ABSTRACT
Blueberry (Vaccinium spp.) is a widely consumed fruit worldwide. Anthocyanins, phenolic acids, and flavonoids are the main bioactive compounds of blueberry. However, little information is available about the changes in phenolic profiles of blueberries during fruit maturation. The aim of this work was to assess the effects of genotype and maturity on phenolic compounds and antioxidant ability of four rabbiteye blueberry cultivars grown in Guizhou, China. The total phenolics, total flavonoid and individual phenolic compound in the rabbiteye blueberries were investigated at five ripening stages based on color. Derivatives of phenolic compounds (gallic acid, epicatechin, rutin, p-coumaric acid, quercetin, catechin, ellagic acid, chlorogenic acid, and ferulic acid) were determined by HLPC and quantified using calibration curves. Antioxidant capacity was evaluated by total reducing power assay (TRPA), 1,1-diphenyl-2- picrylhydrazyl (DPPH), ferric ion reducing antioxidant power (FRAP), and 2,2ʹ-Azinobis-(3-ethylbenzthiazoline-6-sulfonate (ABTS) assay. Gallic acid, ellagic acid, and ferulic acid were the main phenolic compounds in rabbiteye blueberries, and phenolic compound contents of different rabbiteye blueberries cultivars changed with ripening stage. The gallic acid content of all cultivars increased at first and then decreased. The ferulic acid contents of ‘Powderblue’ and ‘Gardenblue’ cultivars gradually increased during ripening. The ellagic acid content of ‘Powderblue’ blueberries increased with ripening but decreased in ‘Baldwin’ blueberries. The total phenolics, total flavonoid, and antioxidant capacity of all cultivars increased nonlinearly with ripening. Phenolic compounds were the main antioxidants found in rabbiteye blueberries. Notably, ‘Gardenblue’ had exceptionally higher phenolics compound and antioxidant activity compared with other cultivars.
Introduction
Blueberry (Vaccinium spp) is a widely consumed fruit worldwide owing in part to its richness in bioactive compounds and excellent antioxidant activity.[1–Citation3] New blueberry cultivars had been bred and selected based on their bioactive compounds and antioxidant activity.[Citation4] Anthocyanins, phenolic acids, and flavonoids are the main bioactive compounds of blueberry. Anthocyanin synthesis has been accelerated through the increasing maturity of highbush blueberry, with fruit skin color steadily becoming darker and bluer, reaching a peak value at a purple-black stage.[Citation5,Citation6] Phenolic acids, mainly located in the cell wall tissue of blueberries, are major secondary metabolites in blueberry fruits with important implications for human health.[Citation7,Citation8] The effects of the genotype, growing seasons, climate conditions, ripeness, and storage conditions on the phenolic content and antioxidant activity in the blueberry fruits have been reported.[Citation1,Citation7,Citation9–Citation13] Previous studies have reported that blueberries are rich in phenolics, which are greatly affected by varieties and producing regions[Citation14,Citation15], and phenolic compounds, including quinic acid, chlorogenic acid, gallic acid, p-coumaric acid, caffeic acid, ferulic acid, ellagic acid, rutin, catechin, epicatechin, myricetin, quercetin, and kaempferol.[Citation13,Citation16–Citation21]
The concentrations of phenolic acids and flavonols in unripe green and fully ripe fruits were high and similar, whereas the lowest levels were found in intermediately ripe fruits. Flavonol content, hydroxycinnamic acid content, and antioxidant activity decreased during ripening. The same trend was observed for total antioxidant activity. This could be owing to the higher phenolic acid at immature fruit stages. Blueberry fruit had high total antioxidant activity during ripening, with green fruit showing the highest total soluble phenolic content, and ripe fruit had higher α-amylase and α-glucosidase inhibitory activity than those of green or green/pink fruit. The high total antioxidant activity in mature fruits could be explained by the elevated amounts of anthocyanin.[Citation1,Citation3,Citation5,Citation11] The phenolic compound and flavonoid contents showed evident antioxidant capacity. All phenolic contents were highly correlated with total antioxidant capacity and reducing power.[Citation2,Citation9,Citation10,Citation16]
Little information is available about the changes in phenolic profiles and antioxidant ability of blueberries during fruits maturation in China. Therefore, the aim of this work was to assess the effects of genotype and maturity on phenolic compounds and antioxidant ability of four rabbiteye blueberry cultivars grown in Guizhou, China. This approach identifies specific cultivars with the aim of maximizing the bioactive compounds for food, nutraceutical, and pharmaceutical industry uses as well as for further blueberry breeding programs.
Materials and methods
Plant materials
Blueberry fruits were collected from four different rabbiteye blueberries cultivars (Vaccinium ashei Reade): ‘Powderblue,’ ‘Gardenblue,’ ‘Baldwin,’ and ‘Bright Well.’ A completely randomized design with three replications was used. The four cultivars were cultivated in the same farm at the Long-Ben planting base in Majiang County (Guizhou Provence, China)(Location: N26°37′, E107°44′, Altitude: 800 m). All cultivars were the same age and were cultivated on homogeneous soil with technical regulations for the cultivation of blueberry in Majiang county, China.
Samples of blueberries were hand-harvested at five ripening stages, based on visual observation of fruit color, ranging from green, green/red, red, red/blue to blue during fruit development (correpresenting to ripening stages R1, R2, R3, R4, and R5). Each 200-g fruit sample was randomly collected from four plants of each cultivar block, from both sides of the canopy, and from different areas of the clusters. After harvest, the fruits were transported to Guizhou Engineering Research Center for fruit processing under refrigeration. The samples were washed with ultra-pure water and then homogenized using a lapping machine (Model A11, IKA, Germany), and stored at −80°C until later analysis.
Extraction and quantification of phenolic compounds
For the quantification of phenolic compounds, sample extraction was performed using the method described by Xie et al.,[Citation21] with modification. Briefly, approximately 10.0 g of frozen fruits were ground into powder in a mortar in an ice bath, and 2.0-g fruit samples were then transferred into 10-mL centrifuge tubes with 4.0 mL 3‰ trifluoroacetic acid (TFA): methanol (1: 49, v/v). The sample was extracted in an ultrasonic wave cleaner at 50°C for 30 min and centrifuged in a PF-24R centrifuge at 12000 rpm for 10 min. After filtration to remove the residual particles, all supernatants were combined in a flask to obtain 10 mL of extract, which was filtered through a 0.45-μm aqueous membrane. The extractions were performed in triplicate.
Analysis and quantification of phenolic compounds
The HPLC-UV/Vis analyses were performed as those described in a previous study.[Citation21,Citation22] HPLC analysis for phenolic compounds was performed using a Prominence CBM-20A system (SHIMADZU, Japan) with an LC-20AT liquid chromatograph, a SIL-20A Prominence autosampler, a CTO-10A SHIMADZU column oven, and an SPD-20A Prominence UV/Vis detector. The analytical column was an XBridge BEH C18 column (250 × 4.6 mm, 5 μm i.d.; Waters, Taunton Ireland) protected with a C18 Van Guard Cartridge (Waters, Taunton Ireland). Eluent A was double distilled water containing 3% TFA; eluent B was methanol. The following elution gradient was used: 88% B at 0 ~ 5 min, 75% B at 10 min, 75% B at 15 min, 70% B at 20 min, 70% B at 25 min, 65% B at 35 min, 65% B at 40 min, 50% B at 50 min, 88% B at 55 min, 88% B at 60 min. For HPLC analysis, 10 μL of analytes was injected with a flow rate of 0.8 mL/min and a constant column temperature of 30°C. Chromatograms were acquired at 280 nm to identify phenolic compounds, and identification was based on the retention time and UV-spectra. The quantification of phenolic compounds in blueberry fruit was based on the linear regression of peak areas of commercial standards. Gallic acid, epicatechin, rutin, p-coumaric acid, quercetin, catechin, ellagic acid, chlorogenic acid, and ferulic acid standards were used for quantification of phenolic compounds. The results of phenolic compound were expressed as mg kg−1 fresh weight of fruit.
Total phenolics and total flavonoid content
Total phenolics content was quantified using Folin – Ciocalteu’s reagent.[Citation23] Briefly, 0.5 mL of extract was transferred into the 25-mL calibration tube, to which 1.5 mL of Folin – Ciocalteu’s agent and 3.0 mL of 12% Na2CO3 were added. After a 30-s reaction, the remainder of the tube was filled up to 25 mL with distilled water. Absorption at 760 nm was measured using a spectrophotometer (model UV-2550; Shimadzu, Japan) after 2 h at 25°C in the dark. Total phenolics were expressed as gallic acid equivalents (GAE) in mg 100 g−1 fresh weight of fruit.
Total flavonoids were determined using the method developed by Dragović-Uzelac et al.[Citation10] Briefly, 0.5 mL of extract was placed in 10-mL calibration tubes, to which 0.3 mL of 5% NaNO2 and 1.0 mL of 10% Al(NO3)3 were added after 5 min. After the mixture was shaken, 4.0 mL of 1.5 mol·L−1 NaOH was added, and 6 min later, the tube was filled up to 25 mL with distilled water. Tube was again well shaken and then centrifuged for 10 min. Absorption at 510 nm was measured by using a spectrophotometer (model UV-2550; Shimadzu, Japan). Total flavonoid content was recorded as rutin equivalents (RE) in mg 100 g−1 fresh weight of fruit.
Antioxidant activity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity of extracts was assessed according to the method described by Tauchen et al.[Citation24] with minor modifications. Briefly, 50 μL of extract was added to 150 μL of DPPH (0.4 mM), and the mixture was shaken vigorously. The free radical scavenging capacity was evaluated by measuring the absorbance at 515 nm using a spectrophotometer (model UV-2550; Shimadzu, Japan) after 30-min of reaction period at 37°C on a biochemical incubator. The results were recorded as trolox equivalents (TE) in mmol 100 g−1 fresh weight of fruit.
The ferric ion reducing antioxidant power (FRAP) assay was conducted according to the method described by Todorovic et al.[Citation25] with some modifications. Briefly, stock solutions were prepared, and they included 300 mM acetate buffers (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl, and 20 mM FeCl3 6H2O solution. The fresh working solution was made by mixing 25 mL acetate buffers, 2.5 mL TPTZ solution, and 2.5 mL FeCl3 6H2O solution and then warmed to 37°C before use. Then, 10-μL extract samples were allowed to react with 190 μL of the FRAP solution for 10 min at 37°C on a biochemical incubator. The absorbance at 593 nm was measured using a spectrophotometer (model UV-2550; Shimadzu, Japan). FRAP was recorded as TE in mmol 100 g−1 fresh weight of fruit.
The total reducing power assay (TRPA) was conducted according to the methods described by Oliveira et al.[Citation26] Briefly, 0.1-mL extract samples were diluted with 1.0 mL of 0.2 mol L−1 PBS (pH 6.6) and mixed with 1.0 mL of 1% (w/v) K3[Fe(CN)6]. The mixture was incubated for 20 min at 50°C. After the addition of 1.0 mL of 10% (w/v) trichloroacetic acid, distilled water was added to reach a volume of 25 mL. Then, the mixture was centrifuged at 3000 rpm in a centrifuge (model PF-24R; Pingfan) for 8 min at 4°C. The upper layer (2.5 mL) was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% FeCl3, and the absorbance at 700 nm was measured using a spectrophotometer (model UV-2550; Shimadzu, Japan). TRPA was recorded as TE in mmol 100 g−1 fresh weight of fruit.
The 2,2ʹ-azinobis-(3-ethylbenzothiazoline)-6-sulfonate (ABTS) scavenging activity of extracts was estimated according to a previously described by Schaich et al.[Citation27] Briefly, the working solution of ABTS was produced by reacting 14 mmol L−1 ABTS with 4.9 mmol L−1 potassium persulfate in the dark at room temperature for 12 h before use. The ABTS radical solution was diluted with phosphate buffer to an absorbance of 0.70 ± 0.02 at 734 nm. After the addition of 50 μL of sample to 250 μL of the working solution of ABTS, the absorbance at 734 nm was taken after 1 min using the spectrophotometer (model UV-2550; Shimadzu, Japan). ABTS was recorded as TE in mmol 100 g−1 fresh weight of fruit.
Statistical analysis
Extraction and all analyses were run in duplicate, and the results were expressed as mean ± standard error. Statistical analyses were performed with the IBM SPSS version 19.0 (SPSS Statistical Software, Inc., Chicago, IL, USA). The one-way ANOVA was performed, and the ducan’s test (P < 0.05) was used to compare the means among maturity and cultivars.
Results and discussion
Total phenolics content and total flavonoid contents
The results for changes in total phenolics and total flavonoid contents of rabbiteye blueberries are shown in . The total phenolics contents of ‘Powderblue’ and ‘Baldwin’ fruits tended to increase with ripening and peaked at the R5 stage. The total phenolics and flavonoid contents of ‘Bright Well’ fruits decreased at the R2 stage and then increased. The total phenolics content of ‘Gardenblue’ fruits increased during the R2 stage, decreased to the R3 stage, and then increased. Kalt et.al (2003) and Castrejo´n et.al (2008) examined total phenolic of highbush blueberries (cvs. ‘Reka’, ‘Puru’, ‘Bluecrop’, ‘Berkeley’, ‘Bergitta’, ‘Bluegold’, and ‘Nelson’) at different stages of ripening and harvest: At comparable maturity stages as in the present study, total phenolic content ranged in average from 70.2 (‘Bluecrop’ at unripe green berries) to 11.7 (‘Bergitta’ at ripe blue) mg GAE/g DM.[Citation1,Citation28] Previously reports indicated that total phenolics contents of ripe fruits are 1.71–2.63, 2.37–4.19, 1.17–1.68, 0.98–2.31 mg GAE · g−1 in ‘Powderblue’, ‘Gardenblue’, ‘Baldwin’, and ‘Bright Well’, respectively.[Citation15,Citation21,Citation29] Those values are lower than our estimates of 3.9–26.25 mg GAE ·g−1. An increase in ‘Bluegold’during ripening was also observed.[Citation5] Differences of the present results in comparison to this report in the literature may decrease due to different cultivations, regional differences, climate conditions, and cultivated technologies.
Figure 1. Total polyphenol and total flavonoids in rabbiteye blueberries during ripening. Different lower case letters within each column represent significant differences among cultivars at the same ripeness stages and (P < 0.05)
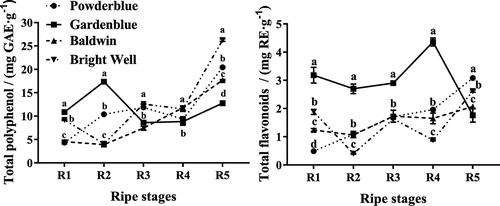
The total flavonoid contents in ‘Powderblue’ and ‘Baldwin’ fruits tended to increase with ripening and peaked at the R5 stage. The total flavonoid content of ‘Gardenblue’ fruits decreased during the R2 stage, increased by R4 stage and then decreased. The total flavonoid contents of ‘Bright Well’ fruits decreased at the R2 stage and then increased. According to Xie et al.[Citation15,Citation21,Citation30] which reported that total flavonoid contents of ripe fruits are 5.12–5.89, 10.84–12.81, 2.77–4.85, 3.40–4.16 mg RE · g−1 in the ‘Powderblue’, ‘Gardenblue’, ‘Baldwin’, and ‘Bright Well’, respectively. These results contrast with our results, as a consequence of differences among varieties, levels of maturity, and regions.[Citation1,Citation28,Citation29]
Profile of phenolic compounds
The phenolic compounds were quantified in rabbiteye blueberries during ripening by HPLC-UV/Vis. Phenolic compounds varied throughout ripening, though the variation profile differed from cultivar to cultivar (). Gallic acid was found to be the major phenolic compound in rabbiteye blueberries, and across all ripening stages, it matched the results of the mature fruits[Citation21], ranged from 27.67 mg·kg−1 in ‘Powderblue’ to1161.62 mg·kg−1 in ‘Bright Well’. The second most abundant phenolic compound was that of ellagic acid, which ranged from 2.41 mg·kg−1 in ‘Powderblue’ to 192.54 mg·kg−1 in ‘Gardenblue’. Other significant phenolic compounds observed in rabbiteye blueberries were ferulic acid, rutin, epicatechin, quercetin, chlorogenic acid, catechin, and p-coumaric. The contents of these compounds in the present study ranged from 0.27 to 128.96 mg·kg−1, 0.44 to 67.40 mg·kg−1, 0.59 to 66.49 mg·kg−1, 0.46 to 36.79 mg·kg−1, 0.39 to 24.59 mg·kg−1, 1.30 to 11.55 mg·kg−1, and 0.01 to 7.58 mg·kg−1, respectively. Studies by Sellappan et al.[Citation16], Colak et al.[Citation31] and Silva et al.[Citation30] have quantified many phenolic compounds in blueberry, such as gallic acid, chlorogenic acid, p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, ferulic acid, and ellagic acid, catechin, epicatechin, myricetin, quercetin, and kaempferol.
Figure 2. Phenolic compounds in rabbiteye blueberries during ripening. Different lower case letters within each column represent significant differences among cultivars at the same ripeness stages and (P < 0.05)
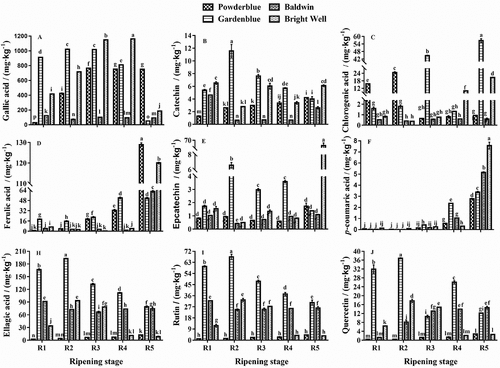
Variation in individual phenolic compounds was investigated during ripening in the four rabbiteye blueberry cultivars. The gallic acid levels in ‘Powderblue’, ‘Baldwin’, and ‘Bright Well’ showed rapid growth in the early stage and then remained essentially stable. The gallic acid, catechin, epicatechin, ellagic acid, rutin, and quercetin contents in ‘Gardenblue’ increased at the R2 stage and then began to decrease. The ferulic acid content in ‘Bright Well’ and catechin, ellagic acid and rutin contents in ‘Baldwin’ decreased until the R3 stage and then increased. The chlorogenic acid and quercetin contents in ‘Baldwin’ and ferulic acid content in ‘Gardenblue’ as well as the catechin, ellagic acid, rutin, and quercetin contents in ‘Powderblue’ and p-coumaric acid content in all studied cultivars tended to increase throughout ripening. The epicatechin content in ‘Powderblue’ and ferulic acid content in ‘Baldwin’ decreased at the R4 stage and then increased. The epicatechin contents in ‘Baldwin’ and ‘Bright Well’ fruits decreased at the R2 stage and then increased by the next ripening stage. The ellagic acid, rutin, and quercetin contents in ‘Bright Well’ increased at the R2 stage and then decreased at the next ripening stage. Silva et al.[Citation31] studied the phenolic compounds in blueberries (ss ‘Duke’, ‘Bluecrop’, ‘Goldtraube’, and ‘Ozarkblue’) throughout the ripening process, and found phenolic acids (chlorogenic, gallic, and p-coumaric acid) tended to decrease as the fruit ripens. Differences of the present results in comparison to this reported in the literature may decrease due to the varieties, climate conditions, levels of maturation, ripening stages, and different extraction solvents in sample preparation.[Citation1,Citation5,Citation31]
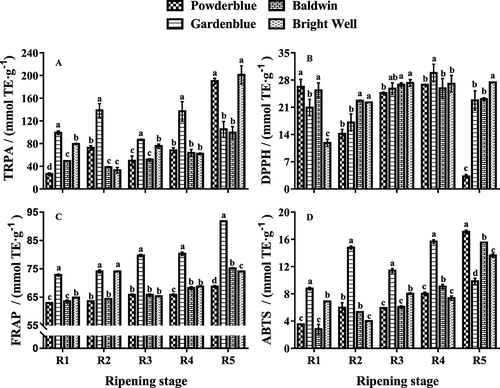
Antioxidant activity
Changes in antioxidant activity, including those detected by TRPA, DPPH, FRAP, and ABTS assays, were observed depending on the ripe stages (). The TRPA in ‘Powderblue’, ABTS in ‘Gardenblue’ ‘Baldwin’, and ‘Bright Well’, and FRAP in all tested cultivars tended to increase during ripening. The TRPA in ‘Gardenblue’ and FRAP in ‘Bright Well’ increased during the R2 stage, decreased by the R3 stage, and then increased. In contrast, the DPPH in ‘Powderblue’ decreased during the R2 stage, increased at the R3 stage and then decreased. However, the TRPA in ‘Baldwin’ and ‘Bright Well’ decreased in the early stage and then increased. The DPPH and ABTS in ‘Gardenblue’, and DPPH in ‘Baldwin’ and ‘Bright Well’ increased in the early stage, then decreased. Phenolic acids contribute significantly to the total antioxidant capacity have reported by Dragovic´-Uzelac et.al. (2010), Kang et.al. (2014), and Cheplick et.al. (2015).[Citation2,Citation3,Citation10] These differences are mainly related to the bioactive components in fruits.[Citation2,Citation10,Citation21]
Correlation of phenolic compound and antioxidant activity
The correlation between antioxidant capacity and phenolic compounds in rabbiteye blueberries during ripening was examined (). A positive correlation between the total antioxidant activity and the phenolic compounds was observed with FRAP, TRPA, DPPH, and ABTS assays. Several studies have previously reported that phenolic acid contributed significantly to the total antioxidant capacity.[Citation2,Citation3,Citation10] The total phenolic content was positively correlated with ferulic acid, p-coumaric acid, and epicatechin, weaker correlation with catechin. The total flavonoid contents were positively correlated with catechin, ferulic acid, quercetin, rutin, and ellagic acid. The TRPA was positively correlated with ferulic acid, total phenolic content, total flavonoid contents, p-coumaric acid, epicatechin, and catechin. The DPPH was positively correlated with chlorogenic acid, p-coumaric acid, rutin, ellagic acid, and quercetin, weaker correlation with ferulic acid and catechin. The ABTS was positively correlated with total flavonoids, total polyphenols content, ferulic acid, p-coumaric acid, and catechin, weaker correlation with quercetin, there is no information available ABTS. The results suggest that phenolic compounds, such as ferulic acid, chlorogenic acid, p-coumaric acid, may be responsible for presenting the most important contributions to the antioxidant activity of the fruits studied.[Citation16,Citation21,Citation32] Other studies have also reported a high correlation between phenolic acid concentration and the total antioxidant capacity.[Citation2,Citation3,Citation10]
Table 1. Correlation coefficient of 15 indices
Conclusion
The results suggest that phenolic compound contents of four rabbiteye blueberries significantly differed throughout the ripening stages. Gallic acid, ellagic acid, and ferulic acid were the main phenolic compounds in the fruits of all the cultivars. The concentration of gallic acid was highest in ‘Bright Well’ fruits at the fourth ripening stage. The concentration of ellagic acid was highest in ‘Gardenblue’ fruits at the second ripening stage. The concentration of ferulic acid was highest in ‘Powderblue’ fruits at the fifth ripening stage. All cultivars at the fourth ripening stage had their highest DPPH, and the antioxidant capacity of FRAP, TRPA, and ABTS were high in all fruits at fifth ripening stage. There was a positive correlation between the total antioxidant activity and phenolic compound content. This study indicated that ‘Gardenblue’ fruits at the fourth ripening stage and other cultivars at the fifth ripening stage had high phenolic compound contents and high antioxidant capacity. The phenolic compounds had a strong contribution to antioxidant capacity. Health-promoting phenolic compounds contributed positively to antioxidant activity, and thus this study has potential applications in the food, nutraceutical, and pharmaceutical industries as well as in breeding programs.
Additional information
Funding
References
- Castrejón, A. D. R.; Eichholz, I.; Rohn, S.; Kroh, L. W.; Huyskens-Keil, S. Phenolic Profile and Antioxidant Activity of Highbush Blueberry (Vaccinium Corymbosum, L.) During Fruit Maturation and Ripening. Food Chem. 2008, 109, 564–572. DOI: 10.1016/j.foodchem.2008.01.007.
- Kang, J.; Thakali, K. M.; Jensen, G. S.; Wu, X. Phenolic Acids of the Two Major Blueberry Species in the US Market and Their Antioxidant and Anti-Inflammatory Activities. Plant Foods Human Nutr. 2014, 70, 56–62. DOI: 10.1007/s11130-014-0461-6.
- Cheplick, S.; Sarkar, D.; Bhowmik, P.; Shetty, K. Phenolic Bioactives from Developmental Stages of Highbush Blueberry (Vaccinium Corymbosum) for Hyperglycemia Management Using in Vitro Models. Can. J. Plant Sci. 2015, 95, 653–662. DOI: 10.4141/cjps-2014-352.
- Vizzotto, M.; Fetter, M. D. R.; Corbelini, D. D.; Pereira, M. C.; Gonzales, T. N. Bioactive Compounds and Antioxidant Activity of Blueberry (Vaccinium Ashei Reade). Acta Hortic. 2013, 972, 111–115. DOI: 10.17660/ActaHortic.2013.972.14.
- Ribera, A. E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G. E.; Mora, M. L. Antioxidant Compounds in Skin and Pulp of Fruits Change among Genotypes and Maturity Stages in Highbush Blueberry (Vaccinium Corymbosum L.) Grown in Southern Chile. J. Soil Sci. Plant Nutr. 2010, 10(4), 509–536. DOI: 10.4067/S0718-95162010000200010.
- Sun, W. C.; Yu, D. J.; Lee, H. J. Changes in Anthocyanidin and Anthocyanin Pigments in Highbush Blueberry (Vaccinium Corymbosum, Cv. Bluecrop) Fruits during Ripening. Hortic. Environ. Biotechnol. 2016, 57, 424–430. DOI: 10.1007/s13580-016-0107-8.
- Yousef, G. G.; Brown, A. F.; Funakoshi, Y.; Mbeunkui, F.; Grace, M. H.; Ballington, J. R.; Loraine, A.; Lila, M. A. Efficient Quantification of the Health-Relevant Anthocyanin and Phenolic Acid Profiles in Commercial Cultivars and Breeding Selections of Blueberries (Vaccinium Spp.). J. Agric. Food Chem. 2013, 61, 4806–4815. DOI: 10.1021/jf400823s.
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M. A.; Masci, A. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium, Spp.) Extracts: Comparison between Hplc-Dad and Cielab Analyses. Food Chem. 2017, 232, 114–123. DOI: 10.1016/j.foodchem.2017.03.153.
- Howard, L. R.; Clark, J. R.; Brownmiller, C. Antioxidant Capacity and Phenolic Content in Blueberries as Affected by Genotype and Growing Season. J. Sci. Food Agric. 2003, 83, 1238–1247. DOI: 10.1002/(ISSN)1097-0010.
- Dragovic´-Uzelac, V.; Savic, Z.; Brala, A.; Levaj, B.; Kovačevic, D. B.; Biško, A. Evaluation of Phenolic Content and Antioxidant Capacity of Blueberry Cultivars (Vaccinium Corymbosum L.) Grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48(2), 214–221.
- Forney, C. F.; Kalt, W.; Jordan, J. A.; Vinqvisttymchuk, M. R.; Fillmore, S. A. E. Compositional Changes in Blueberry and Cranberry Fruit during Ripening. Acta Hortic. 2012, 926, 331–338. DOI: 10.17660/ActaHortic.2012.926.46.
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Human Nutr. 2017, 72, 360–364. DOI: 10.1007/s11130-017-0640-3.
- Kraujalytė, V.; Venskutonis, P. R.; Pukalskas, A.; Česonienė, L.; Daubaras, R. Antioxidant Properties, Phenolic Composition and Potentiometric Sensor Array Evaluation of Commercial and New Blueberry (Vaccinium Corymbosum) and Bog Blueberry (Vaccinium Uliginosum) Genotypes. Food Chem. 2015, 188, 583–590. DOI: 10.1016/j.foodchem.2015.05.031.
- Huang, W. Y.; Zhang, H. C.; Liu, W. X.; Li, C. Y. Survey of Antioxidant Capacity and Phenolic Composition of Blueberry, Blackberry, and Strawberry in Nanjing. J. Zhejiang Univ. Sci. B. 2012, 13(2), 94–102. DOI: 10.1631/jzus.B1100137.
- Xie, G. F.; Tan, Y.; Wang, R.; Wang, Y.; Zhou, X. L.; Ma, L. Z. Evaluation of Main Cultivars and Producing Area on Processing Characteristics of Late-Maturing Blueberry in Guizhou Province. Food Ferment. Ind. 2016, 42, 128–133. [In Chinese, English abstract].
- Sellappan, S.; Akoh, C. C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2440.
- Dastmalchi, K.; Flore, G.; Petrova, V.; Pedraza-Peñalosa, P.; Kennelly, E. J. Edible Neotropical Blueberries: Antioxidant and Compositional Fingerprint Analysis. J. Agric. Food Chem. 2011, 59, 3020–3026. DOI: 10.1021/jf200367j.
- Pertuzatti, B. P.; Barcia, T. M.; Jacques, C.; Vizzotto, A. M.; Godoy, T. H.; Zambiazi, C. R. Quantification of Several Bioactive Compounds and Antioxidant Activities of Six Cultivars of Brazilian Blueberry. Nat. Prod. J. 2012, 2, 188–195. DOI: 10.2174/2210315511202030188.
- Liu, Y. X.; Wu, Y. P.; Liu, G. M.; Cao, M. J.; Lv, Y. C.; Ji, B. P. Identification of Phenolic Acids in Wild Blueberries and Their Scavenging Intracellular ROS Activity. J. Chin. Inst. Food Sci. Technol. 2015, 15, 173–179. [In Chinese, English abstract].
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R. H. Comparison of Phytochemical Profiles, Antioxidant and Cellular Antioxidant Activities of Different Varieties of Blueberry (Vaccinium Spp.). Food Chem. 2017, 217, 773–781. DOI: 10.1016/j.foodchem.2016.09.002.
- Xie, G. F.; Wang, X. H.; Wang, R.; Jin, C. X.; Zhou, X. L.; Liu, Z. G.; Zheng, X. Evaluation of Bioactive Compounds and Antioxidant Activity of Major Blueberry Cultivars in Guizhou Province. Food Ferment. Ind. 2017, 43, 180–184. [In Chinese, English abstract].
- Xie, G. F.; Xu, X. Y.; Wang, R.; Liu, Z. G.; Zhou, X. L.; Yang, H. T. Analysis of Phenolic, Vc and Antioxidant Activity of Fruits and Leaves of Rosa Sterilis D. Shi. Plant Sci. J. 2017, 35, 122–127. [In Chinese, English abstract].
- Nuncio-Jáuregui, N.; Munera-Picazo, S.; Calín-Sánchez, Á.; Wojdyło, A.; Hernandez, F.; Carbonell-Barrachina, A. A. Bioactive Compound Composition of Pomegranate Fruits Removed during Thinning. J. Food Compost. Anal. 2015, 37, 11–19. DOI: 10.1016/j.jfca.2014.06.015.
- Tauchen, J.; Marsik, P.; Kvasnicova, M. In Vitro Antioxidant Activity and Phenolic Composition of Georgian, Central and West European Wines. J. Food Compost. Anal. 2015, 41, 113–121. DOI: 10.1016/j.jfca.2014.12.029.
- Todorovic, V.; Redovnikovic, I. R.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, Methylxanthines, and Antioxidant Capacity of Chocolates Produced in Serbia. J. Food Compost. Anal. 2015, 41, 137–143. DOI: 10.1016/j.jfca.2015.01.018.
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J. A. Influence of Strawberry Tree (Arbutus Unedo L.) Fruit Ripening Stage on Chemical Composition and Antioxidant Activity. Food Res. Int. 2011, 44, 1401–1407. DOI: 10.1016/j.foodres.2011.02.009.
- Schaich, K.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. J. Funct. Foods. 2015, 14, 111–125. DOI: 10.1016/j.jff.2015.01.043.
- Kalt, W.; Lawand, C.; Ryan, D. A. J.; Mcdonald, J. E.; Donner, H.; Forney, C. F. Oxygen Radical Absorbing Capacity, Anthocyanin and Phenolic Content of Highbush Blueberries (Vaccinium Corymbosum L.) During Ripening and Storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. DOI: 10.21273/JASHS.128.6.0917.
- Xie, G. F.; Wang, Y.; Luo, Q. L.; Zhou, X. L.; Liu, Z. G. Comprehensive Factor Evaluation of Blueberry Quality from Different Regions. Food Ferment. Ind. 2018, 44, 254–259. [In Chinese, English abstract].
- Silva, S.; Costa, E. M.; Coelho, M. C.; Morais, R. M.; Pintado, M. E. Variation of Anthocyanins and Other Major Phenolic Compounds Throughout the Ripening of Four Portuguese Blueberry (Vaccinium Corymbosum L) Cultivars. Nat. Prod. Res. 2016, 31, 1.
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Subrtova, M.; Inceer, H. Comparison of Phenolics and Phenolic Acid Profiles in Conjunction with Oxygen Radical Absorbing Capacity (ORAC) in Berries of Vaccinium Arctostaphylos L. And V. Myrtillus L. Pol. J. Food Nutr. Sci. 2016, 66, 85–92. DOI: 10.1515/pjfns-2015-0053.
- Gibson, L.; Rupasinghe, H. P. V.; Forney, C. F.; Eaton, L. Characterization of Changes in Polyphenols, Antioxidant Capacity and Physico-Chemical Parameters during Lowbush Blueberry Fruit Ripening. Antioxidants. 2013, 2(4), 216–229. DOI: 10.3390/antiox2040216.