?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Several studies have demonstrated strong antioxidant activity of date fruits. Nevertheless, limited data is available for the bioactive compounds responsible for the activity. In this study, the antioxidant activity of five different date varieties (Ajwa, Anbara, Piyarom, Rabbi and Deglet Nour) extracted using 80% ethanol was determined using free radical scavenging (DPPH), ferric reducing power (FRAP) and antioxidant capacity (ABTS) assays. The 1H- NMR was employed to profile the bioactive metabolites present in the dates. The obtained results demonstrated that the Piyarom extract had the highest total phenolic content (355 mg GAE/100g DW), total flavonoid content (57.07 mg/100g DW) and exhibited good antioxidant activity with IC50 of 16.2 µg/mL, 26.38 mmol Fe (II)/g and 11.3 µg/mL for DPPH scavenging activity, FRAP antioxidant power and ABTS antioxidant capacity, respectively. The metabolites responsible for the variation between different date’s varieties were successfully identified using 1H-NMR–based metabolomics. The principal component analysis (PCA) and partial least squares (PLS) analysis depicted clear and distinct separations into three clusters representing the five date varieties. The metabolites responsible for the separation were identified as sucrose, betaine, fructose, ascorbic acid, glycine, and arginine. Correlation study from PLS biplot revealed that glucose, ascorbic acid, epicatechin, gallic acid, and citric acid are responsible for the antioxidant activity measured.
Introduction
Great number of diseases including autoimmune disease, cancer, diabetes, coronary heart disease, atherosclerosis, sclerosis, chronic inflammation, and cataracts are induced by free radicals that are reactive oxygen species such as hydrogen peroxide, hydroxyl radical and superoxide anion radical. Damages to cells caused by these free radicals include denaturation of protein, peroxidation of lipids, destabilization of membrane, and DNA mutation which can ultimately lead to chronic diseases of various intensities.[1,Citation2] On the other hand, antioxidants compounds are capable of scavenging free radicals and preventing the oxidation of lipids in a biological.[Citation3,Citation4] They are used as interrupters during the radical-chain oxidation stages, improvement of general health, help in the rejuvenation of cells, as well as in the prevention of cancers.[Citation5] Dietary antioxidants that are naturally found in fruits and vegetables such as flavonoids, polyphenols, ascorbic acid, tocopherols, and carotenoids offer favorable protection to consumers.[Citation2]
Date fruits (Phoenix dactylifera L.) are rich sources of invaluable energy and nutrients. They have been reported to have anti-mutagenic and antioxidant activities as well as aid in reducing the risk of cardiovascular diseases.[Citation6] However, reports on the properties of different date varieties obtained from different origins are still limited. This is more obvious for the bioactive metabolites responsible for the antioxidant activity of dates. One of the most advanced techniques used for the identification of metabolites in a complex extract is using Nuclear magnetic resonance (NMR). The method is widely used due to its ease in sample preparation and the results obtained are reproducible.[Citation7] The NMR techniques coupled with multivariate data analysis (MVA) have been employed extensively in recent time for metabolites profiling as well as to differentiate the samples.[Citation8] The main objective of the study was to determine the antioxidant activity of five different date varieties originated from different regions. Moreover, to identify the bioactive metabolites responsible for the antioxidant activity of selected date varieties using 1H-NMR spectroscopy.
Materials and methods
Sample extraction
Five date varieties commonly consumed in Malaysia were purchased from a local store in Kuala Lumpur, Malaysia, in May 2016. The varieties of dates (Phoenix dactylifera L) used in the study were Piyarom and Rabbi imported from Iran, Anbara and Ajwa from Saudi Arabia and Deglet Nour from Tunisia. The dates were cleaned, the seeds were removed, and the edible parts were crushed into small pieces for drying at 40°C until constant weight was reached. The pieces were further ground to powder using liquid nitrogen and then dispensed aseptically into sterile plastic bags, and kept at −80°C until analysis. A total of 10 g of the date powder was dissolved in 80% ethanol with stirring at room temperature for 24 h. The extracts were then filtered using Whatman’s No 1 filter paper and evaporation of the solvent was done using a rotary evaporator at 40°C under reduced pressure until a viscous extract was obtained. The crude extract was then freeze-dried until constant weight to ensure removal of the residual water (72 h). The dried extract was then kept in the dark at 4°C for further analysis.
Total phenolic content (TPC)
The phenolics are found in many fruits and vegetables, and they have great function against oxidative stress.[Citation9,Citation10] Total phenolic content (TPC) was determined using the Folin-Ciocalteau colorimetric assay[Citation11], with some modifications. Briefly, 0.5 mL of the date extract (1 mg/mL) was mixed with Folin-Ciocalteau (0.5 mL) and 7% sodium carbonate (10 mL) and allowed to react in the dark for 1 h. The absorbance of the resulting blue complex was then measured at 725 nm. The standard curve with concentration range of 0–100 μg/mL was drawn using gallic acid and TPC was expressed as mg GAE/g dry weight (DW).
Total flavonoids content (TFC)
Flavonoids consist of a large group of polyphenolic substances, and their functional hydroxyl groups are well known for antioxidant activity.[Citation12] Total flavonoids content was estimated following the method described by Kim et al.[Citation13] About 0.3 mL of the date extract was mixed with 3.4 mL of 30% methanol, and 0.15 mL of each of 0.5 M NaNO2 & 0.3 M AlCl3.6H2O, sequel to the addition of 1 mL of NaOH (1 M) after 10 min. Absorbance was measured at 506 nm with the aid of UV/Visible Spectrophotometer (UV-1650 PC Shimadzu, Japan) after the solution has been mixed properly. The standard curve was also drawn for TFC using rutin as standard at the range from 0 to 100 μg/mL. Total flavonoids were expressed as mg RE/g DW.
Free radical scavenging assay (DPPH)
DPPH radical scavenging method was used to determine the free radical scavenging ability of the date extracts.[Citation14] Estimation of antioxidant activity was conducted using 2, 2-diphenyl-1-picrylhydrazyl radicals (DPPH) according to the previous study with some modifications.[Citation15] About 0.25 mL of the extract was dissolved in methanol, mixed with 1.75 mL DPPH reagent (6 × 10−5 mol) in a 24-well plate and allowed to react at room temperature for 30 min in darkness. The absorbance was then recorded at 515 nm using Elisa plate reader (Biotek, EL 800). The percentage of inhibition was calculated as follows:
BHA and α-tocopherol were used as positive controls for natural and synthetic antioxidants, respectively.
Ferric reducing antioxidant power (FRAP)
Ferric reducing antioxidant power assay is a method that depends on reducing the ferric-tripyridyltriazine.[Citation16] The assay was employed for the determination of the ferric reducing activity of the date extracts.[Citation16] Fresh FRAP reagent was prepared by mixing FeCl3, 2, 4, 6-tripyridyl-s-triazine (TPTZ) solution and acetate buffer (pH 3.6) in the ratio of 1:1:10 (v/v/v). About 10 μL of the methanol sample was dispensed into the wells of a 96-well microtiter plate. The FRAP reagent (200 μL) was incubated at 37°C for 30 min and was also dispensed into the wells containing the samples. The solution was then mixed thoroughly, incubated for 30 min at room temperature, and the absorbance was then measured at 593 nm. Standard curve was prepared using ferrous sulfate solution (FeSO4.7H2O) with concentrations ranging from 0.1 to 1 mM. Alpha-tocopherol and BHA were used as positive controls. The FRAP was expressed as mmol Fe (II)/g DW.
Antioxidant capacity (ABTS)
The 2, 2´-azino-bis (3-ethylbenzothiazoline sulfate ABTS+) colorimetric antioxidant assay depends on the ability of phenolic compounds to change the color of the reagent measured by spectrophotometer.[Citation17] The ABTS radical cation (ABTS +) inhibition was done to measure the total antioxidant activity as described by Cai et al.[Citation18] ABTS reagent was prepared by mixing 2.45 mM (K2S2O8) with 7 mM ABTS solution and incubated for 12 to 16 h at room temperature in the dark. The resulting solution was diluted to get the absorbance of 0.700 ± 0.005 with 100% methanol at 734 nm. Working solution for the date extracts was prepared with ethanol. The resulting solutions of date extracts were mixed with 2 mL of the diluted ABTS solution and mixed well to obtain a uniform solution. The reaction mixture was kept for 6 min at 23°C, after which the absorbance was read at 734 nm. Different concentrations of Trolox (0 to 15 mM) in 80% methanol were prepared and the absorbance was used to plot a standard curve. The percent inhibition was computed using the following equation:
1H-NMR metabolomics analysis
A total of 10 mg of date extract was mixed with 0.375 mL of CH3OH-d4 without any internal standard along with 0.375 mL of KH2PO4 buffer in D2O (pH 6 adjusted with NaOD) containing 0.1% TSP. Six replicates were examined for each sample. The mixture was vortexed for 1 min and sonicated in an ultra-sonicator at 30°C for 15 min. The solution was then centrifuged at 13000 rpm for l0 min and an aliquot of 600 μL of the supernatant was transferred to NMR tube for 1H-NMR analysis.[Citation19] Spectra were recorded at 26°C on a Varian Unity INOVA 500 MHz spectrometer (Varian Inc, CA), with a frequency of 499.887 MHz. A total of 64 scans conducted for each sample and recorded with an acquisition time of 193 s, pulse width of 3.75 μL and a relaxation delay of 1.0 s. Tetramethylsilane (TMS) was employed as an internal standard and all spectra were manually phased and bucketed using Chenomx software, with standard bins of δ 0.05 ranging from region δ 0.50 to 10.00. The residual methanol region (δ 3.28 to 3.33) and water region (δ 4.70 to 4.96) were excluded from the analysis. Two-dimensional 1H–1H J-resolved and Heteronuclear Multiple-Bond Correlation (HMBC) were employed for the metabolites identification. Principal component analysis (PCA) and partial least squares analysis (PLS) were performed using the SIMCA-P software (Umetrics, Sweden).
Statistical analysis
Data were analyzed using MINITAB version 16. Statistical differences between the samples and the controls were evaluated by one-way analysis of variance (ANOVA). Results are expressed as the mean of three determinations ± standard deviation (SD). Mean values differences at P < 0.05 was considered significant statistically. In addition, multivariate data analysis was performed by the use of SIMCA-P software (Umetrics, Sweden).
Results and discussion
Extraction yield
The health benefits of date fruits are well known as the fruit provide a wide range of nutrients. In this study, five different varieties of dates, namely, Ajwa, Anbara, Piyarom, Rabbi and Deglet Nour was extracted using 80% ethanol. The yields of the extraction were measured to be significant differences between the varieties ranging from 16% to 23% (). The highest extraction yield was measured in Piyarom (23.8%), while the lowest yield was detected in Deglet Nour (16.07%). Al-Farsi et al.[Citation20] reported that high extraction efficiency is attributed to the effectiveness of ethanol that was used as the extraction solvent in. In another study, Trabzuni et al.[Citation21] reported that different date varieties extracted using ethanol had significant differences due to the presence of various chemical compounds found in each variety. The previous studies supported the findings of this study as significant variations were observed for the extraction yield.
Table 1. Percentage of extraction yield from different date varieties
Total phenolic compounds (TPC)
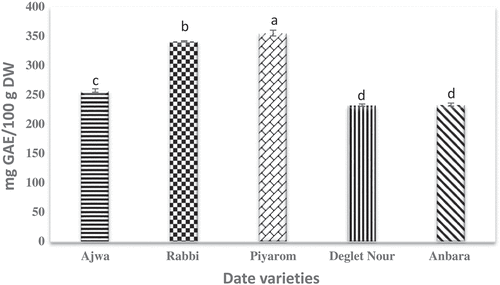
Phenolic compounds are well known for their antioxidant activity that can reduce the production of free radical and lower the oxidative stress. These compounds are responsible for various biological activities of plants and usually present as micro-constituents of the plants.[Citation22] The results demonstrated that selected dates contained different TPC concentrations (). In comparison to all varieties, TPC in Piyarom was the highest measuring at 355 mg/100 g DW. In the earlier study, a significant difference in the TPC content was observed in 14 varieties of date fruit from Iran.[Citation23] Saleh et al.[Citation24] measured the amount of TPC in Ajwa date fruit obtained from Saudi Arabia to be at 245.66 mg/100 g DW. On the other hand, low amount of TPC was detected in Rabbi and Piyarom date fruits measuring only at 4.66 and 6.09 mg/100 g, respectively[Citation2,Citation25]. The TPC result in Deglet Nour date in this study (232 mg/100 g DW) was in agreement with the result published by Emna et al.[Citation26] which reported that the TPC contents of the same variety to be at 230.90 mg/100 g. The results herein showed that the differences for the contents of the TPC are attributed to the origin of the dates and this is further connected to the differences in their genetic makeup.
Total flavonoid content (TFC)
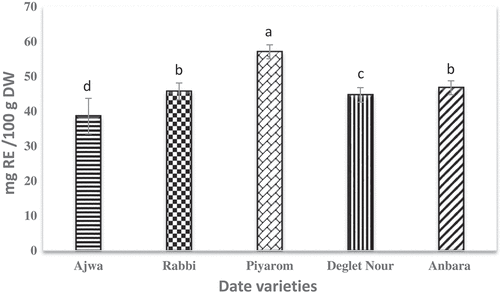
Flavonoids are important metabolites of plants due to its potent antioxidant activity. Aluminum chloride colorimetric methods were employed in the measurement of the total flavonoid content (TFC) of dates in this study. illustrates the TFC found in different date varieties. The results demonstrated high flavonoid content with some variations among the selected date varieties. The highest flavonoid content was observed for Piyarom which was 57.06 mg RE/100 g DW, while the lowest content of 38.63 mg RE/100 g DW was observed for Ajwa. There was no significant difference (p < 0.05) between Rabbi and Anbara in TFC content, while significant difference was observed for Deglet Nour and Ajwa. The differences in TFC of the varieties selected in this study and the date fruits in other studies could be due to various factors such as the solubility of the flavonoids in solvents, methods of analysis used as well as the use of varied source of standards.[Citation27] Flavonoids are very important constituents because they are the most useful compounds contributing to the antioxidant activity of phenolics. Flavonoids were reported in several studies to have a strong relation to the antioxidant activity of plant extracts.[Citation2,Citation28] In a previous study, the date fruit extracts were found to be high in TFC and direct effects on the antioxidant activity of the extracts were observed.[Citation29]
DPPH free radical scavenging
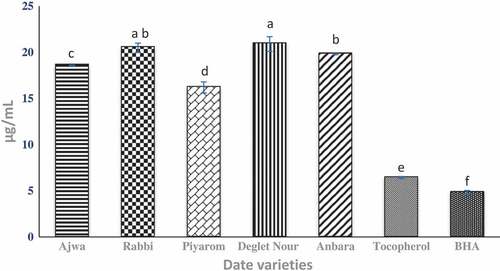
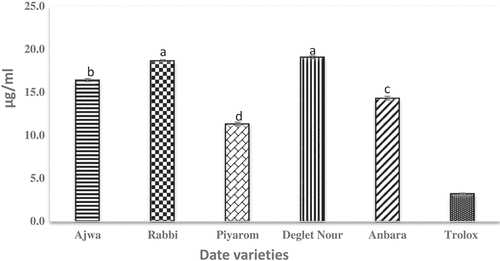
Antioxidant prevents and delays oxidation in oxidizable substances and thus reduce oxidative stress.[Citation30] The radicals scavenging activity of different date extracts are shown in . Results showed that the radical scavenging activity was the highest for Piyarom, followed by Ajwa, and Anbara and are significantly different from each other. These results correlate well with the highest content for both TPC and flavonoid content for Piyarom date. On the other hand, low radical scavenging activity is exhibited by Rabbi and Deglet Nour, but not significantly different from each other. However, the antioxidant activity of Ajwa dates was competitively high in this study as indicated by the DPPH value (IC50 of 18.6 µg/mL). The results are in agreement with a previous study that showed the high antioxidant activity of Ajwa extracted by 80% ethanol.[Citation31] The author suggested that there is a correlation between the antioxidant activity and the phenolic content. Meanwhile, Deglet Nour demonstrated the lowest antioxidant activity (IC50 of 20.9 µg/mL) compared to the other selected date fruits. Deglet Nour was also reported in many studies to have moderate and/or low antioxidant activity among the other date varieties. In the earlier study, the antioxidant activity of Deglet Nour was lower than two other Tunisian date varieties.[Citation32]
FRAP ferric reducing antioxidant power
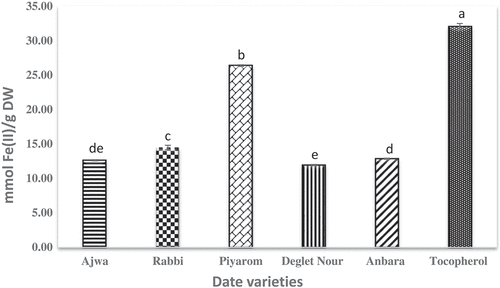
The principle of FRAP assay depends on the reduction of Fe3+ in ferric-tripyridyltriazine[Citation33] into ferrous Fe2+.[Citation16] The FRAP antioxidant assay is a simple technique and normally used for initial evaluation of antioxidant activity in plant extract samples.[Citation34] A significant correlation toward antioxidant activity has been reported for TPC, FRAP and DPPH radical scavenging activities.[Citation35] The results obtained in this study revealed that all extracts have strong antioxidant properties by showing high ferric reducing antioxidant power (). The FRAP value for the date extracts ranged from 11.83 to 26.38 mmol Fe (II)/g dry weight. The highest value for FRAP observed was 26.38 mmol Fe (II)/g dry weight in Piyarom while the lowest was for Deglet Nour extract, 11.83 mmol Fe (II)/g dry weight. Interestingly the result obtained is similar to that of DPPH activity. No significant differences were observed between the extracts of Ajwa and Deglet Nour. Previously, date fruits were reported for strong antioxidant activity in a great number of published manuscripts and the activity was found to be varied among different varieties.[Citation26,Citation36,Citation37]
Antioxidant capacity (ABTS)
ABTS is a well-known assay used to measure the antioxidant activity of plant extracts due to the activity to reduce the production of free radical and lower oxidative stress. The principle of the method depends on reduction of ABTS (2, 2´-azino-bis (3-ethylbenzothiazoline sulfate) radical and the result is expressed in the IC50 value (i.e. concentration of antioxidant required to reduce the initial concentration of ABTS by 50%). Results of ABTS antioxidant activity of different date varieties are displayed in . The results showed that ABTS activity of Piyarom, Rabbi, Ajwa, Anbara, and Deglet Nour were measured at 11.3, 18.7, 16.4, 14.3, and 19.1 mmol TE/100 g dry weight, respectively. Comparison to our finding, strong ABTS activity of Piyarom was also shown in the previous study.[Citation2] Meanwhile, Deglet Nour demonstrated the lowest activity, and the result was also in agreement with the previous study.[Citation32] The results obtained from the ABTS assay have been an asset to confirm the antioxidant activity of the selected date fruits extracts that was shown in preliminary analysis by DPPH and FRAP assays.
1H-NMR spectra and metabolites identification
Many studies reported that date possesses numerous health benefits attributed and various biological activities. However, limited information on the metabolites profile from different date varieties is available. In this study, metabolite differences of five different date varieties were performed utilizing both one-dimensional (1D) and two-dimensional (2D) NMR. Representative spectra from different date varieties studied are shown in –. Full spectra (6A; 1 to 8 ppm) visual observation of the tasted date varieties showed that they were very similar except for the intensities of the peaks. In addition, Deglet Nour showed the presence of high percentage of sucrose, which is not obvious in the other dates. This is in line with that of the previous study which reported sucrose as the major metabolite detected in Deglet Nour.[Citation32] Expansion of the spectra was done from 1 to 3 ppm (), to 6 ppm () and 6 to 8 ppm (), respectively. Based on the expanded spectra, different groups of metabolites were detected including carbohydrates, phenolic compounds, amino acids, and organic acids. Upon visual inspection of the spectrum for each date variety, no differences were revealed except, in the peaks at the sugar region (δ 3.0–5.5) and few smaller peaks at the aromatic region (δ 5.5–8.0). The metabolites identified in these regions covered a variety of compounds including glucose, fructose, xylose, sucrose, leucine, isoleucine, alanine, and D-L-valine as amino acids.
Figure 6. 1H-NMR spectra of different date varieties. (A) Full Spectra of δ 1.0 to 8.5 ppm. (B) Spectra of δ 1.0 to 3.0 ppm. (C) Spectra of δ 3.0 to 6.0 ppm. (D) Spectra of δ 6.0 to 8.0 ppm
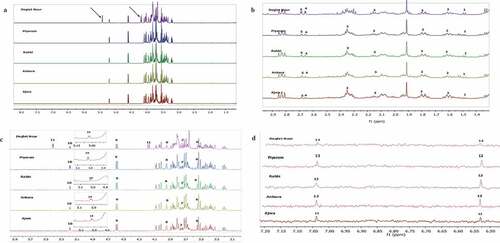
In addition, organic acids, acetic, malic and formic acids, and epicatechin were also identified in the aromatic region. Based on the comparison of 1H-NMR spectra of the date fruits with those in the literature and as well as with their 2D J-resolved spectrophotometry, 13 metabolites were clearly identified as shown in .[Citation38] The metabolites were identified by matching their signals with those in Chenomx database. summarizes the list of the identified metabolites, which include amino acids, organic acids, and various secondary metabolites. The carbohydrate regions (δ 3.0–5.5) typically contained overlapped signals, which complicate the metabolite identification.[Citation39] However, the protons of glucose, sucrose, and fructose were observed at δ 5.19 (d, J = 3.72 Hz), δ 5.41 (d, J = 3.8 Hz) and δ 3.86 (d, J = 9.91 Hz), respectively (). Variations in the intensity of the signals were observed in Deglet Nour which was considerably different from the other varieties. This is in agreement with that of the previous study which showed simple sugars are the main metabolites being detected in dates, where glucose and fructose accumulated around 97% of the total carbohydrates.[Citation11]
Table 2. Assignments of 1H-NMR spectral signals attained from different date varieties
Multivariate analysis of the date extracts
The aim of metabolic biological study is to measure the relative variability between the metabolome of the samples. In this study, multivariate analysis was performed for the characterization of spectral features that are biologically related to additional targeted analyses.[Citation40] The PCA and PLS are the two most commonly employed techniques for differentiating classes in the sets of highly complex data. The fitness of their analysis models was determined via cross-validation, external validation, and variable importance.[Citation41] A cumulative Q2 greater than 0.5 revealed that the model is statistically significant and good fitted. shows that the PCA score plot did not give clear separation among the samples, and substantial overlap of the samples can be seen. However, a distinct separation can be seen between Deglet Nour and the rest of the date varieties, revealing the different metabolites between Deglet Nour and the others. In the effort to get clear separation, principal least square discriminant analysis model (PLS-DA) was applied for further analysis and the result was shown in . The plot revealed three distinct clusters that may be attributed by the presence of variable metabolites in the date varieties.[Citation42] Results from 1H-NMR analysis were used to conduct PCA and PLS in order to perceive similarity between the date varieties. Results from the two models measured the cumulative Q2 values at greater than 0.5, which depicts the model is significant and was good fitted. Specifically, the values of 0.964 and 0.620 were fitted for both the PCA and PLS models, respectively, which indicates the models used were significant statistically. The R2 values for the PCA and PLS models measured at 0.996 and 0.921, respectively, used for external validation, and also confirmed that they are significantly fitted.
Figure 7. Analysis for the date spectra obtained using 1H-NMR. (A) PCA (PC1 vs. PC2), (B) PLS-DA score plot, (C) Loading column plots of PC1 and (D) PC2
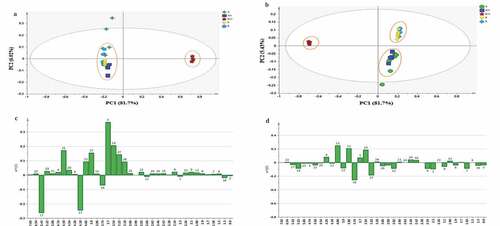
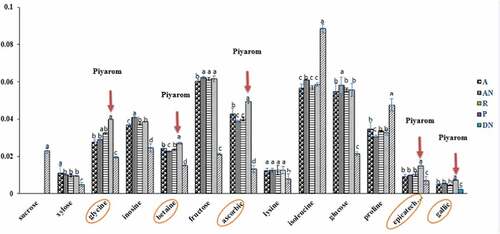
Findings from this study indicated that three of the dominant metabolites played a major role in the discrimination of Deglet Nour, namely, fructose, sucrose and isoleucine. In contrast, fructose, ascorbic acid, glycine, betaine, glucose, and epicatechin were found in the other four dates (). Based on the PC1 and loading plots shown in , the metabolites responsible directly or indirectly for the discrimination of Madinah Ajwa and Anbara, Iranian Piyarom and Rabbi with that of Tunisian Deglet Nour may be sucrose, fructose, glucose, ascorbic acid, betaine, glycine, and epicatechin and others.
Relative quantities of metabolites identified
The relative concentrations of metabolites responsible for discrimination of the dates studied were measured to look at the variations between samples, and the result is presented in . The dominant metabolites signals were picked and quantified relatively. The signal characteristics of epicatechin at δ 5.12 (m), gallic acid at δ 7.03 (s), ascorbic acid at δ 3.72–3.76 (m), and proline at δ 3.40 or δ 2.38 (m) were used to quantify the respective metabolites. Meanwhile, the carbohydrate signals of α-glucose at δ 5.19 (d), β-glucose at δ 4.56 (d), sucrose at δ 5.41 (d), fructose at δ 4.18 (d) and xylose at δ 4.59 (d) were also determined in the result. Additionally, the signals for amino acids were lysine at δ 3.73 (t), glycine at δ 3.55 (s) and isoleucine at δ 3.68 (d), while that of betaine at δ 3.22 (s), and inosine at δ 3.89 (m), respectively. The result for ANOVA showed that there are significant differences (P < 0.05) between the values of these metabolites in Deglet Nour and Ajwa, Anbara, Rabbi, and Piyarom, based also on the varieties and origin of the dates. The result indicated that Piyarom had higher concentrations of ascorbic acid, epicatechin, glycine, betaine, and gallic acid in comparison to the other date varieties. On the other hand, the content of sucrose was significantly higher in Deglet Nour compared to the other varieties.
Correlation between antioxidant activity and metabolites responsible for the activity
A PLS biplot (PLS; R2 = 0.81 and Q2 = 0.718) was conducted to ascertain the possible relationship between antioxidant activity (i.e. DPPH, FRAP, and ABTS), and the metabolites in the extracts of the date varieties. Such correlation will reveal the metabolites that may have contributed to the antioxidant activity that was previously measured. shows the PLS biplot that reveals three different clusters for the date extracts analyzed. The results indicated close proximity of Piyarom extract to the antioxidant activities of DPPH and ABTS. On the other hand, Deglet Nour was separated to the negative side of the plot and led to be deviated from antioxidant activity.
Figure 9. The biplot obtained from PLS describing the relation between the metabolites with antioxidant activities in extract of different date varieties
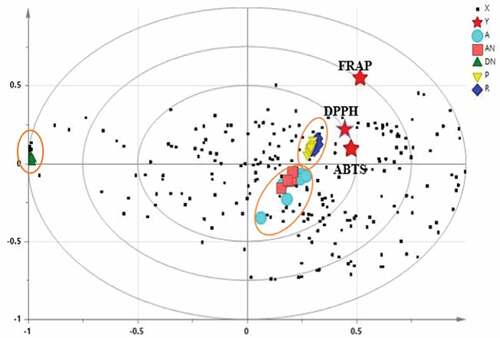
The possible compounds that may have caused the separation in PC1 plot are probably fructose, ascorbic acid, glycine, betaine, glucose, and epicatechin. Majority of these six metabolites were found in Piyarom followed by Ajwa, Anbara, Rabbi extracts. Deglet Nour was distinct from the others since it was the only variety that contains high sucrose concentration. The VIP values were then used to identify the metabolites that attributed to the antioxidant activity. The VIP value is a “calculated sum of squares of the PLS weights, which is a description for the elucidated Y variance in every dimension.” Usually, VIP values greater than 0.7 are considered to be significant in the separation of samples using PLS model.[Citation43] Metabolites and their VIP values contributing to their separation in the plots from the PLS model are shown in . Sucrose, betaine, glycine, lysine, β-glucose, choline, fructose, glutamine, citric acid, α-glucose, arginine, epicatechin, gallic acid, and ascorbic acid were the metabolites found to be responsible for the antioxidant activity measured previously. Some of these metabolites (e.g. epicatechin, gallic acid, ascorbic acid, and citric acid) are known to have potent antioxidant activity. These same metabolites are also found in high concentration in Piyarom.
Table 3. VIP values of the major contributing compounds in the PLS
Conclusion
Piyarom date demonstrated the highest antioxidant activity (i.e. DPPH, FRAP, and ABTS) and the highest concentrations of phenolic compounds (i.e. TPC, TFC). Profiling of metabolites indicated the presence of 17 bioactive compounds in the ethanolic extract of five selected samples with various concentrations. The PLS-DA score plots showed clear separation for the five date extracts. Sucrose, ascorbic acid, fructose, gallic acid, and epicatechin were depicted to be the main constituents of the date extracts that are responsible for the separation. Correlation between antioxidant activity and metabolites of the date varieties showed that Piyarom and Rabbi have the highest antioxidant activities probably attributed by the metabolites that have been identified to be ascorbic acid, epicatechin, citric acid, and gallic acid. The results indicated the antioxidant activity of date extracts that have high potential for medicinal uses to reduce the oxidative stress for consumers.
Acknowledgments
The authors would like to thank Government of Malaysia for the grant provided and Mr. Salahudin Mohd. Raof. (Science Officer, IBS, UPM) for his technical assistance during NMR analyses. The authors would also like to thank the Faculty of Food Science and Technology, Universiti Putra Malaysia for the facilities provided.
References
- Borchani, C.; Besbes, S.; Masmoudi, M.; Blecker, C.; Paquot, M.; Attia, H. Effect of Drying Methods on Physico-Chemical and Antioxidant Properties of Date Fibre Concentrates. Food Chem. 2011, 125(4), 1194–1201. DOI: 10.1016/j.foodchem.2010.10.030.
- Biglari, F.; AlKarkhi, A. F. M.; Easa, A. M. Antioxidant Activity and Phenolic Content of Various Date Palm (Phoenix Dactylifera) Fruits from Iran. Food Chem. 2008, 107(4), 1636–1641. DOI: 10.1016/j.foodchem.2007.10.033.
- Atmani, D.; Chaher, N.; Berboucha, M.; Ayouni, K.; Lounis, H.; Boudaoud, H.; Debbache, N.; Atmani, D. Antioxidant Capacity and Phenol Content of Selected Algerian Medicinal Plants. Food Chem. 2009, 112(2), 303–309. DOI: 10.1016/j.foodchem.2008.05.077.
- Shyamala, B. N.; Gupta, S.; Lakshmi, A. J.; T, J. P. Leafy Vegetable Extracts – Antioxidant Activity and Effect on Storage Stability of Heated Oils. Innov. Food Sci. Emerg. Technol. 2005, 6, 239–245. DOI: 10.1016/j.ifset.2004.12.002.
- Ghiaba, Z.; Yousfi, M.; Hadjadj, M.; Saidi, M.; Dakmouche, M. Study of Antioxidant Properties of Five Algerian Date (Phoenix Dactylifera L) Cultivars by Cyclic Voltammetric Technique. Int. J. Electrochem. Sci. 2013, 9(2014), 909–920.
- Vayalil, P. K.;. Antioxidant and Antimutagenic Properties of Aqueous Extract of Date Fruit (Phoenix Dactylifera Antioxidant and Antimutagenic Properties of Aqueous Extract. J. Agric. Food Chem. 2002, 50(3), 610–617. DOI: 10.1021/jf010716t.
- Kim, H. K.; Choi, Y. H.; Verpoorte, R. NMR-Based Metabolomic Analysis of Plants. Nat. Protoc. 2010, 5(3), 536–549. DOI: 10.1038/nprot.2009.149.
- Gallo, V.; Mastrorilli, P.; Cafagna, I.; Nitti, G. I.; Latronico, M.; Longobardi, F.; Minoja, A. P.; Napoli, C.; Romito, V. A.; Schäfer, H.;; et al. Effects of Agronomical Practices on Chemical Composition of Table Grapes Evaluated by NMR Spectroscopy. J. Food Compos. Anal. 2014, 35(1), 44–52.
- Sarikurkcu, C.; Arisoy, K.; Tepe, B.; Cakir, A.; Abali, G.; Mete, E. Studies on the Antioxidant Activity of Essential Oil and Different Solvent Extracts of Vitex Agnus Castus L. Fruits from Turkey. Food Chem. Toxicol. 2009, 47(10), 2479–2483. DOI: 10.1016/j.fct.2009.07.005.
- Matkowski, A.; Wołniak, D. Plant Phenolic Metabolites as the Free Radical Scavengers and Mutagenesis Inhibitors. BMC Plant Biol. 2005, 5(Suppl 1), S23. DOI: 10.1186/1471-2229-5-S1-S23.
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Compositional and Sensory Characteristics of Three Native Fresh and Sun-Dried Date (Phoenix Dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53(19), 7586–7591. DOI: 10.1021/jf050578y.
- Shashank, K.; Pandey, A. K. Chemistry and Biological Activities of Flavonoids. Hindawi Sci. World J. 2013, 12, 533–548.
- Kim, D. O.; Jeong, S. W.; Lee, C. Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81(3), 321–326. DOI: 10.1016/S0308-8146(02)00423-5.
- Meng, R. G.; Tian, Y. C.; Y., Y.; S., J. Evaluation of DPPH Free Radical Scavenging Activity of Various Extracts of Ligularia Fischeri in Vitro: A Case Study of Shaanxi Region. Indian J. Pharm. Sci. 2016, 78(4), 436–442. DOI: 10.4172/pharmaceutical-sciences.1000137.
- Nadjet, G.; Zaouia, K.; Laboratoire, V. P. R. S.; Ouargla, U. D.; Ouargla, D. G. Comparison of Antioxidant Activity and Phenolic Content of Three Varieties of Algerian Dates. Ajae. 2012, 2(1), 42–48.
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. DOI: 10.1006/abio.1996.0292.
- Re, R.; Pellegrini, N.; Proteggente, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical. Free Radic. Biol. Med. 1999, 26(98), 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74(17), 2157–2184. DOI: 10.1016/j.lfs.2003.11.013.
- Mediani, A.; Abas, F.; Ping, T. C.; Khatib, A.; Lajis, N. H. Influence of Growth Stage and Season on the Antioxidant Constituents of Cosmos Caudatus. Plant Foods Hum. Nutr. 2012, 67(4), 344–350. DOI: 10.1007/s11130-012-0301-5.
- Al-Farsi, M. A.; Lee, C. Y. Nutritional and Functional Properties of Dates: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48(10), 877–887. DOI: 10.1080/10408390701724264.
- Trabzuni, D. M.; Ahmed, S. E. B.; Abu-Tarboush, H. M. Chemical Composition, Minerals and Antioxidants of the Heart of Date Palm from Three Saudi Cultivars. Food Nutc. Sci. 2014, 5(14), 1379–1386.
- Ka, M. P.;. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47(10), 3954–3962.
- Ardekani, M. R. S.; Khanavi, M.; Hajimahmoodi, M.; Jahangiri, M.; Hadjiakhoondi, A. Comparison of Antioxidant Activity and Total Phenol Contents of Some Date Seed Varieties from Iran. Iran. J. Pharm. Res. 2010, 9(2), 141–146.
- Saleh, E. A.; Tawfik, M. S.; Abu-Tarboush, H. M. Phenolic Contents and Antioxidant Activity of Various Date Palm (Phoenix Dactylifera L.) Fruits from Saudi Arabia. Food Nutr. Sci. 2011, 02(10), 1134–1141.
- Sadeghi, Z.; Valizadeh, J.; Shermeh, O. A. Antioxidant Activity and Total Phenolic Contents of Some Date Varieties from Saravan Region, Baluchistan, Iran. J. Med. Plants Res. 2015, 9(4), 78–83. DOI: 10.5897/JMPR2014.5676.
- Saafi, E. B.; El Arem, A.; Issaoui, M.; Hammami, M.; Achour, L. Phenolic Content and Antioxidant Activity of Four Date Palm (Phoenix Dactylifera L.) Fruit Varieties Grown in Tunisia. Int. J. Food Sci. Technol. 2009, 44(11), 2314–2319. DOI: 10.1111/j.1365-2621.2009.02075.x.
- Kchaou, W.; Abbès, F.; Attia, H.; Besbes, S. In Vitro Antioxidant Activities of Three Selected Dates from Tunisia (Phoenix Dactylifera L.). J. Chem. 2014, 2014. DOI: 10.1155/2014/367681.
- Singh, V.; Guizani, N.; Essa, M. M.; Hakkim, F. L.; Rahman, M. S. Comparative Analysis of Total Phenolics, Flavonoid Content and Antioxidant Profile of Different Date Varieties (Phoenix Dactylifera L.) From Sultanate of Oman. Int. Food Res. J. 2012, 19(3), 1063–1070.
- Amira, E. A.; Behija, S. E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the Ripening Stage on Phenolic Profile, Phytochemical Composition and Antioxidant Activity of Date Palm Fruit. J. Agric. Food Chem. 2012, 60(44), 10896–10902. DOI: 10.1021/jf302602v.
- Chen, C. K.; Muhamad, A. S.; Ooi, F. K. Herbs in Exercise and Sports. J. Physiol. Anthropol. 2012, 31(1), 1–7. DOI: 10.1186/1880-6805-31-4.
- Khalid, S.; Ahmad, A.; Kaleem, M. Antioxidant Activity and Phenolic Contents of Ajwa Date and Their Effect on Lipo-Protein Profile. Ffhd. 2017, 7(6), 396–410.
- Souli, I.; Bagues, M.; Lachehib, B.; Ferchichi, A. Nutritional Values and Antioxidant Activities of Juice Extracted from Some Tunisian Date Varieties. J. New Sci. 2016, 35(6), 1976–1985.
- Kim J.; Jung, Y.; Song, B.; Bong, Y. S.; Ryu, D. H.; Lee, K. S.Hwang, G. S. Discrimination of Cabbage (Brassica Rapa Ssp. Pekinensis) Cultivars Grown in Different Geographical Areas Using1H NMR-Based Metabolomics. Food Chem. 2013, 137(1–4),68–75. DOI: 10.1016/j.foodchem.2012.10.012.
- Halvorsen, B. L.; Holte, K.; Myhrstad, M. C. W.; Barikmo, I.; Hvattum, E.; Remberg, S. F.; Wold, A.; Haffner, K.; Baugerød, H.; Andersen, L. F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132(3), 461–471. DOI: 10.1093/jn/132.9.2514.
- Sabeena Farvin, K. H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138(2–3), 1670–1681. DOI: 10.1016/j.foodchem.2012.10.078.
- Zineb, G.; Boukouada, M.; Djeridane, A.; Saidi, M.; Yousfi, M. Screening of Antioxidant Activity and Phenolic Compounds of Various Date Palm (Phoenix Dactylifera) Fruits from Algeria. Med. J. Nutr. Metab. 2012, 5(2), 119–126. DOI: 10.1007/s12349-011-0082-7.
- Allaith, A. A. A.;. Antioxidant Activity of Bahraini Date Palm (Phoenix Dactylifera L.) Fruit of Various Cultivars. Int. J. Food Sci. Technol. 2008, 43(6), 1033–1040. DOI: 10.1111/j.1365-2621.2007.01558.x.
- Khatoon, S.; Rai, V.; Kumar, A.; Rawat, S.; Mehrotra, S. Comparative Pharmacognostic Studies of Three Phyllanthus Species. J. Ethnopharmacol. 2006, 104, 79–86. DOI: 10.1016/j.jep.2005.08.048.
- Shuib, N. H.; Shaari, K.; Khatib, A.; Maulidiani; Kneer, R.; Zareen, S.; Raof, S. M.; Lajis, N. H.; Neto, V. Discrimination of Young and Mature Leaves of Melicope Ptelefolia Using1H NMR and Multivariate Data Analysis. Food Chem. 2011, 126(2), 640–645. DOI: 10.1016/j.foodchem.2010.10.043.
- Schauer, N.; Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A. R. Corrigendum : Gas Chromatography Mass Spectrometry-Based Metabolite Profiling in Plants Corrigendum : Gas Chromatography Mass Spectrometry – Based Metabolite Profiling in Plants Corrigendum : Primary Culture of Ovarian Surface Epithelial Cells and Ascites. Nat. Protoc. 2015, 10(9), 1457. DOI: 10.1038/nprot.2015.094.
- Sun, H.;. A Universal Molecular Descriptor System for Prediction of LogP, LogS, LogBB, and Absorption. J. Chem. Inf. Comput. Sci. 2004, 44(2), 748–757. DOI: 10.1021/ci030304f.
- Gao, M.; Liu, C. Z. Comparison of Techniques for the Extraction of Flavonoids from Cultured Cells of Saussurea Medusa Maxim. World J. Microbiol. Biotechnol. 2005, 21(8–9), 1461–1463. DOI: 10.1007/s11274-005-6809-1.
- Kim, H. S.; Park, S. J.; Hyun, S. H.; Yang, S. O.; Lee, J.; Auh, J. H.; Kim, J. H.; Cho, S. M.; Marriott, P. J.; Choi, H. K. Biochemical Monitoring of Black Raspberry (Rubus Coreanus Miquel) Fruits according to Maturation Stage by 1H NMR Using Multiple Solvent Systems. Food Res. Int. 2011, 44(7), 1977–1987. DOI: 10.1016/j.foodres.2011.01.023.