ABSTRACT
The aim of this study was to demonstrate the effect of different treatments on collagen self-assembly, the morphological and the qualitative analysis of collagen assembly were measured by atomic force microscopy. Collagen samples were treated with 0.02%, 0.04%, 0.10% and 0.20% glutaraldehyde individually at the first steps. Meanwhile, the other experimental groups were treated with 0.05%, 0.10%, 0.20% riboflavin, respectively, the structural change of the collagen network with 15, 30, 45, 60 min UV365 exposure and the fluorescent irradiation as the control group in turn was investigated by atomic force microscopy. The width and height of collagen assembly increased with the concentration of glutaraldehyde, which were changed from 36.45 ± 4.45nm to 64.35 ± 2.58nm and 2.26 ± 0.19nm to 4.93 ± 0.29 nm, respectively. The effect of different UV365 irradiation time on the height of collagen was greater than the effect on the width, the width and height of collagen were varied from 41.36 ± 4.30nm to 55.47 ± 4.53nm and 3.10 ± 0.39nm to 6.20 ± 0.85nm, which were changed 25.44% and 50.00%, respectively. The concentration of riboflavin was more efficiently than irradiation time, and the maximum width and height of collagen reached 106.98 ± 3.37nm and 11.17 ± 1.33 nm, respectively, the control group without a significant change at the fluorescent irradiation by the time. This optimized cross-linking method with riboflavin/UV365 would have great potential for tissue engineering in clinic and food packaging science.
Introduction
Packaging and food industry are increasingly focusing on the development of biodegradable packaging materials, protein films play a very important role in this field. Wang and Rhim reported that collagen was mixed with agar and alginate to obtain a film with strong antibacterial properties.[Citation1] What is more, collagen exhibits excellent biocompatibility and low antigenicity and benefits numerous cellular behaviours, so it has been widely utilized in tissue engineering. Heo and his colleagues suggested that the mechanical properties of collagen scaffold after photo crosslinking had been significantly improved, so it was better used in meniscus tissue engineering.[Citation2] Collagen mainly distributed in the skin, tendons and other organizations. It is composed of three polypeptide chains forming three helical structures, and its amino acids present (Gly-X-Y)n periodic arrangement, where the positions of X and Y are proline and hydroxyproline, which are the specific amino acids of collagen, accounting for about 25%, and it is also the highest content of all kinds of proteins.[Citation3] Collagen shows excellent water vapour permeability and biocompatibility, but low mechanical strength and low elongation. Cross-linking techniques have been reported to enhance the mechanical properties of collagen.[Citation4,Citation5] Glutaraldehyde is a kind of common cross-linking agent applied in protein chemistry and tissue engineering.[Citation6] The free amino acid residues of collagen can react in cross-linking with glutaraldehyde and change the structural stability of collagen assembly by adding the glutaraldehyde to collagen solution.[Citation7] Henriquez and his colleagues reported that UV365 radiation caused physical and chemical changes of collagen molecules, which made the collagen structure change, affecting the mechanical and optical properties of collagen films in some degree.[Citation8] Zhao and his colleagues found that the cross-linking conditions, including irradiation time and modifier concentration, played significant roles on the property of the collagen.[Citation9] At the optimal condition, the collagen exhibited an increased thermal stability and enzyme tolerance capacity.[Citation10] This proves that UV365 irradiation in a short time can induce the increase of cross-linking degree and strengthen the thermal stability of collagen molecules.[Citation11] However, the conformation of collagen changed and sulphide bond broke down when the collagen absorbed a certain amount of high-energy radiation. Therefore, the structural damages of collagen under UV365 irradiation need to be inhibited by adding cross-linking modifier or mixing the collagen with other materials.[Citation12] As one kind of photo-sensitizers, riboflavin can produce free radicals under irradiation, with maximum absorption peaks at 270, 366, and 445 nm, selecting the wavelength at 365 nm can satisfy the maximum absorption value of the riboflavin chromophore.[Citation13] Riboflavin can induce the collagen molecules to form a new linkage with each other under the UV365 irradiation. The riboflavin concentration and the irradiation time showed a significant influence on the degree of crosslinking between collagen molecules,[Citation14] which led to the increase of the density, the diameter and the mechanical strength of collagen fibres.[Citation15,Citation16] Through the above crosslinking method, the defects of collagen film and its mechanical properties were improved, which was of great significance in food packaging.
The effects of glutaraldehyde and riboflavin/UV365 on the crossing-linking of type I collagen molecules were investigated and evaluated with atomic force microscopy (AFM) imaging and the related analysis methods in this study, which was an efficient and widely used technique in molecular and cellular biology for its nano-scale resolution and simple sample preparation methods.
Materials and methods
Materials and chemical reagents
The tendon tissues were peeled from the fresh swine trotter, which was purchased from Yangling Jiafu-Supermarket at Shaanxi, China. Twenty-five percent glutaraldehyde and 36% acetic acid were purchased from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). Sodium chloride and sodium hydroxide were purchased from Guangdong Chemical Reagent Engineering-technological Research and Development Center (Guangdong, China). Riboflavin was provided by Sinopharm Chemical ReagentCo., Ltd (Shanghai, China). All reagents in analytical grade were used as received without further purification. All solutions were prepared with deionized water supplied by the Northwest A&F University.
Preparation of original collagen solutions
Four grams of tendon tissues were firstly cut into 0.1cm3 cubes, and then washed with 0.9% NaCl for more than 3 times, the fresh tendons turbid liquid was mixed with 100ml solution (pH7.5) containing 0.5 M Tris-HCl and 1M NaCl, and immediately placed in 4°C refrigerator for 24 h with stirring every 6h.[Citation17] Then the supernatant was discarded to remove the non-collagenous impurities. After fully washing with distilled water, the collagen was extracted by adding 100 ml 0.5 M acetic acid with stirred (90–1 thermostat magnetic stirrer, Shanghai, China) for 24 h. Then, the collagen solution was filtered and precipitated by adding 20% NaCl successively. When the flow occurred, the above collagen was collected by centrifuged at 10,280 g for 20 min and then was re-dissolved by 0.5 M acetic acid with the final collagen concentration of 4mg/ml. The original collagen solutions were stored at 4°C in order to avoid denaturation and microbial contamination for further processing.[Citation18]
Preparation of the collagen solutions cross-linked with glutaraldehyde
Stock solution of Na2HPO4 and NaH2PO4 was prepared with distilled water and the pH value was adjusted to 9.2 with 0.1 M NaOH in this experiment. The original 4mg/ml solutions of collagen were diluted with stock solution to the final concentration at 0.4 mg/ml. Then, 25% glutaraldehyde solution was diluted to 0.02%, 0.04%, 0.10% and 0.20% with stock solution (pH9.2), respectively. The different concentrations of glutaraldehyde solutions were severally mixed with the same volume of 0.4 mg/ml collagen solution. In the control group, collagen solutions were mixed with phosphate buffer at the same volume. 20 μl mixtures were deposited onto a fresh cleaved mica surface separately. All of these samples were put into 12-well culture plates, dried at room temperature and washed for several times with distilled water before AFM imaging.
Preparation of the collagen solutions cross-linked with riboflavin under UV365 irradiation
The 4mg/ml collagen solution was diluted with phosphate buffer (pH = 9.2) to the final concentration at 0.4 mg/ml. Riboflavin was dissolved by 0.1 M NaoH with the final riboflavin concentration of 0.05%, 0.10%, 0.20% (w/v), and it was kept in dark place to avoid its photolysis. Then, the collagen solutions were divided into 5 groups: collagen solutions mixed with 0.1 M NaOH at the same volume was put in fluorescent as group 1; mixed liquor was exposed to UV365 (6W, ZF-2 UV analyser, Shanghai, China) at a distance of 15 cm as group 2; the collagen solutions mixed with riboflavin 0.05%, 0.10%, 0.20% (w/v), respectively, were exposed to UV365 irradiation as group 3 to 5. The abbreviations of the samples prepared in different riboflavin concentrations with varied irradiation time are shown in . Twenty microliter mixtures were deposited onto the freshly cleaved mica sheets separately after UV365 irradiation. All of these groups were dried in 12-well culture plates at room temperature and washed for several times with distilled water after irradiation by fluorescent or UV365, avoiding the interference of crystals derived from phosphate buffer.
Table 1. The abbreviation of the collagen prepared in different riboflavin concentrations and irradiation time.
SDS-PAGE analysis of collagen in different processing
SDS-PAGE was used to analyse the changes of molecular mass distribution of modified collagen in different riboflavin concentrations under UV365 for 1 h.[Citation19] Thirty microliters collagen samples were subjected to analysis using 10% acrylamide gel and 5% stacking gel following initial characterization experiments. The bands were stained with Coomassie Brilliant Blue R-250 and analysed by WD-9413B type gel imaging system (Liuyi Co., Beijing, China).
AFM imaging and offline analysis
Crosslinking assay was executed on Multimode-8 AFM with SCANASYST-AIR probes (Bruker Co., Santa Barbara, USA) in ScanAsyst mode at 0.997 Hz. Height and error images in 512 × 512 pixels were collected simultaneously after a first order flatten, and noisy line erasing using AFM offline software NanoScopeAnalysis V1.10 (Bruker Co., Santa Barbara, USA).
Statistical analysis
Experiments were analysed using analysis of variance (ANOVA) to determine the significant differences among the groups and p < 0.05 was considered statistically significant. All data were expressed as mean± standard deviations (mean± SD). Analyses were performed using Excel 2010 (Microsoft Co., Redmond, USA) and SPSS22.0 (IBM Co., Armonk, USA).
Results and discussion
Effects of glutaraldehyde concentration on the collagen cross-linking
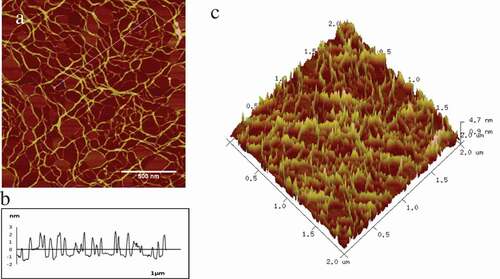
AFM was used to explore the morphology of collagen treated with glutaraldehyde in different concentrations. Typical AFM 2D/3D height images and section analysis profiles of original collagen fibrils were shown in . was shown the loose cross-linking of collagen, and the white line interception was shown in the , which represented the width and height of the collagen fibre. was shown the 3D image of collagen, which expressed the distribution of collagen, was relatively loose and rough. The width, height and roughness (Rq) were 36.45 ± 4.45nm, 2.26 ± 0.19nm and 0.62 ± 0.04 nm, respectively, of collagen fibre as seen in .
Table 2. Width, height, and roughness of the collagen cross-linked with glutaraldehyde in different concentrations.
Figure 1. Typical AFM 2D/3D height images and section analysis profiles of original collagen fibrils. a-c represent original collagen fibrils of 2D images, section analysis profiles and 3D images individually.
The cross-linking collagen displayed a typical fibre structure after treated with different concentrations of glutaraldehyde. The section analysis profiles were posted right below the height images, as shown in . Besides, the data of width, height and roughness (Rq) were calculated automatically, as listed in . was shown the process of collagen crosslinking, which was becoming progressively dense. Meanwhile, as the results shown in , the width and height of fibers were changed from 42.25 ± 3.74nm to 64.35 ± 2.58nm increased 52.30% and 2.30 ± 0.13nm to 4.93 ± 0.29nm changed about 113.04%, respectively, which increased compared with the control group. Cheung and Nimni indicated that the excess glutaraldehyde could react with the ɛ-amino group of lysine or hydroxylysine residues by condensation in phosphate buffer and resulted in the formation of a Schiff base intermediate;[Citation20] besides, the hydroxyl group of hydroxyprolamine formed acetal with the aldehyde group, which could improve the stability of Schiff base in the crosslinking condition. Glutaraldehyde could enhance the stability of collagen by Michael addition reaction, and the reaction of amine groups with the free aldehyde groups to form further cross-linking.[Citation21]
Figure 2. Typical AFM height images and section analysis profiles of collagen self-assembly in different glutaraldehyde concentrations in 512 × 512pixels. Scale bar = 500nm.
(a) 0.02%; (b) 0.04%; (c) 0.10%; (d) 0.20%
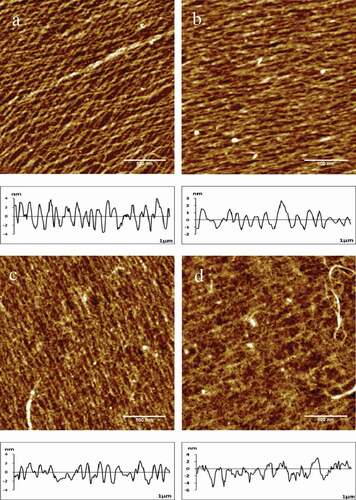
The concentration of glutaraldehyde had a significant effect on the width, height and Rq of collagen fibres. In addition, the width of collagen was first increased from 36.45 ± 4.45nm to 64.35 ± 2.58nm and then decreased from 64.35 ± 2.58nm to 42.25 ± 3.74nm along with the increased of glutaraldehyde concentration; however, the change of height showed an obviously trend increased to the maximum 4.93 ± 0.29nm. The Rq increased mostly from 0.62 ± 0.04nm to 2.76 ± 0.24nm with the concentration, which was caused by the too much glutaraldehyde on the fibre surface initiated the polymerization. The experiment performed that the maximum height of collagen could reach 4.93 ± 0.29nm, which was 2 times in the control group after treated with 0.20% glutaraldehyde. It was implied that the high concentration of glutaraldehyde could enhance the vertical cross-linking degree of fibres.
Collagen fibrils tended to be coupled together at the low concentration of glutaraldehyde, suggesting that crosslinking preferentially appeared between neighbouring collagen molecules. The collagen fibrils chaotically intertwined to each other with the increased concentration of glutaraldehyde and the glutaraldehyde molecules lengthened the side chain by further self-polymerization. In the end, almost all fibrils were linked and huddled together.
Effects of riboflavin/uv365 irradiation on the collagen cross-linking
It was shown that the collagen fibres without a significant change at the fluorescent irradiation by the time. Typical AFM height images of collagen and the section analysis profiles as a control group were shown in , which represented the width and height of the collagen fibre. Collagen was arranged in single fibre and slender in form and the shape of the 4 pictures was similar. Moreover, the data analysis including width, height and roughness (Rq) are shown in . The width of collagen changed slightly over time varied from 31.36 ± 1.72nm to 33.20 ± 2.13nm, and the roughness had hardly changed, which maintained about 0.76 ± 0.01nm; nevertheless, the height of fibres had an important increase after 60 min UV365 irradiation, which changed from 2.41 ± 0.23nm to 4.13 + 0.38 nm. Prolonged exposure would cause the collagen to absorb heat and degeneration, resulting in minor changed in the structure.
Figure 3. Typical AFM height images and section analysis profiles of collagen self-assembly in different irradiation time span as a control group in 512 × 512pixels. Scale bar = 500nm.
Irradiation time span of UV365 on collagen molecules, (a) 15 min; (b) 30 min; (c) 45 min; (d) 60 min
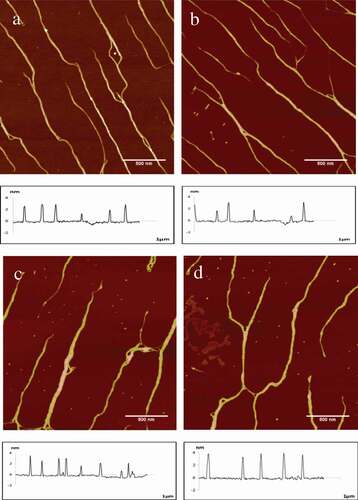
Table 3. Width, height, and roughness of the collagen cross-linking in different riboflavin concentrations and irradiation time span of UV365.
The irradiation time of UV365 had a clear impact on the morphology of collagen, typical AFM height images of collagen and the section analysis profiles were shown in , the data analysis is shown in . The width of collagen presented a rising trend varied from 41.36 ± 4.30nm to 55.47 ± 4.53nm increased 34.12%, which was similar to the height changed from 3.10 ± 0.39nm to 6.20 ± 0.85nm changed 100.00% and the fibres reached a maximum after 60 min irradiation with UV365. However, the roughness of collagen not increased obviously varied from 1.43 ± 0.12nm to 1.67 ± 0.18nm. It was shown that in , collagen assembly underwent a partial to a full physical crosslinking when the radiation time varied from 15 to 60 min. Snibson and his colleagues concluded that the collagen molecules contained some aromatic residues, and the conjugated system absorbed the exogenous energy provided by UV365 after irradiation, then convert into a highly active excited state. Furthermore, the high-energy electrons generated by the excitation were transmitted through the electron transport chain, which would lead to the production of corresponding free radical and triggered free radical reactions, resulted in the cross-linking among collagen fibrils.[Citation22] As a physical crosslinking method, UV365 irradiation was not toxic and would not introduce exogenous substances.
The typical AFM height images of the collagen fibres with riboflavin on different concentrations from 15 to 60min irradiation are shown in for dense cross-linking and loose cross-linking collagen specimens, respectively. The qualitative data of collagen fibres is shown in . As the results shown in , the width of collagen increased from 56.66 ± 3.51nm to 82.41 ± 6.64nm and the height also had a significant increase from 3.84 ± 0.54nm to 9.39 ± 0.93nm, but it was decreased after 30min irradiation as shown in . The possible reason for this phenomenon was that the self-assembly of collagen formed a uniform and dense cross-linking, leading to decreased height. It was shown that in , the degree of crosslinking increased with irradiation time span, small amount of network structure was formed with 0.10% riboflavin irradiated by UV365 for 15min. However, the crosslinking became intensive and formed more obvious reticular structure after 60min irradiation. The width of fibres was shown a rising trend, which increased from 65.54 ± 2.28nm to 103.61 ± 5.79nm and the height changed from 4.87 ± 0.55nm to the maximum of 11.17 ± 1.33nm. It was shown the same trend in as . As the results shown in , the width and height of increased from 74.44 ± 4.31nm to 106.98 ± 3.37nm arrived to 241.14% of control group and 4.24 ± 0.56nm to 11.17 ± 0.92nm reached to 363.49% of original collagen fibrils, respectively. Moreover, the various parameters of collagen seemed to reach a threshold value as irradiation time span and riboflavin concentrations increased simultaneously. Due to the significance analysis, it was demonstrated that the riboflavin concentration had a greater effect on collagen than that induced by irradiation time span. Specifically, the width, height and Rq of collagen self-assembly showed a significant difference (P < 0.05) when the concentrations of riboflavin varied from 0.05% to 0.20% and the irradiation time changed from 15 to 60min. Collagen in the control group showed low width and height; moreover, collagen fibres presented a single strip and there was no network structure appeared. Short time span of irradiation was not enough to inspire all riboflavin to promote collagen cross-linking and low concentrations of riboflavin at short exposure was insufficient to crosslink all collagen fibrils, which led to low reaction efficiency or crosslinking degree.[Citation23] However, high concentrations of riboflavin would lead to higher UV365 absorption and generate more singlet oxygen to trigger a cross-linking reaction.[Citation24] van Best and his colleagues reported that the prolonged exposure of UV365 would destroy the triple helical structure of collagen fibrils and lead to a local fracture, after which the ɛ-amino was gradually exposed and then the free α-amino was generated.[Citation25] The aim of the crosslinking was to create additional chemical bonds inside the collagen fibres by means of a photo-sensitizer induced with UV light at 365nm.[Citation26] In the presence of oxygen and UV365, riboflavin could be stimulated to interact with oxygen, leading to intrahelical crosslinking of amino acids, such as histidine, lysine and arginine. Referring to Fraser and his colleagues’ studies,[Citation27] riboflavin was a photo-sensitizer that produced free radicals under UV irradiation. Firstly, it was excited and formed excited singlet riboflavin, and then became a triplet excited riboflavin and interacted with triplet oxygen in the air, moreover, very reactive singlet oxygen was produced, which was an oxygen radical that constants to interact with the carbonyl group of fibres.[Citation28] From the relationship between parameters and morphological changed during crosslinking, it was concluded that new chemical bonds were introduced at high concentrations of riboflavin and long reaction time led to form dense cross-linking between collagen fibres.[Citation29]
Effects of riboflavin/uv365 irradiation on the mass distribution of collagen molecules
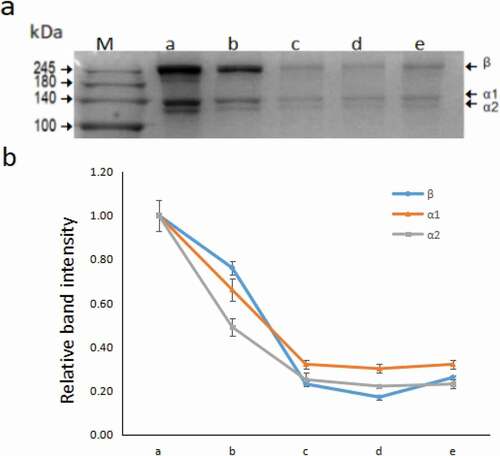
It was shown that UV365 irradiation had better crosslinking effect in 60 min from the above results, which was chosen for SDS-PAGE validation. As the results shown in and , after 60 min of UV365 irradiation, the width and height of collagen reached a relatively large value, and the degree of cross-linking of fibres was relatively dense. The SDS-PAGE data of mass distribution of type I collagen molecules consisted of three chains, including α1 (140 kDa), α2 (130 kDa) and β (250 kDa), as shown in . Compared with the control group, the lane b-e group had relatively shallow staining bands, especially in the riboflavin treatment group. This phenomenon could be interpreted as riboflavin has a protective effect on the form of collagen fibres, resulting in a longer chain of collagen. Then, Image J (National Institutes of Health, Bethesda, USA) was used to analyse the intensity of each band. In order to make statistics more convenient, the intensity values of the three bands (α1, α2, β) of the control group were recorded as a reference and then made the relative intensity of each band. As shown in , the lines of different colours had a similar tendency to decrease, the relative band intensity of lane a to lane b dropped from 1.00 to about 3.00, lane c to lane e tended to be parallel and eventually maintained around 0.30. This phenomenon could be explained as the collagen fibres had a head and tail connection, increasing the length of the chain and the molecular weight of collagen, molecules in the high weight region did not enter the separation gel. Due to the dense cross-linking of collagen fibres under the irradiation of 60 min, there was no significant difference between the lane c to lane e. Based on the above analysis, it was confirmed that a great deal of cross-linking occurred between the fibres after UV365 and riboflavin treatment.
Figure 4. Typical AFM height images of collagen self-assembly in different riboflavin (RF) concentrations and irradiation time in 512 × 512pixels. Scale bar = 500nm. a-d represent riboflavin concentration of 0, 0.05%, 0.10%, and 0.20% individually, and the subscript 1–4 means UV365 irradiation time of 15 min, 30 min, 45 min, and 60 min, respectively.
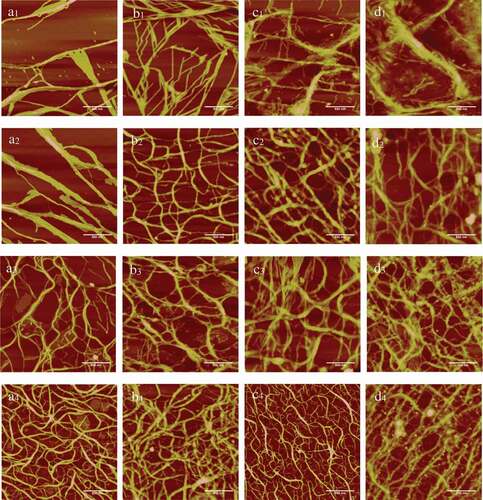
Figure 5. a: SDS-PAGE imaging of collagen molecules processed by riboflavin and UV365 irradiation. Lane M-e is Marker, control, UV365, 0.05% riboflavin, 0.10% riboflavin, 0.20% riboflavin, respectively. b: Line chart shows the relative intensity of the bands (n = 3), β (blue), α1 (orange), α2 (grey)(relative values of the band intensity took control group as a reference).
Conclusion
This study was demonstrated that different concentrations of glutaraldehyde had a significant impact on crosslinking of collagen fibres, Schiff bases, which was generated by the reaction of glutaraldehyde with free amine groups of lysine, were formed rapidly in the beginning of the cross-linking processing. The width, height and Rq of collagen fibres increased along with the increase of glutaraldehyde concentration till 0.20%, showing a maximum height. To evaluate the effect of UV365 and riboflavin on collagen self-assembly, this study chose UV365, a wavelength that one of the absorption maxima of the riboflavin chromophore. Photocrosslinking of collagen with riboflavin at 0.05%, 0.10%, 0.20% (w/v) was also examined, and different irradiation time had a great influence on the structure of collagen. From all the above analysis,a significant increase in the degree of crosslinking could be achieved through the procedure, when the exposure time and the concentration of riboflavin increased. The modification of collagen could improve its mechanical properties and had a wide application prospect in medicine and food processing.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This project was supported by Key Research and Development Plan of Shaanxi Province (2018NY-110, JZ), National Natural Science Foundation of China (11202170, JZ), International S&T Cooperation Foundation of Northwest A&F University (2018, JZ), College Students’ Entrepreneurship Training Program of Northwest A&F University (2201810712098, ML), College Students’ Innovation Training Program of Northwest A&F University (1201810712131 & 1201710712146, WK)
Additional information
Funding
References
- Wang, L.F.; Rhim, J.W. Preparation and Application of Agar/Alginate/Collagen Ternary Blend Functional Food Packaging Films. International Journal of Biological Macromolecules 2015, 80, 460–468. DOI: 10.1016/j.ijbiomac.2015.07.007.
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-Induced Photo-Crosslinking of Collagen Hydrogel and Its Application in Meniscus Tissue Engineering. Drug Delivery & Translational Research 2016, 6(2), 148–158. DOI: 10.1007/s13346-015-0224-4.
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annual Review of Biochemistry 2009, 78, 929–958. DOI: 10.1146/annurev.biochem.77.032207.120833.
- Wihodo, M.; Moraru, C.I. Physical and Chemical Methods Used to Enhance the Structure and Mechanical Properties of Protein Films: A Review. Journal of Food Engineering 2013, 114(3), 292–302. DOI: 10.1016/j.jfoodeng.2012.08.021.
- Wang, Z.W.; Xiao, Q.; Song, X.; Wan, Y.F.; Cation-Specific, Z.J. Effects on the Self-Assembly of Collagen Molecules Mediated by Acetate on Mica Surface Observed with Atomic Force Microscopy. Journal of Food Quality 2017, 2017, 1–10.
- Chvapil, M.; Gibeault, D.; Wang, T.F. Use of Chemically Purified and Cross‐Linked Bovine Pericardium as a Ligament Substitute. Journal of Biomedical Materials Research Part A 1987, 21(12), 1383–1393. DOI: 10.1002/jbm.820211204.
- Roe, S.C.; Milthorpe, B.K.; Schindhelm, K. Collagen Cross-Linking and Resorption: Effect of Glutaraldehyde Concentration. Artificial Organs 1990, 14(6), 443–448.
- Henriquez, M.A.; Izquierdo, L.; Bernilla, C.; Zakrzewski, P.A.; Mannis, M. Riboflavin/Ultraviolet A Corneal Collagen Cross-Linking for the Treatment of Keratoconus: Visual Outcomes and Scheimpflug Analysis. Cornea 2011, 30(3), 281–286. DOI: 10.1097/ICO.0b013e3181eeaea1.
- Zhao, X.; Long, K.; Liu, Y.; Li, W.; Liu, S.; Wang, L.; Ren, L. To Prepare the Collagen Based Artificial Cornea with Improved Mechanical and Biological Property by ultraviolet‐A/riboflavin Crosslinking. Journal of Applied Polymer Science 2017, 134(38), 1–10. DOI: 10.1002/app.45226.
- Ahn, J.I.; Kuffova, L.; Merrett, K.; Mitra, D.; Forrester, J.V.; Li, F.; Griffith, M. Crosslinked Collagen Hydrogels as Corneal Implants: Effects of Sterically Bulky Vs. Non-Bulky Carbodiimides as Crosslinkers. Acta Biomaterialia 2013, 9(8), 7796–7805. DOI: 10.1016/j.actbio.2013.04.014.
- Liu, Y.; Ren, L.; Wang, Y. Crosslinked Collagen–Gelatin–Hyaluronic Acid Biomimetic Film for Cornea Tissue Engineering Applications. Materials Science & Engineering C Materials for Biological Applications 2013, 33(1), 196–201. DOI: 10.1016/j.msec.2012.08.030.
- Hovakimyan, M.; Guthoff, R.F.; Stachs, O. Collagen cross-Linking: Current Status and Future Directions. Journal of Ophthalmology 2012, 2012(9), 1–12. DOI: 10.1155/2012/406850.
- Bueeler, M.; Spoerl, E.; Seiler, T.; Mrochen, M. UV Collagen Cross-Linking of the Cornea: Safety Aspects and Design of a UV Illumination System. Bruce E. Stuck; Michael Belkin; Fabrice Manns; Per G. Söderberg (eds). In Proceedings of SPIE - the International Society for Optical Engineering, Ophthalmic Technologies XVIII. doi: 10.1117/12.764769.
- Fawzy, A.; Nitisusanta, L.; Iqbal, K.; Daood, U.; Beng, L.T.; Neo, J. Characterization of Riboflavin-Modified Dentin Collagen Matrix. Journal of Dental Research 2012, 91(11), 1049–1054. DOI: 10.1177/0022034512459053.
- Mori, H.; Shimizu, K.; Hara, M. Dynamic Viscoelastic Properties of Collagen Gels in the Presence and Absence of Collagen Fibrils. Materials Science & Engineering C Materials for Biological Applications 2012, 32(7), 2007–2016. DOI: 10.1016/j.msec.2012.05.022.
- Mori, H.; Shimizu, K.; Hara, M. Dynamic Viscoelastic Properties of Collagen Gels with High Mechanical Strength. Materials Science & Engineering C Materials for Biological Applications 2013, 33(6), 3230–3236. DOI: 10.1016/j.msec.2013.03.047.
- Song, X.; Wang, Z.W.; Tao, S.Y.; Li, G.X.; Zhu, J. Observing Effects of Calcium/Magnesium Ions and pH Value on the Self-Assembly of Extracted Swine Tendon Collagen by Atomic Force Microscopy. Journal of Food Quality 2017, 2017(24), 1–8. DOI: 10.1155/2017/9257060.
- Zeugolis, D.I.; Paul, R.G.; Attenburrow, G. Factors Influencing the Properties of Reconstituted Collagen Fibers Prior to Self-Sembly: Animal Species and Collagen Extraction Method. Journal of Biomedical Materials Research Part A 2008, 86(4), 892–904. DOI: 10.1002/jbm.a.31694.
- Kato, Y.; Uchida, K.; Kawakishi, S. Aggregation of Collagen Exposed to UVA in the Presence of Riboflavin: A Plausible Role of Tyrosine Modification. Photochemistry & Photobiology 1994, 59(3), 343–349. DOI: 10.1111/php.1994.59.issue-3.
- Cheung, D.T.; Nimni, M.E. Mechanism of Crosslinking of Proteins by Glutaraldehyde I: Reaction with Model Compounds. Connective Tissue Research 1982, 10(2), 187–199.
- Hardy, P.M.; Nicholls, A.C.; Rydon, H.N. The Nature of the Cross-Linking of Proteins by Glutaraldehyde. Part I. Interaction of Glutaraldehyde with the Amino-Groups of 6-Aminohexanoic Acid and of alpha-N-acetyl-lysine. Journal of the Chemical Society Perkin Transactions 1976, 9(9), 958–962.
- Snibson, G.R.;. Collagen Cross-Linking: A New Treatment Paradigm in Corneal Disease-A Review. Clinical and Experimental Ophthalmology 2010, 38(2), 141–153. DOI: 10.1111/j.1442-9071.2010.02228.x.
- Kanellopoulos, A.J.;. Long Term Results of A Prospective Randomized Bilateral Eye Comparison Trial of Higher Fluence, Shorter Duration Ultraviolet A Radiation, and Riboflavin Collagen Cross Linking for Progressive Keratoconus. Clinical Ophthalmology 2012, 6(6), 97–101. DOI: 10.2147/OPTH.S27170.
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z, Dionne, M.J.; Clement, E.M.; Conrad, G.W. Mechanisms of Corneal Tissue Cross-Linking in Response to Treatment with Topical Riboflavin and Long-Wavelength Ultraviolet Radiation (UVA). Invest Ophthalmol Vis Sci 2010, 51(1), 129–138. DOI: 10.1167/iovs.09-3738.
- Van Best, J.A.; Bollemeijer, J.G.; Sterk, C.C. Corneal Transmission in Whole Human Eyes. Experimental Eye Research 1988, 46(5), 765–768.
- Kadler, K.;. Extracellular Matrix 1: Fibril-Forming Collagens. Protein Profile 1995, 2(5), 491–619.
- Fraser, R.D.; Macrae, T.P.; SuzukiFraser, E. Chain Conformation in the Collagen Molecule. Journal of Molecular Biology 1979, 129(3), 463–481.
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, Thermal and Chemical Degradation of Riboflavin. Beilstein Journal of Organic Chemistry 2014, 10, 1999-2012. DOI:10.3762/bjoc.10.208
- Zhu, Y.; Reinach, P.S.; Zhu, H.; Tan, Q.; Zheng, Q.; Qu, J.; Chen, W. High-Intensity Corneal Collagen Crosslinking with Riboflavin and UVA in Rat Cornea. Plos One 2017, 12(6), 1–14.
