ABSTRACT
This study was conducted to characterize the lipid fraction of 15 chia seed samples originating from five countries (Argentina, Paraguay, Uganda, Bolivia, and Peru). On average, chia seeds contained 34.5 g oil per 100 g dry-solids, in which the average contents of sterols, tocopherols, squalene, carotenoids, and phenolic compounds were 7,061, 600, 17.7, 2.2, and 9.7 mg/kg of oil, respectively. Alpha-linolenic acid share varied from 54.35% to 60.48%, and was accompanied by declining shares of linoleic, palmitic, oleic, and stearic acid, respectively. Principal component analysis showed that chia oil induction time was positively correlated with tocopherols and phenols, while negatively with quality indices and squalene content.
Introduction
Salvia hispanica L., commonly known as chia, is a plant native to Mexico and Guatemala[Citation1] and widespread in late pre-Columbian time also to Honduras and Nicaragua.[Citation2,Citation3] The Spanish conquest drastically limited the cultivation of chia and this prohibition was the highest between 1550 and 1810.[Citation3] Due to confirmation of the high nutritional value of chia seeds, a renaissance of this plant was observed. In 1991, a research project was started called the “Western Argentina Regional Project¨ which integrated chia in modern cultivation[Citation4] and resulted in an increase in the worldwide area cultivated for chia from approximately 450 ha per year or less (only in Mexico) in 1994[Citation5] to 370,000 ha in 13 countries in 2014.[Citation3] At present, the main cultivation areas of chia are in Bolivia, Ecuador, Peru, Paraguay, Ghana, Colombia, Thailand, the United States and Australia.[Citation3] The biggest issue facing the USA and other countries is that chia is cultivated in a tropical area with short day lengths and only in agricultural zones between 20°55ʹN to 25°05ʹ S,[Citation4] and at higher latitudes, the plant cannot produce seeds.[Citation6] For this reason, the new varieties adapted to days longer than 12.5 h were developed and registered. This resulted in an increase in chia cultivation area in Argentina from 100 ha in 2010 to 120,000 ha in 2014.[Citation3]
Currently, chia seeds are popular components of various functional food products all over the world. However, in the European Union, their use is restricted by the European Food Safety Authority (EFSA) since chia is recognized as a “novel food”. This term means a food or ingredient that was not consumed to a significant degree in the EU prior to May 15, 1997.[Citation7] According to EFSA recommendations, chia seeds can be added up to 10% to bread and bakery products, breakfast cereals, fruit, nut, and seed mixtures and sold as seeds with a recommended daily intake of up to 15 g. In 2015, the use of chia seeds for fruit juices and fruit juice mixtures in the amount of 15 g per 450 ml was allowed. Currently, EFSA proceeds application by The Ministry of Agriculture of the Czech Republic, which deals with the extension of the use of chia seeds in fermented milk products flavoured with fruit components.[Citation8]
High market interest in chia is due to the special, valuable chemical composition of the seeds. They contain up to 39% fat[Citation5] with an extremely high share (44–69%) of α-linolenic acid.[Citation1,Citation9] Chia seeds are also rich in protein (up to 25%) and crude fibre (up to 30%), which prevails among chia carbohydrates.[Citation10] This indicates that chia seeds can be declared as a source of n-3 fatty acid and/or dietary fibre and that their consumption can balance the n-6/n-3 fatty acid ratio in diet and deliver a significant amount of dietary fibre. Chia seeds also contain significant amounts of other valuable compounds such as minerals (mainly potassium, calcium, magnesium, phosphorus) and vitamins (mainly A, C, E, B1, B2, B3, B6).[Citation10]
One of the primary chia-derived products is chia oil, which is used as a food, pharmaceutical and cosmetic ingredient. However, reports on its characteristics differ widely both in fatty acid composition and in accompanying lipophilic phytochemicals, which are co-extracted when obtaining chia oil. Good examples of this variability can be phytosterols (determined content from ca. 4,000 to 12,600 mg/kg of oil),[Citation11,Citation12] tocopherols (from ca. 150 to 750 mg/kg of oil)[Citation11,Citation13–Citation15] and carotenoids (from ca. 0.5 to 11.6 mg/kg of oil).[Citation9,Citation13] These differences are caused both by the varied origin of the examined chia seeds (South America, Italy, Australia) and the extraction procedures employed (Soxhlet with hexane or petroleum ether, Folch mixture, pressing, supercritical extraction with CO2 and subcritical extraction with n-propane). For example, in the author’s previous study, it was found that the final characteristic of chia oil is highly related to the extraction method, which mostly affects polyphenols and squalene content and oil oxidative stability.[Citation15] Unfortunately, the use of different techniques for oil extraction makes it difficult to compare the results of chia oil composition of different geographical origin in cited studies.
The main aim of this study was to evaluate the content and composition of lipid fraction of 15 batches of chia seeds available on market in Poland originating from five countries (Argentina, Paraguay, Uganda, Bolivia, and Peru) which vary in cultivation areas and latitudes – from ca. 4°N (Uganda) to ca. 24°S (Argentina). To the best of our knowledge, it is a first study, which compares the characteristic of chia seed oils originated from such diversified cultivation regions obtained by the same technique of extraction. Additionally, the relationships between determined lipophilic compounds and oil quality and oxidative stability were also determined.
Material and methods
The material in this study constituted chia seeds available on the local market in Olsztyn (Poland). The countries of origin declared cultivation method and permitted shelf life are presented in .
Table 1. Seed material used in the study.
Oil extraction
Before oil extraction, chia seeds were aligned in moisture by storing 48 h at ca. 20°C and 65% of relative air humidity. Next, they were carefully ground in an A20 laboratory mill (IKA-Werke, Staufen, Germany) and transferred to a Soxhlet thimble. Lipids were extracted and determined by the Soxhlet method with hexane according to Polish Standard PN-EN ISO 659:2010.[Citation16] Extraction was repeated 3-fold for each seed sample. The obtained oils were used in all further determinations.
Determination of fatty acid composition
Before analysis, fatty acid methylation was carried out according to the method described by Zadernowski and Sosulski.[Citation17] The obtained methyl esters were analysed by gas chromatography with mass spectrometry using a GC-MS QP2010 PLUS (Shimadzu, Kyoto, Japan) system according to the parameters described by Czaplicki et al.[Citation18] Separation was performed on a BPX70 (25 m × 0.22 mm × 0.25 µm) capillary column (SGE Analytical Science, Victoria, Australia) with helium as a carrier gas at a flow rate of 1.3 mL/min. The column temperature was programmed as follows: a subsequent increase from 150°C to 180°C at the rate of 10°C/min, to 185°C at the rate of 1.5°C/min, to 250°C at the rate of 30°C/min, and then a 10-min hold. The interface temperature of GC-MS was set at 240°C. The temperature of the ion source was 240°C and the electron energy was 70 eV. The total ion current (TIC) mode was used in the 50–500 m/z range.
Determination of phytosterols and squalene
The content of sterols and squalene was determined by gas chromatography coupled with mass spectrometry (GC-MS QP2010 PLUS, Shimadzu, Kyoto, Japan) according to the method described by Vlahakis and Hazebroek.[Citation19] After saponification and extraction, compounds were re-dissolved in 1.5 mL of n-hexane and a 0.2 mL 5α-cholestane (Sigma-Aldrich, Poznań Poland) internal standard solution was added (0.83 mg/mL). After evaporation, the residues were re-dissolved in 100 µL of pyridine and 100 µL BSTFA (N,O-bis (trimethylsilyl) trifluoroacetamide) with 1% TMCS (trimethylchlorosilane) and left at 60°C for 60 min to complete derivatization. After silylation, the compounds were separated on a ZB-5MSi (Phenomenex Inc., Torrance, CA, USA) capillary column with helium as a carrier gas (0.9 mL/min). The injector temperature was set at 230°C and the column temperature was programmed as follows: 70°C for 2 min, a subsequent increase to 230°C at the rate of 15°C/min, to 310°C at the rate of 3°C/min, and then a 10-min hold. The interface temperature of GC-MS was set at 240°C. The temperature of the ion source was 220°C and the electron energy was 70 eV. The total ion current (TIC) mode for quantification (100–600 m/z range) was used. The quantifications using an internal standard method were then conducted.
Determination of tocopherols
Analysis of the content of tocopherols was carried out using high-performance liquid chromatography with fluorescence detection (HPLC-FLD), according to the method described by Czaplicki et al.[Citation20] The oil–in–n-hexane solution (1%, m/v) was injected into the chromatographic system. The analysis was performed using a 1200 series liquid chromatograph manufactured by Agilent Technologies (Palo Alto, CA, USA), equipped with a fluorescence detector. The separation was done on a Merck LiChrospher Si 60 column, 250 mm × 4 mm, 5 µm. A 0.7% isopropanol solution in n-hexane at a 1 mL/min flow rate was used as a mobile phase. The fluorescence detector was set at 296 nm for excitation and 330 nm for emission. Peaks were identified based on retention times determined for α, β, γ, and δ tocopherol standards (Merck, Darmstadt, Germany) separately, and their content was calculated using external calibration curves.
Determination of carotenoids
Carotenoids were analysed using reversed-phase high-performance liquid chromatography (RP-HPLC) technique according to Czaplicki et al.[Citation18] Briefly, the analysis was carried out using a 1200 series liquid chromatograph of Agilent Technologies (Palo Alto, CA, USA), equipped with a diode array detector (DAD) from the same manufacturer. Separation was performed at 30°C on a YMC-C30 150 × 4.6 mm, 3 µm column and YMC-C30 10 × 4.6 mm, 3 µm precolumn (YMC-Europe GmbH, Dinslaken, Germany). The binary mobile phase consisted of methanol (solvent A) and methyl tert-butyl ether (MTBE) (solvent B). The solvent gradient was as follows: 0–5 min, 95% A, 1 mL/min; 25 min, 72% A, 1.25 mL/min; 33 min, 5% A, 1.25 mL/min; 40–60 min, 95% A, 1 mL/min. The absorbance was measured at the wavelength of 450 nm. Compounds were identified based on retention times of commercially available standards (Sigma-Aldrich, Poznań, Poland) and their content was calculated using an external calibration curve prepared for β-carotene.
Determination of total phenolic compounds
Phenolic compounds were isolated using 4-fold methanolic (80%, v/v) extraction. Their content was determined spectrophotometrically according to the method described by Czaplicki et al.,[Citation20] with the Folin–Ciocalteu phenol reagent (Sigma–Aldrich, Poznań, Poland). Briefly, the extract was evaporated to dryness using a type R210 rotary evaporator. 0.25 mL of Folin–Ciocalteu reagent solution was then added to the dry residue, together with 1.5 mL of 14% (m/v) sodium carbonate water solution and 3.25 mL of deionized water. After 1 h, the absorbance of reaction mixtures was measured at 720 nm using a UV-8000S type spectrophotometer (Shanghai Metash Instruments, Shanghai, China) against a blank sample. The phenolic compound content was calculated based on the D-catechin (Sigma–Aldrich, Poznań, Poland) calibration curve.
Determination of chia oil quality
The acid, peroxide, and p-anisidine values were determined in accordance with procedures of CEN ISO 660:2010,[Citation21] CEN ISO 3960:2012[Citation22] and CEN ISO 6885:2006,[Citation23] respectively. The content of conjugated dienes and trienes was determined according to the AOCS method.[Citation24]
Determination of induction time
The induction time of oils was tested on a Rancimat apparatus 743 (Metrohm, Herisau, Switzerland). The analysis was performed according to the method described in PN-EN ISO 6886:2016–04[Citation25] standard. Briefly, 2.5 g of oil in a reaction vessel was weighed and, after capping, the vessel was placed in a thermostated electric heating block at a temperature of 110°C. An airflow rate of 20 L/h was provided. Determination of the induction time was based on the conductometric detection of volatile oxidation products. The time that elapsed until these oxidation products appeared was saved as the induction time.
Statistical methods
Statistical analysis of the results was performed using STATISTICA version 12.5 (StatSoft, Kraków, Poland). Principal components analysis (PCA) and analysis of variance (ANOVA) with Tukey’s test for homogenous groups were also conducted (all analyses at the significance level p ≤ 0.05).
Results and discussion
Oil content and fatty acid composition of chia oils
The chia seeds only slightly varied regarding fat content (33.7–35.8 g/100 g of dry basis), with an average value of 34.5 g/100 g dry basis (). The variability between samples of various origin was relatively low and slightly exceed 2%. As expected, α-linolenic acid prevailed among fatty acids, reaching a medium share of 55.84% with variation from 54.35% (Uganda No 10) to 60.48% (Argentina No 5). Chia seeds also contained linoleic, palmitic, oleic and stearic acids, listed in descending order of shares: 22.34%, 8.68%, 8.61%, and 3.95%, respectively. The oils also contained 4 unidentified minor fatty acids, with overall share from 0.27 to 0.87% of total fatty acids. In the case of major fatty acids the variability between samples of various origin was the highest for stearic (CV = 14.11%) and oleic acid (CV = 8.64%), while the lowest for linoleic acid (CV = 2.43%). The average n-6/n-3 fatty acid ratio was 0.40, with variation from 0.36 to 0.42.
Table 2. Oil recovery from chia seeds and the fatty acid composition of chia seed oils.
The content of oil in chia seeds determined in the present study overlapped with values 30–35% summarized in the EFSA report.[Citation10] However, Ayerza[Citation26] showed that cultivation location highly affects oil content and, for a single genotype, it can vary from 26.0% to 33.5%. The highest value can be obtained for cultivation areas with a medium year temperature of 15–17°C, rainfall of 300–560 mm and elevation of 1,156–2,200 m, while higher temperature and rainfall, followed by cultivation at lower altitudes, diminished oil deposition.[Citation27] Although, chia seeds/oils are recommended as one of the richest plant sources of α-linolenic acid in the human diet (besides flax, perilla and cannabis sativa seeds), the composition of fatty acids in chia oil can be highly variable. Alfa-linolenic acid is always predominant, but its share varies from ca. 44% to 66%.[Citation12,Citation15,Citation26–Citation28] The share of linoleic acid varies from 16.6% to 30.5% and is negatively correlated with the share of α-linolenic.[Citation26,Citation27] In the case of minor fatty acids such as palmitic, oleic and stearic acid, their shares can vary from 5.5% to 14.0%, from 3.9% to 11.1% and from 2.4% to 4.4%.[Citation1,Citation9,Citation26] Chia oil can also contain trace amounts of saturated fatty acids of carbon chain length from C14 to C24, monounsaturated from C16 to C20, and trans fatty acids (C18:2 and C18:3), which in summary consist up to 1.5% of total fatty acids.[Citation29] In general, α-linolenic acid share is increased in seeds cultivated in areas which favour oil accumulation (relatively cold, dry and of high elevation) and this explains the relatively low share of this fatty acid in seeds originating from Uganda. However, the extraction method can also be decisive. Ixtaina et al.[Citation1] showed that variation in SC-CO2 extraction parameters (temperature, duration, pressure) can change the share of α-linolenic acid (from 44.4% to 63.4%). In the cited study, a significant effect of extraction time on the percentage of linoleic and linolenic acids and n-6/n-3 relationship was found. There was an increase in linolenic acid at increasing extraction times whereas higher levels of linoleic acid were observed at intermediate times.[Citation1] Such impacts should be taken into consideration during the choice of seed origin or method of oil extraction, since a high share of α-linolenic acid is crucial for the pro-healthy value of chia seeds. Alfa-linolenic acid is a precursor of the long-chain n-3 fatty acids: docosahexaenoic acid (DHA) and eicosapentaenoic (EPA), which are linked to various benefits, including cardiovascular and neurological health.[Citation30]
Sterols in chia oils
The sterol content varied from 6,653 (Peru No 13) to 7,665 mg/kg of oil (Paraguay No 6), with an average value of 7,061 mg/kg (). Among them, β-sitosterol prevailed with a share of ca. 55%. Chia oils also contained campesterol, 25-hydroxy-24-methylcholesterol, and stigmasterol (with average shares of 10.8%, 8.9%, and 4.2%, respectively). Additionally, approximately 20.7% of sterol-like compounds were not identified in this study. In general, sterol content and composition were relatively slightly varied by seed origin, with CV value not exceeding 5.18%.
Table 3. Phytosterol contents (mg/kg) in chia seed oils.
Scientific references to sterol content in chia oil are scarce and inconclusive. Álvarez-Chávez et al.[Citation12] determined their content in oil from seeds originating from Mexico (states of Jalisco and Sinaloa) as 12,600 and 8,159 mg/kg, respectively. In contrast, Ciftci et al.[Citation11] found these compounds in amounts 2–3-fold lower (4,132 mg/kg of oil from seeds cultivated in Peru). Even lower values (up to 3,300 mg/kg oil) were found by Zanqui et al.[Citation14] This suggests that the typical sterol content in chia oil is still uncertain. However, it is worth noting that the sterol content is highly dependent on the method of oil extraction. For example, Zanqui et al.[Citation14] found these compounds in amounts from 1,610 to 3,300 mg/kg of chia oil in relation to the method of oil extraction (the lowest was found in oil after subcritical extraction with n-propane, while the highest was in oil extracted by the Soxhlet procedure). According to Dąbrowski et al.,[Citation15] extraction-dependent (solvent extraction, pressing, SC-CO2 extraction) variability of sterol content in chia oil varies in a significantly lower range (from 4,794 to 5,522 mg/kg).
Besides the high variability of sterols content in chia oil, there is also still an inconsistency regarding their composition. In the present study, four compounds were identified: β-sitosterol (ca. 55%), campesterol (ca. 11%), 25-hydroxy-24-methylcholesterol (ca. 9%), and stigmasterol (ca 4%), with an additional share of ca. 21% of unidentified homologues. Ciftci et al.[Citation11] found that the share of β-sitosterol (ca. 50%) and campesterol (ca. 11%) was similar, while these sterols were accompanied by a significantly higher share of stigmasterol (30%) and a 9% share of ∆5-avenasterol. In contrast, in a study by Zanqui et al.[Citation14] β-sitosterol was predominant, reaching ca. 82%, followed by ca. 12% of campesterol and ca. 6% of stigmasterol. In turn, Álvarez-Chávez et al.[Citation12] identified only β-sitosterol (56–64%), stigmasterol (14– 17%), and stigmastanol (23–27%), while Bodoira et al.[Citation31] found ca. 60% of β-sitosterol, ca. 10% of campesterol, and ca. 30% of 4,6-cholestadien-3β-ol. Summarizing these results, the data indicate that chia oils contain ca. 50–80% of β-sitosterol and probably ca. 10% of campesterol. In the case of the other sterols (25-hydroxy-24-methylcholesterol, ∆5-avenasterol, stigmastanol, or 4,6-cholestadien-3β-ol), a conclusive study on their identification and quantification is still needed.
Tocopherols in chia oils
The results presented in show that the content of tocopherols in chia oils was highly variable, ranging from 129 mg/kg (Argentina No 1) to 735 mg/kg oil (Uganda No 10), with an average value of 600 mg/kg of oil. Among them, γ-tocopherol accounted for ca. 92% of the total, with an additional contribution of α- (ca. 5%) and δ-homologues (ca. 3%). Sample No 1 (Argentina) was unique in this regard, with an extremely low amount of total tocopherols (3–6-fold lower than in remaining samples) and an absence of α-tocopherol. Unlike fatty acids and sterols, the tocopherols content varied depending on the seed origin, with inter-sample variation of 30.02%, 27.45% and 10.47%, for α-, γ-, and δ-tocopherol, respectively.
Table 4. Tocopherol contents (mg/kg) in chia seed oils.
Only a few previous studies have focused on tocopherols in chia oils. Ixtaina et al.[Citation9] determined their content from 238 to 427 mg/kg oil; Ciftci et al.[Citation11] as 446 mg/kg; Zanqui et al.[Citation14] from 150 to 273 mg/kg of oil; Amato et al.[Citation13] from 472 to 510 mg/kg; Dąbrowski et al.[Citation15] from 498 to 739 mg/kg, and Bodoira et al.[Citation31] as 717 mg/kg. This indicates, in summary, that the tocopherol content can vary from 130 to 740 mg per kg of chia oil and this variability is highly dependent on the oil extraction method.[Citation14,Citation15] Current study complements this knowledge, showing that the content of these compounds varies depending on the origin of the seeds, with a determined range from 129 to 735 mg/kg of oil. It is worth noting that all cited studies found γ-tocopherol to be the main homologue (share from 91% to 99%), with only a small contribution of other homologues.
Minor chia oil components: squalene, carotenoids, and polyphenols
Squalene content varied from 11.08 (Paraguay No 7) to 29.86 mg/kg chia oil (Argentina No 2), with an average value of 17.67 mg/kg (). To the best of the authors’ knowledge, only our studies have found this compound in chia oil. In a previous study by Dąbrowski et al.,[Citation15] squalene content reached values of 58–99 mg/kg in relation to the method of extraction. The highest concentrations were determined in oils obtained by SC-CO2 extraction conducted at 70°C (ca. 99 mg/kg) and by Soxhlet extraction using acetone (ca. 91 mg/kg). However, the results of the present study with a higher number of seed samples showed that squalene content in typical chia oil seems to be closer to 20 mg/kg. For such a squalene value, the extraction procedure can change this value in the range of 18.7–63.5 mg/kg.[Citation32]
Table 5. Polyphenol, carotenoid, and squalene contents in chia seed oils (mg/kg).
In tested chia oils, carotenoids were minor phytochemicals and accounted for 2.20 mg/kg, with variation from 1.34 (Paraguay No 8) to 3.98 mg/kg (Bolivia No 11). Previous studies showed that these compounds in concentrations from 0.53[Citation9] to 11.6 mg/kg.[Citation13] Similar to the previously discussed compounds, the carotenoid content in chia oil also depends on the extraction method, with possible variation from 4.10 to 8.40 mg/kg.[Citation15] Chia carotenoid composition is also still unknown. The authors’ previous study with the use of supercritical extraction with CO2 enriched with acetone showed that, among them, ca. 2/3 constitutes lutein, with an approximately 30% share of β-carotene and small amounts of 9-cis-β-carotene.[Citation32] However, the present study does not confirm this finding, since β-carotene was found as the main representative in all 15 various samples, with its share from 27 (Argentina No 5) to 79% (Argentina No 4). Besides this compound, 14 samples contained detectable amounts of lutein and 6 samples of 9-cis-β-carotene. Additionally, chia oils contained two or three unidentified carotenoid-like compounds.
The polyphenol content in chia oils ranged from 0.35 (Argentina No 2) to 25.32 mg/kg oil (Paraguay No 9), with an average value of 9.66 mg/kg. Despite the high impact on oil oxidative stability, polyphenols are rarely assayed in chia oils. Amato et al.[Citation13] determined only traces of these compounds in chia oils, Bodoira et al.[Citation31] determined ca. 42 mg/kg, while Oliveira-Alves et al.[Citation33] the level of 20 mg/kg. The authors’ previous results showed that the method of oil extraction is crucial. By changing the procedure/type of extraction, the concentration of polyphenols in oil can vary in the range of 0–172 mg/kg.[Citation15] The cited study showed that polyphenols can be preferentially extracted with the use of acetone. However, the relatively high variation of phenolic compounds in oils extracted in this study by the same manner (Soxhlet procedure with hexane use) is difficult to explain. It is thought that part of the polyphenols, especially phenolic acids like caffeic, chlorogenic, and rosmarinic found in chia seeds[Citation34] become lipid soluble after release from lignocellulose matrix by specific esterases.[Citation35] On the other hand, phenolic acids and other chia phenols like myricetin, quercetin and kaempferol[Citation29,Citation36] can be derivatized to more polar compounds of poorer solubility in oil. It may explain the phenomenon of sample No 1, characterized by the highest rancidity indices (discussed in the next section) with the lowest content of phenolic compounds.
Quality indices of chia oils and their correlations with phytochemical content
The quality indices () of chia oils differed widely in regard to acid (AV), peroxide (PV) and p-anisidine (p-AV) values and the contents of conjugated dienes (K232) and trienes (K270). In summary, the variation of AV ranged from 1.56 to 25.69 mg KOH/g of oil; PV ranged from 1.60 to 7.30 mEq O2/kg of oil; p-AV ranged from 0.53 to 14.59 and conjugated dienes and trienes ranged from 0.157% to 0.344% and 0.000% to 0.013%, respectively. Codex Alimentarius Commission for cold-pressed and virgin oils recommended a maximum level of 4 mg KOH/g and 15 mEq O2/kg (Codex Stan 210–1999 and 211–1999). Among the tested chia seeds, samples coded 1, 4, 8, and 9 (originating from Argentina and Paraguay) exceeded the recommended level of fatty acidity. Although oxidative deterioration was at an acceptable level, two samples (coded 1 and 8) were relatively abundant in aldehydes, ketones (products of hydroperoxide decomposition) and conjugated fatty acids (products of oxidative migration of double bonds). The variability of these oil indices resulted in high variation of induction times between 0.14 (Argentina No 1) and 3.45 h (Uganda No 10), which pointed to a ca. 25-fold difference in the oxidative stability of these two chia oils. A previous study showed that chia oil quality can be differentiated by seed origin (Mexican, Argentinian or Guatemalan seeds), with a variation of peroxide value from 1.64 to 17.5 mEq O2/kg oil and an acidity value from 1.64 to 2.05 mg KOH/g of oil.[Citation37] Much higher acidity of fresh-pressed chia oil from seeds cultivated in Argentina (0.13 g of oleic acid/g oil), accompanied by a unique lack of primary oxidation products (not detected PV) was determined by Bodoira et al.[Citation31] This indicates that cultivation conditions and post-harvest seed treatments can probably be decisive in maintaining the quality of the lipid fraction of chia seeds. The main reason for the high acid value of chia oil can be the prolonged time of activity of native seed lipases.[Citation38]
Table 6. Quality features of chia seed oils.
The correlations between chia oil quality indices and the total content of determined lipophilic compounds are presented in . As expected, the induction time of oil was positively correlated with the total content of polyphenols and tocopherols. Recently, strong correlations between induction time and total phenolics in various oils were determined, e.g. by Dąbrowski et al.,[Citation15] Gruzdiene and Anelauskaite,[Citation39] Farhoosh et al.[Citation40] and Roszkowska et al.[Citation41] Similarly, tocopherols are known as the main antioxidants of vegetable oils, although their antioxidant activity is highly dependent on the concentration, relative proportions of isomers and oxidation/storage temperature.[Citation40,Citation42] In general, the highest antioxidant activity was determined for δ and γ forms[Citation43,Citation44] which prevail in chia oils.
Figure 1. Two-dimensional graph for a PCA (correlation circle for the composition and quality features of chia seed oils).
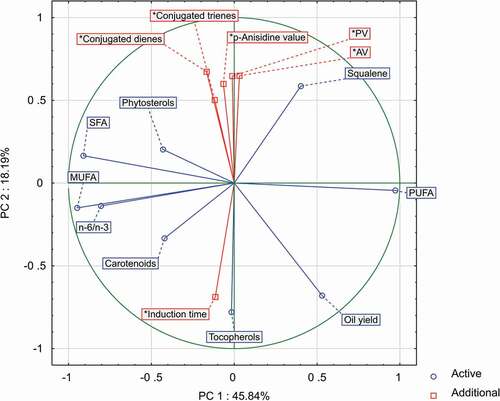
In contrast, the main negative correlations () were found between chia oil induction time and all indices of initial oil deterioration (AV, PV, K232, and K270). Increased concentration of free fatty acids favours oxidation,[Citation45,Citation46] while an increased content of hydroperoxides and conjugates of fatty acids indicates the propagation stage of oxidation. As a result, oils with initial high values of hydrolytic and oxidative deterioration are of significantly lower shelf life.[Citation47] The present study also found a negative correlation of chia oil induction time with squalene content. Although squalene is considered to be a potentially strong antioxidant, it mainly acts as a quencher of singlet oxygen.[Citation48] However, in the case of the used Rancimat test (low light impact and sensitizer concentration), oxidation proceeded mainly by triplet oxygen (in this case, squalene can be less active). Additionally, the observed correlation could be concentration-related, since in chia oil squalene was only a minor ingredient. In comparison, the best plant sources of squalene with high oxidative resistance are olive oil (1700–4600 mg/kg)[Citation49] and amaranthus oil (10400–73000 mg/kg).[Citation50]
Conclusion
The results of this study showed significant differences between chia seeds available on market. The mostly variable was oil quality, with some samples characterized by rancidity indices exceeding the limits recommended by Codex Alimentarius Commission. It is supposed that the observed deterioration of fatty acids proceeded at the stage of seed production or storage before market distribution. It indicates that more scientific attention should be paid to processes related to harvesting, drying, and storage of chia seeds. This can help to protect seeds against the activity of lipases, which open routes for other enzymes and undesirable oxidation processes.
Acknowledgement
We thank Izabela Lotkowska for helpful technical support in laboratory.
Additional information
Funding
References
- Ixtaina, V. Y.; Vega, A.; Nolasco, S. M.; Tomás, M. C.; Gimeno, M.; Bárzana, E.; Tecante, A., Supercritical Carbon Dioxide Extraction of Oil from Mexican Chia Seed (Salvia Hispanica L.): Characterization and Process Optimization. The Journal of Supercritical Fluids 2010, 55, 192–199. DOI: 10.1016/j.supflu.2010.06.003.
- Jamboonsri, W.; Phillips, T. D.; Geneve, R. L.; Cahill, J. P.; Hildebrand, D. F., Extending the Range of an Ancient Crop, Salvia Hispanica L.-A New Ω3 Source. Genetic Resources and Crop Evolution 2012, 59, 171–178. DOI: 10.1007/s10722-011-9673-x.
- Sosa, A.;. Chia Crop (Salvia Hispanica L.): Its History and Importance as A Source of Polyunsaturated Fatty Acids Omega-3 around the World: A Review. Journal of Crop Researcher Fertility. 2016, 1–9. DOI:10.17303/jcrf.2016.104.
- Al-Bakri. Assessment of Oil Quantification Methods in Soybean and Chia Seeds and Characterization of Oil and Protein in Mutant Chia (Salvia Hispanica L.) Seeds. https://uknowledge.uky.edu/cgi/viewcontent.cgi?referer=http://www.google.pl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=45&ved=0ahUKEwi6psjAzcDYAhW0hKYKHfxmC2w4KBAWCEkwBA&url=http%3A%2F%2Fuknowledge.uky.edu%2Fcgi%2Fviewcontent.cgi%3Farticle%3D1100%26context%3D (accessed Dec 20, 2017).
- Ayerza, R.;, Oil Content and Fatty Acid Composition of Chia (Salvia Hispanica L.) From Five Northwestern Locations in Argentina. Journal of the American Oil Chemists’ Society 1995, 72, 1079–1081. DOI: 10.1007/BF02660727.
- Ayerza, R.; Coates, W. Chia: Rediscovering a Forgotten Crop of the Aztecs; University of Arizona Press: Tucson, 2005.
- European Parliament and the Council. Applications under Regulation (EC) N° 258/97 of the European Parliament and of the Council. https://ec.europa.eu/food/safety/novel_food_en (accessed Sep 12, 2017).
- European Commission. Novel Food. https://ec.europa.eu/food/sites/food/files/safety/docs/novel-food_applications-status_en.pdf (accessed Sep 12, 2017).
- Ixtaina, V. Y.; Martínez, M. L.; Spotorno, V.; Mateo, C. M.; Maestri, D. M.; Diehl, B. W. K.; Nolasco, S. M.; Tomás, M. C., Characterization of Chia Seed Oils Obtained by Pressing and Solvent Extraction. Journal of Food Composition and Analysis 2011, 24, 166–174. DOI: 10.1016/j.jfca.2010.08.006.
- EFSA. Opinion on the Safety of ‘Chia Seeds (Salvia Hispanica L.) And Ground Whole Chia Seeds’ as a Food Ingredient. EFSA Journal. 2009, 996, 1–26. DOI: 10.2903/j.efsa.2009.996.
- Ciftci, O. N.; Przybylski, R.; Rudzińska, M., Lipid Components of Flax, Perilla, and Chia Seeds. European Journal of Lipid Science and Technology 2012, 114, 794–800. DOI: 10.1002/ejlt.201100207.
- Álvarez-Chávez, L. M.; Valdivia-López, M. D. L. A.; Aburto-Juárez, M. D. L.; Tecante, A., Chemical Characterization of the Lipid Fraction of Mexican Chia Seed (Salvia Hispanica L.). International Journal of Food Properties 2008, 11, 687–697. DOI: 10.1080/10942910701622656.
- Amato, M.; Caruso, M. C.; Guzzo, F.; Galgano, F.; Commisso, M.; Bochicchio, R.; Labella, R.; Favati, F., Nutritional Quality of Seeds and Leaf Metabolites of Chia (Salvia Hispanica L.) From Southern Italy. European Food Research and Technology 2015, 241, 615–625. DOI: 10.1007/s00217-015-2488-9.
- Zanqui, A. B.; De Morais, D. R.; Da Silva, C. M.; Santos, J. M.; Chiavelli, L. U. R.; Bittencourt, P. R. S.; Eberlin, M. N.; Visentainer, J. V.; Cardozo-Filho, L.; Matsushita, M., Subcritical Extraction of Salvia Hispanica L. Oil with n-Propane: Composition, Purity and Oxidation Stability as Compared to the Oils Obtained by Conventional Solvent Extraction Methods. Journal of the Brazilian Chemical Society 2015, 26, 282–289. DOI: 10.5935/0103-5053.20140278.
- Dąbrowski, G.; Konopka, I.; Czaplicki, S.; Tańska, M. Composition and Oxidative Stability of Oil from Salvia Hispanica L. Seeds in Relation to Extraction Method. European Journal of Lipid Science and Technology 2017, 119. DOI:10.1002/ejlt.201600209.
- PN-EN ISO 659:2010. Oilseed Meals - Determination of Oil Content (Reference Method) (In Polish).
- Zadernowski, R.; Sosulski, F., Composition of Total Lipids in Rapeseed. Journal of the American Oil Chemists’ Society 1978, 55, 870–872. DOI: 10.1007/BF02671409.
- Czaplicki, S.; Tańska, M.; Konopka, I., Sea-Buckthorn Oil in Vegetable Oils Stabilisation. Italian Journal of Food Science 2016, 28, 412–425. DOI: 10.14674/1120-1770%2FIJFS.V252.
- Vlahakis, C.; Hazebroek, J., Phytosterol Accumulation in Canola, Sunflower, and Soybean Oils: Effects of Genetics, Planting Location, and Temperature. Journal of the American Oil Chemists’ Society 2000, 77, 49–53. DOI: 10.1007/s11746-000-0008-6.
- Czaplicki, S.; Ogrodowska, D.; Derewiaka, D.; Tańska, M.; Zadernowski, R., Bioactive Compounds in Unsaponifiable Fraction of Oils from Unconventional Sources. European Journal of Lipid Science and Technology 2011, 113, 1456–1464. DOI: 10.1002/ejlt.201000410.
- ISO 660:2010. Animal and Vegetable Fats and Oils. Determination of Acid Value and Acidity.
- ISO 3960:2012. Animal and Vegetable Fats and Oils. Determination of Peroxide Value. Iodometric (Visual) Endpoint Determination.
- ISO 6885:2006. Animal and Vegetable Fats and Oils. Determination of Anisidine Value.
- AOCS. Official Methods Cd 7-58. Poly-Unsaturated Acids. Ultraviolet Spectrophotometric Method. AOCS Press: Urbana, Illinois, 2017.
- ISO 6886:2016–04. Animal and Vegetable Fats and Oils - Determination of Oxidative Stability (Accelerated Oxidation Test).
- Ayerza, R.;. The Seed’s Protein and Oil Content, Fatty Acid Composition, and Growing Cycle Length of a Single Genotype of Chia (Salvia Hispanica L.) As Affected by Environmental Factors. Journal of Oleo Science 2009, 58, 347–354. DOI: 10.5650/jos.58.347.
- Ayerza, H. R.; Coates, W., Protein Content, Oil Content and Fatty Acid Profiles as Potential Criteria to Determine the Origin of Commercially Grown Chia (Salvia Hispanica L.). Industrial Crops and Products 2011, 34, 1366–1371. DOI: 10.1016/j.indcrop.2010.12.007.
- De Mello, B. T. F.; dos Santos Garcia, V. A.; Da Silva, C., Ultrasound-Assisted Extraction of Oil from Chia (Salvia Hispânica L.) Seeds: Optimization Extraction and Fatty Acid Profile. Journal of Food Process Engineering 2017, 40, e12298e12298. DOI: 10.1111/jfpe.12298.
- Marineli, R. D. S.; Moraes, É. A.; Lenquiste, S. A.; Godoy, A. T.; Eberlin, M. N.; Maróstica, M. R., Chemical Characterization and Antioxidant Potential of Chilean Chia Seeds and Oil (Salvia Hispanica L.). LWT - Food Sciences Technological 2014, 59, 1304–1310. DOI: 10.1016/j.lwt.2014.04.014.
- Cassiday, L.;. Sink or Swim: Fish Oil Supplements and Human Health. International News Fats, Oils Related Materials 2016, 27, 6–13.
- Bodoira, R. M.; Penci, M. C.; Ribotta, P. D.; Martínez, M. L., Chia (Salvia Hispanica L.) Oil Stability: Study of the Effect of Natural Antioxidants. LWT - Food Sciences Technological 2017, 75, 107–113. DOI: 10.1016/j.lwt.2016.08.031.
- Dąbrowski, G.; Konopka, I.; Czaplicki, S. Supercritical CO2 Extraction in Chia Oils Production: Impact of Process Duration and Co-Solvent Addition. Food Science and Biotechnology 2018, 27, 677-686. DOI: 10.1007/s10068-018-0316-2.
- Oliveira-Alves, S. C.; Vendramini-Costa, D. B.; Betim Cazarin, C. B.; Maróstica Júnior, M. R.; Borges Ferreira, J. P.; Silva, A. B.; Prado, M. A.; Bronze, M. R., Characterization of Phenolic Compounds in Chia (Salvia Hispanica L.) Seeds, Fiber Flour and Oil. Food Chemistry 2017, 232, 295–305. DOI: 10.1016/j.foodchem.2017.04.002.
- Mohd Ali, N.; Yeap, S. K.; Ho, W. Y.; Beh, B. K.; Tan, S. W.; Tan, S. G., The Promising Future of Chia, Salvia Hispanica L. Journal of Biomedicine and Biotechnology 2012, 2012, 1–9. DOI: 10.1155/2012/171956.
- de Paiva, L. B.; Goldbeck, R.; dos Santos, W. D.; Squina, F. M. Ferulic Acid and Derivatives: Molecules with Potential Application in the Pharmaceutical Field. Brazilian Journal of Pharmaceutical Sciences 2013, 49, 395–411. DOI: 10.1590/S1984-82502013000300002.
- Martínez-Cruz, O.; Paredes-López, O., Phytochemical Profile and Nutraceutical Potential of Chia Seeds (Salvia Hispanica L.) By Ultra High Performance Liquid Chromatography. Journal Chromatographic A 2014, 1346, 43–48. DOI: 10.1016/j.chroma.2014.04.007.
- Segura-Campos, M. R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D., Physicochemical Characterization of Chia (Salvia Hispanica) Seed Oil from Yucatán, México. Agricultural Sciences 2014, 5, 220–226. DOI: 10.4236/as.2014.53025.
- Barros, M.; Fleuri, L. F.; MacEdo, G. A., Seed Lipases: Sources, Applications and Properties - A Review. Brazilian Journal of Chemical Engineering 2010, 27, 15–29. DOI: 10.1590/S0104-66322010000100002.
- Gruzdiene, D.; Anelauskaite, E. Chemical Composition and Stability of Rapeseed Oil Produced from Various Cultivars Grown in Lithuania. Food Process Engineering in a changing world: the 11th International Congress of Engineering and Food, Athens, Greece, May 22-26, 2011; pp 1–4.
- Farhoosh, R.; Hoseini-Yazdi, S.-Z., Shelf-Life Prediction of Olive Oils Using Empirical Models Developed at Low and High Temperatures. Food Chemistry 2013, 141, 557–565. DOI: 10.1016/j.foodchem.2013.03.024.
- Roszkowska, B.; Tańska, M.; Czaplicki, S.; Konopka, I., Variation in the Composition and Oxidative Stability of Commercial Rapeseed Oils during Their Shelf Life. European Journal of Lipid Science and Technology 2015, 117, 673–683. DOI: 10.1002/ejlt.201400271.
- Kamal-Eldin, A.;, Effect of Fatty Acids and Tocopherols on the Oxidative Stability of Vegetable Oils. European Journal of Lipid Science and Technology 2006, 108, 1051–1061. DOI: 10.1002/ejlt.200600090.
- Choe, E.; Min, D. B., Mechanisms and Factors for Edible Oil Oxidation. Comprehensive Reviews in Food Science and Food Safety 2006, 5, 169–186. DOI: 10.1111/j.1541-4337.2006.00009.x.
- Chen, B.; Mcclements, D. J.; Decker, E. A., Minor Components in Food Oils: A Critical Review of Their Roles on Lipid Oxidation Chemistry in Bulk Oils and Emulsions. Critical Reviews in Food Science and Nutrition 2011, 51, 901–916. DOI: 10.1080/10408398.2011.606379.
- Miyashita, K.; Takagi, T., Study on the Oxidative Rate and Prooxidant Activity of Free Fatty Acids. Journal of the American Oil Chemists’ Society 1986, 63, 1380–1384. DOI: 10.1007/BF02679607.
- Frega, N.; Mozzon, M.; Lercker, G., Effects of Free Fatty Acids on Oxidative Stability of Vegetable Oil. Journal of the American Oil Chemists’ Society 1999, 76, 325–329. DOI: 10.1007/s11746-999-0239-4.
- Lacoste, F.; Lagardere, L., Quality Parameters Evolution during Biodiesel Oxidation Using Rancimat Test. European Journal of Lipid Science and Technology 2003, 105, 149–155. DOI: 10.1002/ejlt.200390030.
- Tikekar, R. V.; Ludescher, R. D.; Karwe, M. V., Processing Stability of Squalene in Amaranth and Antioxidant Potential of Amaranth Extract. Journal of Agricultural and Food Chemistry 2008, 56, 10675–10678. DOI: 10.1021/jf801729m.
- Grigoriadou, D.; Androulaki, A.; Psomiadou, E.; Tsimidou, M. Z., Solid Phase Extraction in the Analysis of Squalene and Tocopherols in Olive Oil. Food Chemistry 2007, 105, 675–680. DOI: 10.1016/j.foodchem.2006.12.065.
- He, H. P.; Corke, H., Oil and Squalene in Amaranthus Grain and Leaf. Journal of Agricultural and Food Chemistry 2003, 51, 7913–7920. DOI: 10.1021/jf030489q.
