?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The factors affecting intracellular ice formation (IIF) and growth is essential to the mechanistic understanding of cellular damage through freezing. In the aid of high speed and high-resolution cryo-imaging technology, the broad bean intracellular ice formation and growth processes were successfully captured during freezing. Cytochalasin B(CB)was used to solubilize the cytoskeleton. Images of IIF were compared between cells with and without cytoskeleton. The behavior of intracellular ice crystal formation in plant tissues with or without CB was evaluated using changes of cell areas, the probability of crystallization, and growing rate of intracellular ice crystal. Moreover, light intensity figures were used to determine cell damage. This study showed that the cytoskeleton was involved in ice crystal nucleation mechanism during freezing responses of the plant cells.
Introduction
Cryopreservation of living materials such as foods, Biological organs and tissues can reduce bacterial and plant spoilage due to respiration. [Citation1] However, since living substances contain moisture, when they are placed at a temperature lower than the freezing point, they will spontaneously freeze by being overcooled. The response of cells to freezing could lead to cell damage [Citation2,Citation3] because IIF mechanically directly organelles and membranes. [Citation4,Citation5]
Intracellular freezing did not only lead to cell death [Citation2,Citation3,Citation6,Citation7], but also some other problems, such as it was believed that ice crystals formed both outside and within a cell caused the mollification of the plant tissue of vegetables and fruits. [Citation8,Citation9] More and more evidence showed that the understanding of ice nucleation theory was key for the cryopreservation of cells. [Citation10] There were a lot of standard texts describing the processes and mechanism of ice nucleation and crystal growth in different kinds of cells. [Citation11–Citation14] Ken Muldrew and Locksley E. McGann proposed that the osmotic rupture hypothesis of cryoinjury was introduced to explain the phenomenon of intracellular freezing due to the failure of prevailing theories to account for several experimental challenges. [Citation15,Citation16]
The control of ice nucleation was exploited in a lot of process technologies including deformation degree of food after freezing [Citation17], cryogelation of polymers [Citation18] and formation of uniform porosity materials. [Citation19] Kasper thought the ice nucleation temperature was recognized as a key factor in determining both the processing time and recovery of biological activity on rehydration. [Citation20] Duohua Xu [Citation21] studied the crystallization process of onion cells and showed different numbers of ice in different cells. However, due to the limitations of imaging technology, the influence of the internal structure of cells on the growth of ice crystals has not been analyzed. In this paper, a high-speed camera was used instead of an ordinary CCD to further observe the changes in the internal structure of the cell. The structure that controls the growth of ice crystals in cells and exerts regulatory effects should be learned.
Shigehiko [Citation22] studied freezing injury of agricultural products, and he found that the freezing process of cells was affected by the cell wall and the intercellular structure. And cytoskeletons in the cell also played a significant role in maintaining the cell structure and affected significantly the quality and physiochemical property of foods. Learning about how the cytoskeletons adjust the intracellular Ice growth during freezing was important to develop better freezing methods, control freezing processes, and improve the quality of the frozen products. When studying the role of the cytoskeleton in experiments of the antifreeze properties of plants, cytochalasin was used to destroy the cytoskeletal component to observe cellular responses. [Citation23,Citation24]
Therefore, this paper aimed to provide detailed information on the effect of cell structure on the mechanism of intracellular ice crystal growth. Specifically, disruption of the actin cytoskeleton with CB lowered the mechanical strength and stiffness. Microscopic freezing behaviors, such as freezing patterns, crystallization temperature, and cell deformability, were observed under different cooling conditions. Next, morphological changes of the cells after freezing and thawing were observed in order to quantify typical morphological changes: transmitted light intensity. The research results expand our understanding of the cytoskeleton in cells during freezing.
Materials and methods
Sample preparation
Ripe broad beans were purchased from vegetable gardens in Tianjin, China. Broad bean seeds were selected and cut into 10 × 10 mm cubes. The cubes were placed under the vibrating microtome (Leica VT1200) and cut into thin slices of 80 μm as the research object. Finally, the broad bean seed cells were immersed in 10% CB for 20 min to achieve complete lysis of the cytoskeleton, which were used as an experimental group and normal cells were used as a control group.
Cryo-microscopy study
The cryomicroscopic system used in this study consisted of a microscope (BX51, Olympa, Japan) equipped with a cooling stage (BCS196, Linkam, Britain) and a high-speed camera (the Cooke Corporation, USA), as shown in . The high-speed camera could capture images at a maximum frame rate of 2000 frames per second (fps) and a resolution of 512 × 512 pixels using Phantom Camera Control software package (Version9.0.640.0-C PhCon:640) and was used to document the IIF behavior during intracellular freezing. Fluorescent lamps are used instead of halogen lamps to illuminate plant samples through condensers. Finally, Intracellular freezing in the plant specimens was then captured by the high-speed camera through an objective lens.
The combined air was poured into a cooling station for cooling by using liquid nitrogen and a heater, and the cooling rate was maintained at 1°C/min by a computer. Nitrogen gas was passed to the cooling table to prevent turbidity in the viewing window.
Experimental method
The sample cells were placed between two coverslips and then were placed onto a cryostage, which was charged with liquid nitrogen and connected to a computer. During the freezing process, the temperature was lowered to 0°C at a cooling rate of 5, 20, 60, and 100°C/min, respectively. The sample was held at 0° C for 1 min to reach a steady state and continue to be cooled to −40°C. Finally, the sample was warmed to room temperature with a heating rate of 20°C/min.
The above parameters were controlled by Linksys32 software. In order to achieve effective and consistent experimental results, experiments were performed at least 8 times at each cooling rate. The whole freezing and thawing processes were observed with a 20× long working distance objective and the experimental pictures were recorded by a camera system. The resolution size and time settings were adjusted according to various image requirements. [Citation25]
Image processing
All the images of broad bean cells taken during the process of freezing and thawing were processed and analyzed by using Image-Pro plus software.
Results and discussion
Effect of cytoskeleton on cells
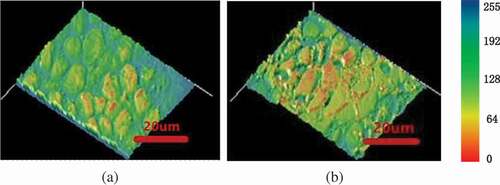
The growth of intracellular ice crystals changed the cell structure. Similarly, changes in cell structure also affected the growth of ice crystals. As shown in , the effect of cytoskeleton was observed on the cell before and after freezing. shows the cells treated with CB for 20 min at room temperature, comparing with the control group as shown in . Two sets of experimental pictures were compared and it is found that intercellular space became larger and the light transmission of the cells increased. To further compare the effects of cytoskeleton on cell and intercellular space, the generated microstructure from the microscopic image was presented. shows the microstructure of cells with CB and shows the microstructure of normal cells. In order to represent the size of the pore changes between cells, the total area () in the picture and sum of all cell areas (
) were measured and defined the cell porosity (ε):
Figure 2. In experimental pictures, changes of intercellular space and light transmission at room temperature in two states: (a) cells with CB, (b) normal cells.

Figure 3. The intercellular space became larger after adding cytoskeleton B. The generated microstructure images at room temperature were presented: (a)cells with CB, (b) normal cells. (c) The variety of the cell porosity at room temperature.
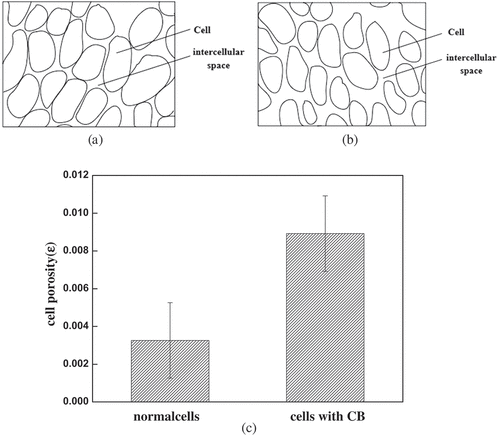
The calculation of cell porosity based on statistical principles is shown in ). The normal cell-to-cell porosity accounted for approximately 0.326%. The cells treated with CB reached 0.621%, which was about 2 times that of normal cells. The pictures of light intensity at room temperature were obtained by Image-Pro Plus software (). The green area represents the light that passes through. The smaller the green area distributed, the greater the light intensity. The light transmission intensity of cells without cytoskeleton was higher than that of normal cells ( vs4b). The increase in cell translucency indicated that cells treated with CB were damaged to a certain extent before freezing cells.
Role of cytoskeleton in freezing process
Probability of crystallization
A typical intracellular ice formation and growth process of plant cells with cooling rate of 60℃/min is shown in . The exposure time of the camera was set at 5 ms. Extracellular ice was seeded with a pre-cooled needle at 0℃ (), and intracellular ice occurred at −20℃ (). The red circle labeled the site where intracellular ice started. As the temperature was further reduced, intracellular ice grew toward the center of the cell (). Ice crystals stopped growing at around −36℃ in the cell (). The growth of ice crystals without cytoskeleton in cells is shown in . The cells started to appear ice crystals at −15°C (), ice crystals grew to the center of the cells at −24°C (), and ice crystals grew completely at −31℃(). By contrast, cells without cytoskeleton had higher crystallization temperature and shorter crystallization time than normal cells. The cytoskeleton had a quite important role in determining the growth of the intracellular ice crystals. However, if some subtle evidence of ice formation is hidden by the subtle changes of images, the gray-level variation detection method may help to discover some unnoticed evidence. [Citation26] and show the processed images of the intracellular ice growth in two kinds of cells by this method. The red zone represented the ice crystal, which displayed the relative gray-level variation between the image and the reference image. According to this method, the growth process of ice crystals was observed clearer and the growth area of ice crystals was calculated more accurately.
Figure 5. Progress of intracellular freezing in normal cells at a cooling rate of 20℃/min. These images were processed using filtering, and the contrast and brightness were adjusted using image analysis software. Temperature elapsed since the start of intracellular freezing: (a) 0℃, (b) −20℃, (c) −30℃, (d) −36℃.
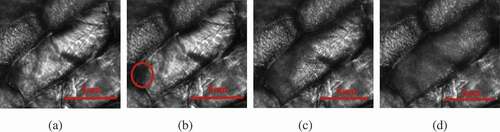
Figure 6. Progress of intracellular freezing in cells with CB at a cooling rate of 20℃/min. These images were processed using filtering, and the contrast and brightness were adjusted using image analysis software. Temperature elapsed since the start of intracellular freezing: (a) 0℃, (b) −20℃, (c) −24℃, (d) −31℃.
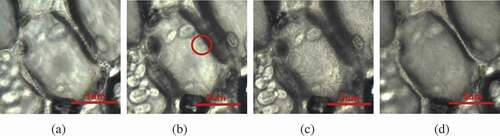
Figure 7. The relative gray-level variation of intracellular freezing in normal cells at a cooling rate of 20℃/min. Temperature elapsed since the start of intracellular freezing: (a) 0℃, (b) −20℃, (c) −30℃, (d) −36℃.

Figure 8. The relative gray-level variation of intracellular freezing in cells with CB at a cooling rate of 20℃/min. Temperature elapsed since the start of intracellular freezing: (a) 0℃, (b) −20℃, (c) −24℃, (d) −31℃.

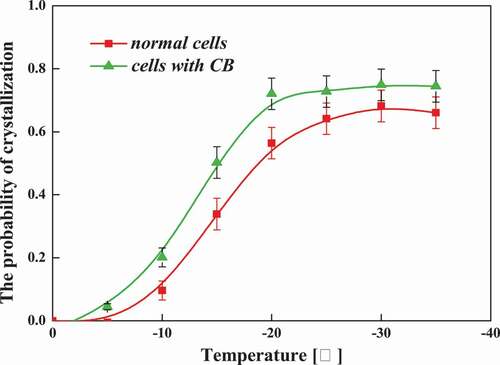
To further compare the cellular dependence of the IIF, and to analyze more rigorously the influence of the cytoskeleton on the ice crystal growth process. The probability of crystallization as a function of temperature was studied in broad bean cells at a cooling rate of 60°C/min. Geer Yang [Citation27] used Equation 2 to calculate the amount of intracellular ice growth when he was experimenting with ice seeding on the occurrence and effects of cancer cells. Similarly, we define the probability of crystallization as follows:
Results are shown in . The cytoskeleton has an important effect on the occurrence of intracellular ice crystals, because the cytoskeleton affects water transport. Obviously, the intracellular ice seems to be formed more easily in cells without cytoskeleton than in the normal ones under the same frozen condition. The probability of IIF in normal cells was higher than that in cells without cytoskeleton at the temperature over the whole range from −6 to −30℃, especially above −20℃. It indicates that the cytoskeleton effect was significant in the initiation of intracellular ice. The presence of a cytoskeleton resisted the growth of intracellular ice and further protected cells during freezing.
Growth rate of intracellular ice
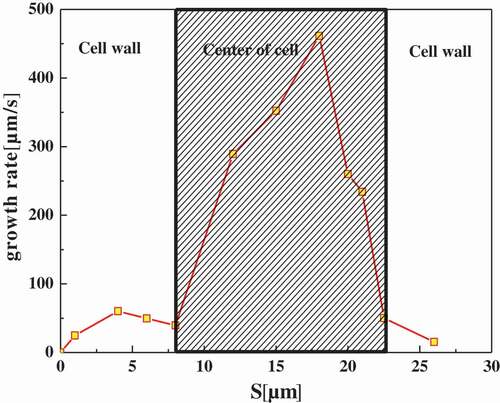
Further, the effect of cytoskeleton on intracellular ice crystal growth, the area of intracellular ice was measured using Image-Pro plus by quantifying the inter-distances of dendrites, to calculate the growth rate of intracellular ices. The transient growth rates of intracellular ice were given in and . At the beginning, the intracellular ice growth rate was relatively smaller, less than 100 μm/s, and then increased slowly right after the ice crystal initiation. When The ice front approached the edge of the cell, the propagation velocity slightly decreased, followed by a sharp increase as it passed through the center of cell. After reaching the peak, the ice growth velocity dropped to a lower level similar to that in the cell wall. At the beginning as the ice arrived nearly on the other side of the cell. Obviously, the average growth rate of intracellular ice in the center of the cell was much larger than in cell wall. By comparing whether the cells are treated with CB ( vs ), it can be seen that the cells without cytoskeleton grow faster than the normal cells.
Figure 10. Intracellular ice crystal growing rate at different cooling rates in normal cells. The shaded area in the graphs distinguished between the cell wall and the center of a cell.
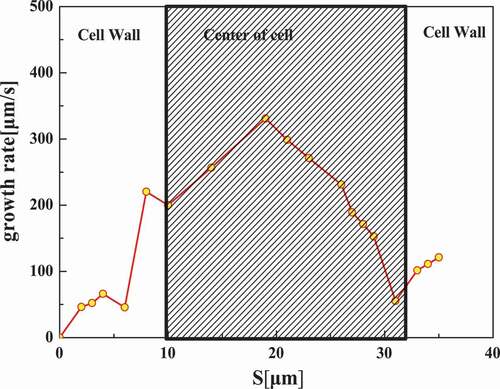
Figure 11. Intracellular ice crystal growing rate at different cooling rates in a cell with CB. The shaded area in the graphs distinguished between the cell wall and the center of the cell.
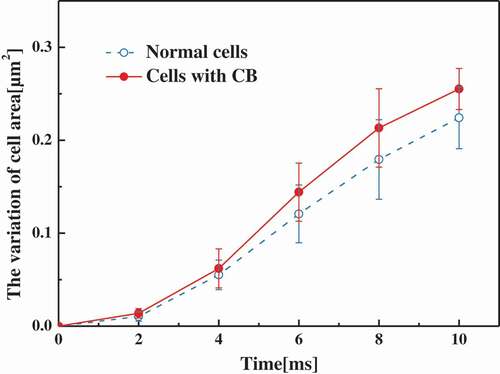
The difference in moisture provides the motivation for the growth of ice crystals. [Citation28–Citation30] Cell areas in different conditions were measured by Image-Pro plus. The amount of change in the area indicated how much the cells lose water. Variation of the ratio of cell area to original cell area with time increasing as shown in . It was found that cell area without cytoskeleton changed less than normal cells, so cells without cytoskeleton lost less water than normal cells. The diffusion rate of water molecules in cells treated with CB became lower. At the same time, the disappearance of the cytoskeleton further increases the differences in the water content across the membrane. Correspondingly, during the cooling process, the degree of supercooling of the liquid in the cell is much greater than that in the extracellular. A larger degree of undercooling results in a faster rate of ice crystal growth [Citation31], which also explains to some extent the spatial differences in the growth of ice crystals inside the cell. Since the intracellular environment is anisotropic, intracellular ice crystal growth is affected by many microscale or nanoscale structural changes inside the cell. This experiment found that the presence of a cytoskeleton reduces the growth rate of intracellular ice crystals. To explain this experimental phenomenon more accurately, it is necessary to further study these micro-scale or nanoscale instantaneous details. However, it can be found that the intracellular microenvironment and microstructure were very important influencing factors for intracellular ice crystal formation and growth.
Conclusion
In this study, the cryo-microscopic observation was performed to investigate intracellular cellular formation and growth in plant cells during freezing. The cytoskeleton affected the cell porosity and light transmission before freezing. The cell porosity of cells treated with CB became larger, about twice that of normal cells. And by comparing the light intensity figures, it was found that the cells without the skeleton structure became light transmissive and the cell structure changed significantly. In the freezing process, the method of gray scale detection was used to observe the growth of ice crystals in cells. The cytoskeleton affected the initiation of intracellular ice. At the same temperature, IIF of cell treated with CB was more likely to occur than normal cells. Cells without cytoskeleton structure have a higher crystallization temperature and a shorter crystallization time. However, the cytoskeleton did not affect the growth process of intracellular ice. Similar to normal cells, ice crystals first appeared in the cell wall and grew along the cell wall toward the cell center. The growth rate of ice crystals was an important indicator for evaluation during the study of intracellular ice growth. The ice crystal growth rate in cells without the skeleton structure was higher than that in the normal cells. The growth rate of ice crystals is generally affected by differences in intracellular water. Therefore, the variation of cell area in the cell area was calculated. At the same time, the change in cell area was greater than that of normal cells. This also proved that the increase in the growth rate of intracellular ice crystals is caused by an increase in water loss. The microstructure inside the cell was an important factor in the growth of ice crystals, but most methods were in the early stages of development. Therefore, more research is needed in the future. It is hoped that the research methods and results on cytoskeleton in this paper should shed more lights on techniques and methods for improving frozen food quality for the food industry.
Acknowledgments
The support by the National Natural Science Foundation of China (Grant No.51676139) is greatly acknowledged.
Additional information
Funding
References
- Fonseca, S.C.; Gil, L.; Manso, M.C.; Cunha, L.C. Modelling the Influence of Storage Temperature and Time after Cutting on Respiration Rate of Diced Red Onions (Allium Cepa L. Cv. Vermelha Da Póvoa). Postharvest Biology & Technology. 2018, 140(C), 27–33. DOI: 10.1016/j.postharvbio.2018.02.003.
- Hoffmann, N.E.; Bischof, J.C. The Cryobiology of Cryosurgical Injury. Urology. 2002, 60(2), 40–49.
- Lagerveld, B.W.;. Cryosurgical Induced Injury of Human Cancerous Tissues – How It Works? British Journal of Medical & Surgical Urology. 2012, 5(5), S24–S27. DOI: 10.1016/S1875-9742(12)60006-8.
- Fujikawa, S.;. Freeze-Fracture and Etching Studies on Membrane Damage on Human Erythrocytes Caused by Formation of Intracellular Ice. Cryobiology. 1980, 17(4), 351–362.
- Acker, J.P.; Mcgann, L.E. Membrane Damage Occurs during the Formation of Intracellular Ice. Cryo Letters. 2001, 22(4), 241–254.
- Ninagawa, T.; Eguchi, A.; Kawamura, Y.; Konishi, T. A Study on Ice Crystal Formation Behavior at Intracellular Freezing of Plant Cells Using A High-Speed Camera. Cryobiology. 2016, 73(1), 20–29. DOI: 10.1016/j.cryobiol.2016.06.003.
- Levitt, J. Responses of Plants to Environmental Stress, 2nd Edition, Chilling, Freezing, and High Temperature Stresses. Physiological Ecology, Ademic Press, New York. 1980, 1, 134.
- Brown, M.S.;. Texture of Frozen Vegetables: Effect of Freezing Rate on Green Beans. Journal of the Science of Food & Agriculture. 2010, 18(2), 77–81. DOI: 10.1002/jsfa.2740180209.
- Barbosacanovas, G.V.; Altunakar, B.; Mejialorio, D. Freezing of Fruits and Vegetables. An Agribusiness Alternative for Rural and Semi-Rural Areas. Fao Agricultural Services Bulletin. 2005, 36(9), 3911–3915.
- Morris, G.J.; Acton, E. Controlled Ice Nucleation in Cryopreservation–A Review. Cryobiology. 2013, 66(2), 85–92. DOI: 10.1016/j.cryobiol.2012.11.007.
- Bartelsrausch, T.; Bergeron, V.; Cartwright, J.H.E.; Escribano, R. Ice Structures, Patterns, and Processes: A View across the Ice-Fields. Review of Modern Physics. 2012, 84(2), 885–944. DOI: 10.1103/RevModPhys.84.885.
- Zaragotas, D.; Liolios, N.T.; Anastassopoulos, E. Supercooling, Ice Nucleation and Crystal Growth; a Systematic Study in Plant Samples. Cryobiology. 2016, 72(3), 239–243. DOI: 10.1016/j.cryobiol.2016.03.012.
- Matsumoto, M.;. Molecular Dynamics of Ice Nucleation and Crystal Growth[J]. 冷凍. 2012, 87, 554–559.
- Amaral, M.; Miller, A. L.; Magee, N. B. Environmental Scanning Electron Microscopy of Ice Crystal Nucleation and Growth// AGU Fall Meeting. AGU Fall Meeting Abstracts, San Francisco, 2012.
- Muldrew, K.; Mcgann, L.E. The Osmotic Rupture Hypothesis of Intracellular Freezing Injury. Biophysical Journal. 1994, 66(2 Pt 1), 532–541.
- Steponkus, P.L.; Dowgert, M.F.; Gordon-Kamm, W.J. Destabilization of the Plasma Membrane of Isolated Plant Protoplasts during a Freeze-Thaw Cycle: The Influence of Cold Acclimation. Cryobiology. 1983, 20(4), 448–465.
- Petzold, G.; Aguilera, J.M. Ice Morphology: Fundamentals and Technological Applications in Foods. Food Biophysics. 2009, 4(4), 378–396. DOI: 10.1007/s11483-009-9136-5.
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The Potential of Polymeric Cryogels in Bioseparation. Bioseparation. 2001, 10(4–5), 163–188.
- Ware, C.B.; Nelson, A.M.; Blau, C.A. Controlled-Rate Freezing of Human ES Cells. Biotechniques. 2005, 38(6), 879–880. DOI: 10.2144/05386ST01.
- Kasper, J.C.; Friess, W. The Freezing Step in Lyophilization: Physico-Chemical Fundamentals, Freezing Methods and Consequences on Process Performance and Quality Attributes of Biopharmaceuticals. European Journal of Pharmaceutics & Biopharmaceutics. 2011, 78(2), 248–263. DOI: 10.1016/j.ejpb.2011.03.010.
- Xu, D.; Wang, H.; Wang, Y.; Zhang, Z. Ice Crystal Growth of Living Onion Epidermal Cells as Affected by Freezing Rates. International Journal of Food Properties. 2018, (11). DOI: 10.1080/10942912.2018.1439506.
- Ohnishi, S.; Fujii, T.; Miyawaki, O. Electrical and Rheological Analysis of Freezing Injury of Agricultural Products. International Journal of Food Properties. 2002, 5(2), 317–332. DOI: 10.1081/JFP-120005788.
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early Steps in Cold Sensing by Plant Cells: The Role of Actin Cytoskeleton and Membrane Fluidity. Plant Journal. 2010, 23(6), 785–794. DOI: 10.1046/j.1365-313x.2000.00845.x.
- Tuteja, N.; Gill, S. S. 6 Abiotic Stress Response in Plants: Role of Cytoskeleton// Abiotic Stress Response in Plants. Wiley‐VCH Verlag GmbH & Co. KGaA. New Jersey, USA. 2016, 835-7.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nature Methods. 2012. DOI: 10.1038/nmeth.2089.
- Wang, Y.; Zhu, K.; Zhang, X.; Ji, H. Applications of Gray-Level Variation Detection Method to Intracellular Ice Formation. Cryobiology. 2018, 81–87. DOI: 10.1016/j.cryobiol.2018.02.006.
- Yang, G.; Zhang, A.; Xu, L.X. Intracellular Ice Formation and Growth in MCF-7 Cancer Cells. Cryobiology. 2011, 63(1), 38–45. DOI: 10.1016/j.cryobiol.2011.04.007.
- Päuser, S.; Zschunke, A.; Khuen, A.; Keller, K. Estimation of Water Content and Water Mobility in the Nucleus and Cytoplasm of Xenopus Laevis, Oocytes by NMR Microscopy. Magnetic Resonance Imaging. 1995, 13(2), 269–276.
- Morrill, G.A.; Kostellow, A.B.; Osterlow, K.; Gupta, R.K. Differences in Hydration State of Nucleus and Cytoplasm of the Amphibian Oocyte. Journal of Membrane Biology. 1996, 153(1), 45–51.
- Hallett, J.;. Experimental Studies of the Crystallization of Supercooled Water. Journal of the Atmospheric Sciences. 1964, 21(21), 671–682. DOI: 10.1175/1520-0469(1964)021<0671:ESOTCO>2.0.CO;2.
- Mazur, P.;. Freezing of Living Cells: Mechanisms and Implications. American Journal of Physiology. 1984, 247(3 Pt 1), C125. DOI: 10.1152/ajpcell.1984.247.3.C125.