmortem storage, as well as the correlation between ATP content and caspase-3 activity in the present study, are consistent with previous findings by Cao and others[Citation28], who also demonstrated that the activation of caspases depends on the availability of intracellular ATP. However, according to the studies of Kemp and others[Citation21] and Huang and others[Citation29], the highest activities of caspase-3 were found at 2 h in pork and 12 h in beef muscles during postmortem storage. The apoptotic pathway and the hypoxia-induced activation of caspase-3 seem to occur earlier in fish than they did in pork or beef muscle. This may be owing to the differences in blood volume among these animals, which imply different time for oxygen deprivation. The time taken for ATP consumption in pork or beef is longer than that in fish; therefore, the caspase-3 activity deprivation takes more time in pork or beef than in fish.
Table 1. Correlations among ATP, caspase-3 activity, and different apoptosis factors.
Expression levels of cytochrome c in mitochondria and cytosol
The expression levels of cytochrome c in the mitochondrial fraction significantly increased initially, followed by a significant decrease, during 36 h of storage (P < 0.05). The highest expression level was observed at 5 h (,)). The expression of cytochrome c in the cytosol fraction showed mild changes before 5 h, and a significant increase after 5 h (P < 0.05) (,)). The expression of cytochrome c in the cytosol was negatively correlated with that in mitochondrial fraction (r = −0.862, P < 0.01), caspase-3 activity (r = −0.883, P < 0.01), and ATP content (r = −0.911, P < 0.01) ().
Figure 3. Western blot analyses of relative changes in mitochondrial cytochrome c (a), cytosolic cytochrome c (b), GAPDH of mitochondrial protein fractions (c), and GAPDH of cytosolic protein fractions (d) in tilapia skeletal muscles during 36 h of storage. The expression levels of cytochrome c in the mitochondria and cytosol are expressed in terms of ratios to the protein density at 0 h (e). Bars represent the standard error of the mean.
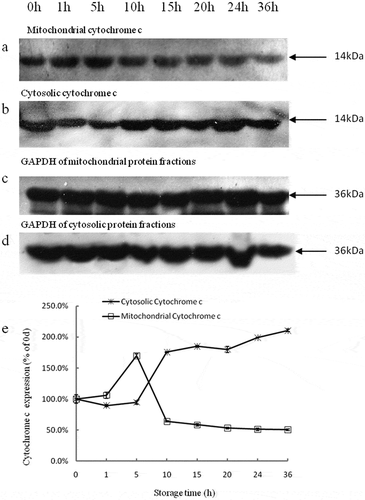
Cytochrome c belongs to the cytochrome c family of proteins, and is an essential component of the mitochondrial respiratory chain involved in electron transport.[Citation30] Mitochondria, which provide 90% of the energy from ATP consumption to the cell, can activate caspases by spontaneously releasing cytochrome c. This process, which is involved in apoptosis, leads to fodrin cleavage and apoptotic nuclear morphology.[Citation22,Citation31] Therefore, the release of cytochrome c from the mitochondria into the cytosol is a key initial step to trigger apoptosis.[Citation29] Environmental stress may affect the morphology of mitochondria. For example, the deprivation of oxygen after animal slaughter will permeabilize the outer membrane of the mitochondria, resulting in the release of cytochrome c.[Citation21,Citation32,Citation33] Therefore, insufficient oxygen would plunge muscle cells into an ischemia-like state, activating apoptosis, and eventually causing cell death, which is known as the mitochondrial pathway.[Citation34,Citation35] Once the mitochondria receives the apoptotic signal, cytochrome c will be released and combined with apoptotic activating factor-1 (Apaf-1) to form a complex in the cytosol, called apoptosome. The apoptosome activates caspase-9 and is limited by the hydrolysis of ATP.[Citation36] And then, the activated caspase-9 activates the downstream caspase-3, which in turn activates additional caspase-9 molecules.[Citation20] Therefore, the increase in cytosolic cytochrome c levels should be associated with the increase in caspase-3 activity. However, negative correlations were observed between cytochrome c and both ATP and caspase-3 activity in the present study. It is may be due to the depletion of ATP prevents the release of cytochrome c, and thus inhibiting the activation of caspase-3. In the current study, we confirmed that cytochrome c was released from the mitochondria into the cytosol when apoptosis initiated, which occurred at approximately 5 h postmortem.
Expression of Bcl-2 family proteins
A single band representing the expression of Bcl-2 was detected ()). Stable expression levels of Bcl-2 were observed at 1, 5, and 10 h, and were significantly lower than that at 0 h (P < 0.05). The Bcl-2 levels were found to be further down-regulated at 15 and 20 h (P < 0.05), before being up-regulated again at 24 and 36 h (P < 0.05) to the same levels as at 1, 5, and 10 h () and ). The changes in Bax levels were exactly the opposite of those in Bcl-2 levels. The highest Bax level was observed at 20 h, followed by that at 15 h () and ). The ratio of Bcl-2/Bax showed a slight decrease from 0 to 5 h, and then decreased significantly until 20 h (P < 0.05), after which it significantly increased (P < 0.05) (). In addition, the expression levels of Bax were negatively correlated to those of Bcl-2 (r = −0.828, P < 0.05)(). It is known that Bcl family proteins may deteriorate and remove the mitochondrial membrane potential, causing the release of cytochrome c to induce apoptosis.[Citation33,Citation37] Bax, a member of the Bcl-2 family, is involved in pro-apoptotic processes and regulates mitochondria-mediated apoptosis by modulating the mitochondrial membrane permeability.[Citation38] In contrast, Bcl-2 is an anti-apoptotic protein that functions in situ on mitochondria, and its overexpression can inhibit the translocation of cytochrome c to block the activation of caspases and apoptosis.[Citation31]
Table 2. Changes in band density ratios of Bax to GAPDH, Bcl-2 to GAPDH, and Bcl-2 to Bax during 36 h of storage.
Figure 4. Western blot analyses of Bcl-2 (a), Bax (b), and GAPDH (c) in tilapia skeletal muscles during 36 h of storage.
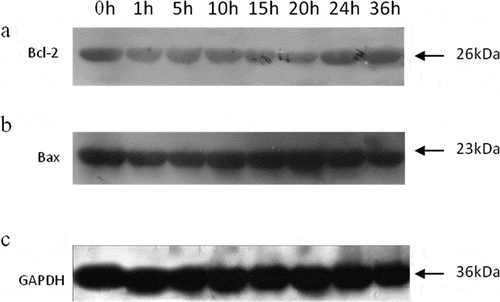
After the cells are stimulated by death signals, the pro-apoptotic protein of the Bcl-2 family undergoes conformational changes upon activation by a protease, and translocates from the cytoplasm to organelles, especially to the outer membrane of the mitochondria. In addition, the interaction between pro-apoptotic protein and the anti-apoptotic protein causes the loss of inhibition by anti-apoptotic protein to apoptosis, causing loss of organelles and the release of various pro-apoptotic factors, and ultimately triggering apoptosis.[Citation39] Many researchers have suggested that the ratio of Bcl-2/Bax is more important than the single apoptosis factor in determining apoptosis; the ratio of Bcl-2/Bax can act as a lever to regulate apoptotic cells; when it increases, apoptosis is inhibited, otherwise apoptosis is promoted when the ratio decreases.[Citation33,Citation40] The western blot analyses in the current study showed a slight decline in the ratio of Bcl-2/Bax postmortem, suggesting a weak activation of apoptosis immediately after fish slaughter. The ratio kept decreasing until 20 h, and then increased again, suggesting that apoptosis occurred until 20 h. Green[Citation25] reported that it often takes about 10 min to activate the caspases, while the entire apoptosis process takes hours or even days. The present results indicated that both Bcl-2 and Bax play important roles in the apoptosis process in muscles during postmortem storage. In addition, Bax was found to induce mitochondrial permeability transition and the release of cytochrome c.[Citation41] Ouali and others[Citation42] speculated that the ratio of Bcl-2/Bax determined the speed of cytochrome c release from mitochondria into the cytosol, allowing us to predict the apoptotic process.
Conclusion
Caspase-3 is known to affect meat quality. We therefore investigated the activation of the apoptosis pathway and its potential influencing factors in tilapia muscle during postmortem storage in an attempt to explore the mechanisms of apoptotic pathways and their regulation. Postmortem hypoxia causes a decrease in available intracellular ATP, inducing the activation of caspase-3. The exhausted ATP in turn limits the apoptotic enzyme activity. Deterioration of the mitochondrial membrane by the pro-apoptotic Bax proteins causes the release of cytochrome c from mitochondria into the cytosol, triggering the apoptosis. The constant decline in the ratio of Bcl-2/Bax until 20 h and the subsequent increase suggested the occurrence of apoptosis during the first 20 h of postmortem storage. These results could help understand the mechanisms behind the apoptotic process and further attempt to develop novel methods for improving the quality of stored fish by delaying apoptosis.
Additional information
Funding
References
- D’Alessandro, A.; Marrocco, C.; Rinalducci, S.; Mirasole, C.; Failla, S.; Zolla, L. Chianina Beef Tenderness Investigated through Integrated Omics. Journal of Proteomics 2012, 75, 4381–4398. DOI: 10.1016/j.jprot.2012.03.052.
- Addis, M.-F.; Cappuccinelli, R.; Tedde, V.; Pagnozzi, D.; Porcu, M.-C.; Bonaglini, E.; Roggio, T.; Uzzau, S. Proteomic Analysis of Muscle Tissue from Gilthead Sea Bream (Sparus Aurata, L.) Farmed in Offshore Floating Cages. Aquaculture 2010, 309, 245–252. DOI: 10.1002/pmic.201100073.
- Kjaersgård, I.-V.-H.; Jessen, F. Proteome Analysis Elucidating Post-Mortem Changes in Cod (Gadus Morhua) Muscle Proteins. Journal of Agricultural and Food Chemistry 2003, 51, 3985–3991. DOI: 10.1021/jf0340097.
- Chéret, R.; Delbarre-Ladrat, C.; Lamballerie-Anton, M.-D.; Verrez-Bagnis, V. Calpain and Cathepsin Activities in Post Mortem Fish and Meat Muscles. Food Chemistry 2007, 101, 1491–1496. DOI: 10.1016/j.foodchem.2006.04.023.
- D’Alessandro, A.; Zolla, L. Meat Science: From Proteomics to Integrated Omics Towards System Biology. Journal of Proteomics 2013, 78, 558–577. DOI: 10.1016/j.jprot.2012.10.023.
- Kemp, C.-M.; Sensky, P.-L.; Bardsley, R.-G.; Buttery, P.-J.; Parr, T. Tenderness – An Enzymatic View. Meat Science 2010, 84, 248–256. DOI: 10.1016/j.meatsci.2009.06.008.
- Thompson, D.-L.; Hopkins, J.-M. Factors Contributing to Proteolysis and Disruption of Myofibrillar Proteins and the Impact on Tenderisation in Beef and Sheep Meat. Crop & Pasture Science 2002, 53, 149–166. DOI: 10.1071/AR01079.
- Huang, M.; Huang, F.; Xue, M.; Xu, X.; Zhou, G. The Effect of Active Caspase-3 on Degradation of Chicken Myofibrillar Proteins and Structure of Myofibrils. Food Chemistry 2011, 128, 22–27. DOI: 10.1016/j.foodchem.2011.02.062.
- Kitamura, Y.; Inden, M.; Miyamura, A.; Kakimura, J.; Taniguchi, T.; Shimohama, S. Possible Involvement of Both Mitochondria- and Endoplasmic Reticulum-Dependent Caspase Pathways in Rotenone-Induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. Neuroscience Letters 2002, 333, 25. DOI: 10.1016/S0304-3940(02)00964-3.
- Brunelle, J.-K.; Chandel, N.-S. Oxygen Deprivation Induced Cell Death: An Update. Apoptosis 2002, 7, 475–482.
- Becila, S.; Herrera-Mendez, C.-H.; Coulis, G.; Labas, R.; Astruc, T.; Picard, B.; Bremaud, L.; Ouali, A. Postmortem Muscle Cells Die through Apoptosis. European Food Research and Technology 2010, 231, 485–493. DOI: 10.1007/s00217-010-1296-5.
- Huang, J.-C.; Yang, J.; Huang, F.; Huang, M.; Chen, K.-J.; Xu, X.-L.; Zhou, G.-H. Effect of Fast pH Decline during the Early Postmortem Period on Calpain Activity and Cytoskeletal Protein Degradation of Broiler M. Pectoralis Major. Poult Science 2016, 95, 2455–2463. DOI: 10.3382/ps/pew206.
- Rami, A.; Agarwal, R.; Botez, G.; Winckler, J. μ-Calpain Activation, DNA Fragmentation, and Synergistic Effects of Caspase and Calpain Inhibitors in Protecting Hippocampal Neurons from Ischemic Damage*1. Brain Research 2000, 866, 299–312. DOI: 10.1016/S0006-8993(00)02301-5.
- Kemp, C.-M.; Parra, T. The Effect of Recombinant Caspase 3 on Myofibrillar Proteins in Porcine Skeletal Muscle. Animal 2008, 2, 1254–1264. DOI: 10.1017/S1751731108002310.
- Vanags, D.; PornAres, M.-I.; Coppola, S.; Burgess, D.-H.; Orrenius, S. Protease Involvement in Fodrin Cleavage and Phosphatidylserine Exposure in Apoptosis. Journal of Biological Chemistry 1996, 271, 31075–31085. DOI: 10.1074/jbc.271.49.31075.
- Waterhouse, N.-J.; Finucane, D.-M.; Green, D.-R.; Elce, J.-S.; Kumar, S.; Alnemri, E.-S.; Litwack, G.; Khanna, K.; Lavin, M.-F.; Watters, D.-J. Calpain Activation Is Upstream of Caspases in Radiation-Induced Apoptosis. Cell Death and Differentiation 1998, 5, 1051–1061. DOI: 10.1038/sj.cdd.4400425.
- Huang, F.; Huang, M.; Zhang, H.; Guo, B.; Zhang, D.; Zhou, G. Cleavage of the Calpain Inhibitor, Calpastatin, during Postmortem Ageing of Beef Skeletal Muscle. Food Chemistry 2014, 148, 1–6. DOI: 10.1016/j.foodchem.2013.10.016.
- Zhang, L.; Shen, H.; Luo, Y. A Nondestructive Method for Estimating Freshness of Freshwater Fish. European Food Research and Technology 2011, 232, 979–984. DOI: 10.1007/s00217-011-1467-z.
- Quadrilatero, J.; Rush, J.-W. Increased DNA Fragmentation and Altered Apoptotic Protein Levels in Skeletal Muscle of Spontaneously Hypertensive Rats. Journal of Applied Physiology 2006, 101, 1149–1161. DOI: 10.1152/japplphysiol.00194.2006.
- Zhang, X.; Pan, D.; Cao, J.; Wu, Z. Changes in the Major Caspases Involved in Cytoskeletal Degradation of Goose Muscle during Prolonged Aging. Food Research International 2013, 51, 603–610. DOI: 10.1016/j.foodres.2013.01.015.
- Kemp, C.-M.; Bardsley, R.-G.; Parr, T. Changes in Caspase Activity during the Postmortem Conditioning Period and Its Relationship to Shear Force in Porcine Longissimus Muscle. Journal of Animal Science 2006, 84, 2841–2846. DOI: 10.2527/jas.2006-163.
- Nieminen, A.-L. Apoptosis and Necrosis in Health and Disease: Role of Mitochondria. International Review of Cytology - A Survey of Cell Biology 2003, 224, 29–55. DOI: 10.1016/S0074-7696(05)24002-0.
- Du, M.; Shen, Q.-W.; Zhu, M.-J. Role of Beta-Adrenoceptor Signaling and AMP-activated Protein Kinase in Glycolysis of Postmortem Skeletal Muscle. Journal of Agricultural and Food Chemistry 2005, 53, 3235–3239. DOI: 10.1021/jf047913n.
- Henckel, P.; Karlsson, A.; Jensen, M.-T.; Oksbjerg, N.; Petersen, J.-S. Metabolic Conditions in Porcine Longissimus Muscle Immediately Pre-Slaughter and Its Influence on Peri- and Post Mortem Energy Metabolism. Meat Science 2002, 62, 145–155. DOI: 10.1016/S0309-1740(01)00239-X.
- Green, D.-R. Apoptotic Pathways: Ten Minutes to Dead. Cell 2005, 121, 671–674. DOI: 10.1016/j.cell.2005.05.019.
- Boatright, K.-M.; Salvesen, G.-S. Mechanisms of Caspase Activation. Current Opinion in Cell Biology 2003, 15, 725–731. DOI: 10.1016/j.ceb.2003.10.009.
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP Levels Determine Cell Death Fate by Apoptosis or Necrosis. Cancer Research 1997, 57, 1835–1840.
- Cao, J.; Yu, X.; Khan, M.-A.; Shao, J.; Xiang, Y.; Zhou, G. The Effect of Calcium Chloride Injection on Shear Force and Caspase Activities in Bovine Longissimus Muscles during Postmortem Conditioning. Animal 2011, 6, 1018–1022. DOI: 10.1017/S1751731111002047.
- Huang, F.; Huang, M.; Zhang, H.; Zhang, C.; Zhang, D.; Zhou, G. Changes in Apoptotic Factors and Caspase Activation Pathways during the Postmortem Aging of Beef Muscle. Food Chemistry 2016, 190, 110–114. DOI: 10.1016/j.foodchem.2015.05.056.
- Fujimura, M.; Morita-Fujimura, Y.; Murakami, K.; Kawase, M.; Chan, P.-H. Cytosolic Redistribution of Cytochrome C after Transient Focal Cerebral Ischemia in Rats. Journal of Cerebral Blood Flow and Metabolism 1998, 18, 1239–1247. DOI: 10.1097/00004647-199811000-00010.
- Kluck, R.-M.; Bossy-Wetzel, E. The Release of Cytochrome C from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis. Science 1997, 275, 1132–1136. DOI: 10.1126/science.275.5303.1132.
- Earnshaw, W.-C.; Martins, L.-M.; Kaufmann, S.-H. Mammalian Caspases: Structure, Activation, Substrates, and Functions during Apoptosis. Annual Review of Biochemistry 1999, 68, 383–424. DOI: 10.1146/annurev.biochem.68.1383.
- Ouali, A.; Herrera-Mendez, C.-H.; Coulis, G.; Becila, S.; Boudjellal, A.; Aubry, L.; Sentandreu, M.-A. Revisiting the Conversion of Muscle into Meat and the Underlying Mechanisms. Meat Science 2006, 74, 44–58. DOI: 10.1016/j.meatsci.2006.05.010.
- Kiang, J.-G.; Tsen, K.-T. Biology of Hypoxia. Chinese Journal of Physiology 2006, 49, 223–233.
- Mishra, O.-P.; Randis, T.; Ashraf, Q.-M.; Delivoria-Papadopoulos, M. Hypoxia-Induced Bax and Bcl-2 Protein Expression, Caspase-9 Activation, DNA Fragmentation, and Lipid Peroxidation in Mitochondria of the Cerebral Cortex of Newborn Piglets: The Role of Nitric Oxide. Neuroscience 2006, 141, 1339–1349. DOI: 10.1016/j.neuroscience.2006.05.005.
- Zou, H.; Henzel, W.-J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1,A Human Protein Homologous to C.Elegans CED-4, Participates in Cytochrome c–Dependent Activation of Caspase-3. Cell 1997, 90, 405–413. DOI: 10.1016/S0092-8674(00)80501-2.
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple Pathways of Cytochrome C Release from Mitochondria in Apoptosis. Biochimica Et Biophysica Acta 2006, 1757, 639–647. DOI: 10.1016/j.bbabio.2006.03.016.
- Mizuta, T.; Shimizu, S.; Matsuoka, Y.; Nakagawa, T.; Tsujimoto, Y. A Bax/Bak-independent Mechanism of Cytochrome C Release. Journal of Biological Chemistry 2007, 282, 16623–16630. DOI: 10.1074/jbc.M611060200.
- Gross, A.; McDonnell, J.-M.; Korsmeyer, S.-J. BCL-2 Family Members and the Mitochondria in Apoptosis. Genes & Development 1999, 13, 1899–1911. DOI: 10.1101/gad.13.15.1899.
- Haghir, H.; Varasteh, A.; Ahmadpour, S.; Sankian, M.; Anarkooli, I.-J. Evaluation of Bcl-2 Family Gene Expression and Caspase-3 Activity in Hippocampus STZ-Induced Diabetic Rats. Journal of Diabetes Research 2008, 2008, 638467. DOI: 10.1155/2008/638467.
- Renault, -T.-T.; Floros, K.-V.; Chipuk, J.-E. BAK/BAX Activation and Cytochrome C Release Assays Using Isolated Mitochondria. Methods 2013, 61, 146–155. DOI: 10.1016/j.ymeth.2013.03.030.
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.-H.; Sentandreu, M.-A. Biomarkers of Meat Tenderness: Present Knowledge and Perspectives in Regards to Our Current Understanding of the Mechanisms Involved. Meat Science 2013, 95, 854–870. DOI: 10.1016/j.meatsci.2013.05.010.