?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Here we report a specific method for the determination and validation of distinguishable standard marker compounds and processed mature silkworm powder with the aim of standardizing the powder to uniformly control it as a raw functional food ingredient. The content of three specific amino acids–serine, glycine, and alanine–were determined using high-performance liquid chromatography. The compounds met the requirement of marker compounds due to their unique repetitive amino acid sequence. The variations of the amounts of serine, glycine, and alanine in the powder were considered to depend on the cultivation and production conditions of the raw ingredient with the sum of three amino acid contents giving the unique quantitative marker compound level.
Introduction
Standardization in health/functional foods involves managing technology and information used during the entire process of food production, from raw material production to manufacturing procedures, for the consistent production of a certified ingredient. Components intrinsic to a food item should not fluctuate as a result of inconsistent production methods. Marker compounds should be designated as indexes of standardization; these should be selective and specific compounds representing the characteristics of the ingredient to be standardized, irrespective of whether they are functional. These marker compounds should be identified and quantitatively analyzed using appropriate methods, the results of which should be accurate, reproducible, and verifiable.[Citation1–Citation3]
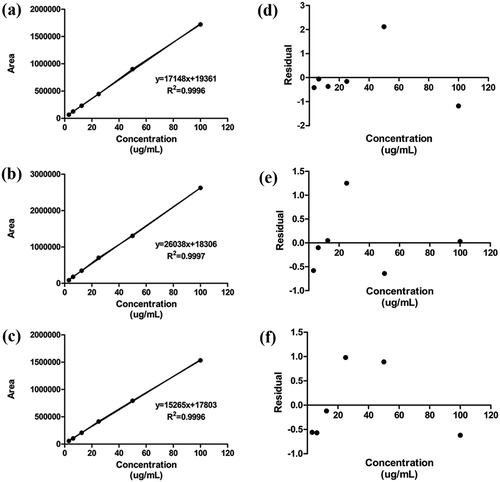
Figure 3. Calibration curves and residual distribution plots of serine, glycine, and alanine. (a), (b), and (c) show calibration curve of serine, glycine, and alanine, respectively. (d), (e), and (f) show residual distribution plot of serine, glycine, and alanine, respectively.
Silkworm (Bombyx mori) undergoes four distinctive developmental stages–embryo, larva, pupa, and adult moth. Mature silkworms are defined as those in which the larvae have been cultured till the end of the 3rd day of the 5th instar. A characteristic of mature silkworms is their silk glands, which contains silk proteins.[Citation4]
Mature silkworm powder is primarily composed of two primary proteins: fibroin (approximately 75%) and sericin (approximately 25%). The amino acid composition of fibroin is mainly glycine (43%), alanine (30%), and serine (12%), and its distinctive, repetitive hexapeptide sequence of glycine-alanine-glycine-alanine-glycine-serine (Gly-Ala-Gly-Ala-Gly-Ser) forms stable anti-parallel β-sheet crystallite structure. In contrast, sericin contains approximately 30% serine, which functions as an adhesive binder to maintain the structural integrity of protein fibers.[Citation5–Citation10]
In contrast to mature silkworm, immature silkworm has been reported to be effective in controlling blood glucose levels.[Citation10,Citation11,Citation12] Furthermore, recently mature silkworm has also been reported to have diverse health benefits.[Citation13–Citation17] Mature silkworm powder is processed from mature silkworms by steam-mediated thermal treatment followed by freeze-drying.
The purpose of this study was to determine and characterize marker compounds for the standardization of the functional food ingredient, mature silkworm powder, proposing a new standard, and specification levels suitable for controlling its quality, when applied to health/functional foods. The method validated in this study used high-performance liquid chromatography (HPLC) with dabsyl chloride as a derivative agent to specifically and precisely measure the serine, glycine, and alanine content in processed mature silkworm powder. To control the quality of the processed mature silkworm powder harvested under different conditions through the validated method, the standards and specifications of the raw materials as well as manufacturing process for the standardization of the raw materials were established.
Materials and methods
Materials
The amino acid standards L-Serine (Ser), L-Glycine (Gly), and L-Alanine (Ala) and the derivatization agent dabsyl chloride were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA) and acetonitrile was purchased from J.T Baker (Phillipsberg, NJ, USA). All other chemicals and the solvents used were HPLC-grade. Hydrochloric acid (HCl; 6 N) used for acid hydrolysis was obtained from Daejung (Siheung, Gyeonggi-do, Korea). Prior to analysis, a mixture of 35 mM sodium acetate buffer and 90% acetonitrile was prepared for use in mobile phases. Processed mature silkworm powder was cultivated and produced in various areas in the Republic of Korea including Sancheong Kyungnam, Boeun Chungnam, and Buan Jeounbuk, different seasons, spring and fall, of the year.
Manufacturing process of mature silkworm powder
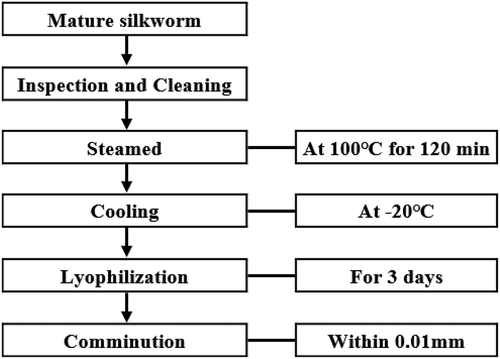
The manufacturing process of mature silkworm powder is shown in . Silkworms that had been cultivated till the 3rd day of the 5th instar were collected, washed, and steamed at 100°C. for 120 min. Thereafter, it was cooled at −20°C. and lyophilized for 3 days to grind to about 0.01 mm. In the Republic of Korea, mature silkworm is harvested in spring (May) and fall (August), and harvesting is affected by climate, and soil for 1 year and to derive comprehensive results to secure the standardized manufacturing process of processed raw materials.
Preparation of sample solution
For amino acid analysis, proteins were subjected to elevated temperatures under acidic conditions to break down their peptide bonds and produce free amino acids.[Citation18] Approximately 0.1 g of the processed mature silkworm powder was suspended in 1 mL of 6 N HCl, and the capped tubes containing these were treated at 105°C. for 24 h to achieve complete hydrolysis.[Citation19] After hydrolysis, the residue was diluted to 50 mL with distilled water (DW) and filtered through a 0.45–μm nylon membrane filter (Hyundai Micro Co., Seoul, Korea). The hydrolysate was diluted 20-fold with 0.84% sodium bicarbonate solution (w/v) and used as the sample solution.
Preparation of amino acid standard solutions
Each amino acid standard stock solution was prepared by dissolving 10 mg of serine, glycine, and alanine separately in 10 mL 0.84% sodium bicarbonate solution (w/v) to obtain a final concentration of 1 mg/mL. The working solution was diluted with 0.84% sodium bicarbonate (w/v) to obtain 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL solutions.
Derivatization with dabsyl chloride
The derivative agent, dabsyl chloride was dissolved in acetonitrile and prepared at a concentration of 3.0 mg/mL. Totally, 1 mL of the sample solution was mixed with 1 mL of the dabsyl chloride solution, and the mixture was reacted at 65°C. for 30 min. It was then cooled to room temperature; then 2 mL of acetonitrile was added, and the solution was mixed and filtered through a 0.45–μm polytetrafluoroethylene (PTFE) membrane filter (Millipore, Billerica, MA, USA). The filtrate was injected into the HPLC apparatus (Nanospace SI-2, Shiseido, Tokyo, Japan), which was equipped with a UV detector (UVD).
Instrumentation and conditions
HPLC analysis was performed on Shiseido Nanospce SI-2 (Shiseido, Tokyo, Japan) coupled with a UVD. The amino acid standards and samples were analyzed on a Capcell Pak C18 MG column with dimensions of 3.0 mm × 150 mm and 3 μm of particle diameters (Shiseido, Tokyo, Japan). The flow rate was maintained at 0.4 mL/min, and a 5 μL sample volume was injected in all experiments. Separation was achieved through gradient elution with a mixture of 35 mM sodium acetate buffer and 90% acetonitrile and was monitored by a UVD set at 436 nm. The column temperature was 40°C. and the run time was 55 min. The HPLC operation conditions for amino acid analysis are shown in .
Table 1. HPLC operation conditions for amino acid analysis.
Method validation
The analysis method described was validated to ensure that test conditions and results were reliable, consistent, and reproducible. Parameters including specificity, linearity, precision, accuracy, limit of quantification (LOQ), and limit of detection (LOD) were assessed according to the International Council on Harmonization (ICH) guidelines.[Citation20]
Results and discussion
Background
Due to the repetitive amino acid sequence of Gly-Ala-Gly-Ala-Gly-Ser in fibroin, the sum of the levels, rather than the individual levels, of serine, glycine, and alanine was calculated. An analytical method was established and validated by the ICH guidelines, demonstrating specificity, linearity, precision, accuracy, LOQ, and LOD, and was applied to both a standard amino acid mixture and a processed mature silkworm powder hydrolysate. Summarizing these analytical data, a new standard and specification level could be presented for the quality control of mature silkworm powder as a standardized functional food ingredient.
Method validation
Specificity
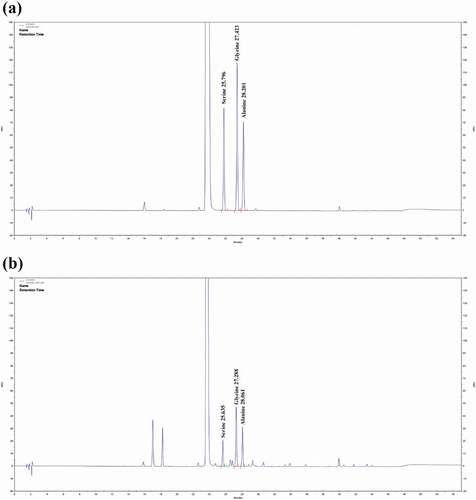
Specificity is the ability to unequivocally assess the analyte in the presence of any other components that may be present. This attribute was assessed by comparing retention times obtained in the standard amino acid mixture with those of the hydrolyte obtained in processed mature silkworm powder and was evaluated based on the chromatogram obtained by HPLC analysis of the treated preparations. Peaks were clearly distinguishable from the baseline (), which confirmed that serine, glycine, and alanine were selectively separated. The retention time of serine, glycine, and alanine were 25.796, 27.423, and 28.201 min in the standard, respectively, and 25.635, 27.288, and 28.061 min in the samples, respectively.
Linearity
Linearity denotes the ability of a given method to provide results proportional to the concentration of a substance within a given application range.[Citation20] The linearity of the method detailed in this study was assessed by analyzing six concentrations ranging from 3.125 to 100 μg/mL and each sample was trialed three times. Linearity was confirmed by the correlation coefficient values obtained from the prepared calibration curve and residual distribution plot. The correlation coefficients ( of serine, glycine, and alanine were over 0.999 and the residual distribution of each was plotted (). Evidence of linearity for serine, glycine, and alanine is supported by the random scatter shown on the residual distribution plots.[Citation1]
Precision and accuracy
Overall precision was measured by repeatability (intra-day) and intermediate precision (inter-day). Intra-day precision was analyzed three times per day, and inter-day precision was analyzed on three different days. Precision of the method was defined as the measurements from the three different samples under the same conditions that contained targeted analytes spiked at three concentrations (6.25, 25, and 100 μg/mL) covering the working range and was ultimately quantitatively expressed as relative standard deviation, i.e., the ratio of standard deviation to the average of each result. The relative standard deviations of serine, glycine, and alanine were calculated to be ≤6% (). Additionally, the % recovery, an index of accuracy, of each amino acid was ranged from 90.94% to 105%. Recovery is defined as the recovered concentration of the estimated measurements against the actual concentration and represents consistency between the true value and the estimated value; a range of 90–110% recovery is considered acceptable.[Citation21]
: Concentration of the spiked sample,
: Concentration of the sample,
: Concentration of the standard
Limit of quantification (LOQ) and limit of detection (LOD)
The LOQ and LOD of serine, glycine, and alanine were 0.88, 3.37, and 2.79 μg/mL and 0.33, 1.11, and 0.92 μg/mL, respectively (). The LOQ and LOD were calculated using the following equation:[Citation20]
Table 2. Precision and accuracy of serine, glycine, and alanine analysis in processed mature silkworm powder.
σ: Standard deviation of the y-intercept, S: Slope of the regression line determined from the calibration curve.
Application of the validated method
The validated method was applied to the targeted analytes, the results of which are shown in . Considering the variations in the processed mature silkworm powder produced depending on the producing area and season, the content of the three specific amino acids–serine, glycine, and alanine–representing the distinguished processed mature silkworm powder was analyzed. Based on the analyzed amino acid contents under various production the sum of the three amino acids–serine, glycine, and alanine–was considered to be the marker for the standardization, with the value 200–300 (± 50) mg/g of processed mature silkworm powder set as the threshold.
Table 3. Limit of quantification (LOQ) and limit of detection (LOD) of serine, glycine, and alanine.
Table 4. Effect of producing area and season on the contents of serine, glycine, and alanine in processed mature silkworm powder.
Conclusion
In the present study, based on data from six batches under different conditions, the manufacturing process of mature silkworm powder was standardized, proving the regularity of qualities. A method for the simultaneous determination of serine, glycine, and alanine contents in processed mature silkworm powder using HPLC with UVD was evaluated and validated, the purpose of which was to establish distinguished marker compounds that represent the characteristic of processed mature silkworm powder and to determine the standard and specification values to uniformly control the quality of the ingredient as functional food uses. The validated method was proven to be reproducible and accurate based on the analyses of parameters including specificity, precision, accuracy, linearity, and the LOD and LOQ, according to the ICH guidelines; this allowed the determination of the serine, glycine, and alanine contents as candidates of the distinguishable marker compounds in the ingredient. Based on the analyzed amino acid contents under various production conditions, the sum of the contents of the three amino acids–serine, glycine, and alanine–was considered and the marker and the quantitative level of the sum was to be proposed as a standard. These findings indicate that due to the unique repetitive amino acid sequence Gly-Ala-Gly-Ala-Gly-Ser in fibroin in Bombyx mori, the sum of the contents of the three specific amino acids–serine, glycine, and alanine–is defined as a specific marker for the standardization of processed mature silkworm powder, and the threshold value for the standardization was considered to be 200–300 (± 50) mg/g of the processed mature silkworm powder for practical regulatory consideration of standardization.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Development of Health Functional Food and Industrialization funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA), 317004041HD030 (grant number).
Additional information
Funding
References
- Korea Food & Drug Administration. Guideline for Standard of Health Functional Food. 2008, 1–146.
- Korea Food & Drug Administration. Standardization of Functional Ingredient in Health and Functional Food, Ministry of Food and Drug Safety: Osong, ChungChungBuk-do, Republic of Korea, 2006.
- Korea Food & Drug Administration. Functional Material Standardization for Health Functional Food Developers. Ministry of Food and Drug Safety: Osong, ChungChungBuk-do, Republic of Korea 2008, 1–148.
- Liu, S.; Zhang, L.; Li, Q. MicroRNA Expression Profiling during the Life Cycle of the Silkworm. BMC Genomics 2009, 10, 1–16. DOI: 10.1186/1471-2164-10-455.
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q.A., Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. International Journal Molecular Sciences 2017, 18, 1–21. DOI: 10.3390/ijms18030237.
- Reddy, B.V.; Divya, P.; Anitha, M., Quantitative Profile Analysis of Mulberry Silkworm Bombyx Mori. L (CSR2XCSR4). International Letters of Natural Sciences 2015, 34, 34–41. DOI: 10.18052/www.scipress.com/ILNS.34.34.
- Schroeder, W.A.; Kay, L.M.; Lewis, B.; Munger, N., The Amino Acid Composition of Bombyx Mori Silk Fibroin and of Tussah Silk Fibroin. Journal of the American Chemical Society 1955, 77, 3908–3913. DOI: 10.1021/ja01619a066.
- Padamwar, M.N.; Pawar, A.P.; Sericin, S.; Application, I. A Review. Journal Sciences Industrial Researcher 2004, 63, 323–329.
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Researcher International. 2016, 2016, 8175701 Epub 2016 Nov 14 DOI: 10.1155/2016/8175701.
- Gupta, D.; Agrawal, A.; Rangi, A. Extraction and Characterization of Silk Sericin. Indian Journal Fibre Text Researcher 2014, 39, 364–372.
- Ryu, K.S.; Lee, H.S.; Chung, S.H.; Kang, P.D. An Activity of Lowering Blood-Glucose Levels Accoring to Preparative Contitions of Silkworm Powder. Journal of Sericultural and Entomological Science 1997, 39, 79–85.
- Ryu, K.S.; Lee, H.S.; Kim, K.Y.; Sumg, G.B.; Ji, S.D.; Kang, P.D. 1-Deoxynojirimycin Content and Blood Glucose-Lowering Effect of Silkworm (Bombyx Mori) Extract Powder. International Journal of Industrial Entomology 2013, 27, 237–242. DOI: 10.7852/ijie.2013.27.2.237.
- Ji, S.D.; Kim, N.S.; Kweon, H.Y.; Choi, B.H.; Yoon, S.M.; Kim, K.Y.; Koh, Y.H., Nutrient Compositions of Bombyx Mori Mature Silkworm Larval Powders Suggest Their Possible Health Improvement Effects in Humans. Journal Asia-Pac Entomological 2016, 19, 1027–1033. DOI: 10.1016/j.aspen.2016.08.004.
- Mun, J.; Lee, H.; Lee, K.; Kweon, H.; Jo, Y.; Yeo, J., Effects of Matured Silkworm Hemolymph on Suppressing Melanin Synthesis. Journal of Sericultural and Entomological Science 2013, 51, 51–54. DOI: 10.7852/jses.2013.51.2.207.
- Napavichayanun, S.; Lutz, O.; Fischnaller, M.; Jakschitz, T.; Bonn, G.; Aramwit, P., Identification and Quantification and Antioxidant Activity of Flavonoids in Different Strains of Silk Cocoon, Bombyx Mori. Archives of Biochemistry and Biophysics 2017, 631, 58–65. DOI: 10.1016/j.abb.2017.08.010.
- Cho, J.-M.; Kim, K.-Y.; Ji, S.-D.; Kim, E.-H., Protective Effect of Boiled and Freeze-Dried Mature Silkworm Larval Powder against Diethylnitrosamine-Induced Hepatotoxicity in Mice. Journal of Cancer Prevention 2016, 21, 173–181. DOI: 10.15430/JCP.2016.21.3.173.
- Yin, H.; Shi, X.; Sun, B.; Ye, J.; Duan, Z.; Zhou, X.; Cui, W.; Wu, X., Accumulation of 1-Deoxynojirimycin in Silkworm, Bombyx Mori L.. Journal of Zhejiang University SCIENCE B 2010, 11, 286–291. DOI: 10.1631/jzus.B0900344.
- Walker, J.M.; Rapley, R. Molecular Biomethods Handbook: Second Edition, Walker, J.M.; Rapley, R. Eds.; Human Press: New York, United States, 2008.
- Hess, S.; Van Beek, J.; Pannell, L.K. Acid Hydrolysis of Silk Fibroins and Determination of the Enrichment of Isotopically Labeled Amino Acids Using Precolumn Derivatization and High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. Analytical Biochemistry 2002, 311, 19–26.
- ICH. Harmonised Tripartiete Guideline. Validation of Analytical Procedure: Text and Methodology Q2(R1). 2005, 1–13.
- Bartolomeo, M.P.; Maisano, F. Validation of a Reversed-Phase HPLC Method for Quantitative Amino Acid Analysis. Journal of Biomolecular Techniques : JBT 2006, 17, 131–137.