ABSTRACT
Three pomegranate clutivars (Tunisia soft seed, Taishanhong, and Qingpiruanzi) were used to evaluate their seed germination percentage, potential, germination time, and shoot growth indices along with antioxidant capacity, bioactive components (i.e., oil, crude fat, soluble sugar, protein, total phenolic and flavonoid, and Vitamin C (VC) content, and mineral element contents of their seeds and sprouts). ‘Tunisia soft seed’ exhibited the highest germination percentage (86.33%) and potential (39.33%) while ‘Qingpiruanzi’ had the shortest germination time (15d) and lowest rate (23.33%). Soluble sugar content of sprouts (16.93–25.81 mg/g) was 1–2-folds that of seeds (8.29–14.06 mg/g). The soluble protein content of seeds (102.05–161.37 mg/g) was 10-fold higher than that of sprouts (14.71–21.46 mg/g), while the VC content of sprout (29.11–32.83 mg/100g) was more than 10 times higher than that of seed (1.89–3.25 mg/100g). Oil content of three varieties's seeds ranged from 164.44 to 183.52 mg/g. Pomegranate seed oil contains 14 fatty acids, the most abundant of which is punicic acid (71.85–77.78%). Pomegranate seeds and sprouts are also rich in mineral elements (calcium 99.49–238.25 mg/100g) and magnesium (97.41–187.59 mg/100g). Total phenols (14.68–16.29 mg/g) and flavonoids (1.97–2.48 mg/g) content in sprouts were significantly higher than that of seeds (3.88–4.96 and 0.51–0.68 mg/g). Antioxidant capacity detected by ABTS and DPPH, FRAP assay was positively associated with total phenolic (ABTS: r = 0.946, DPPH: r = 0.977, FRAP: r = 0.985). These preliminary results were essential to investigate the possibilities of commercial natural health food utilization of pomegranate products in China.
Introduction
Pomegranate, a fruit tree of the Lythraceae family, has nutritional, medicinal, ornamental, and ecological values.[Citation1] The pomegranate fruit is a biological treasure with its peel’s effective antibacterial activity against Staphylococcus aureus and Bacillus cereus; flowers’ great ornamental value, fruits’ high antioxidant activity, and eaten fresh or processed into juice or wine.[Citation2] Pomegranate seeds have strong antioxidant activity, and when consumed as a dietary supplement they reduce obesity[Citation3], blood lipid levels[Citation4], and the risk of cancer.[Citation5] Pomegranate seeds contain rich oil that contains 64–83% punicic acid (PA) which is a ‘super conjugated linoleic acid’ (CLA) with a more potent effect than ordinary conjugated linoleic acid.[Citation6]
Additionally, pomegranate sprouts as a natural health food that is rich in bioactive substances such as with lower fat content, thus offer substantial market potential.[Citation7] Vinokur et al.[Citation8] was the first to study the phytochemical compositions of pomegranate sprouts and examined the main components of pomegranate phenols. According to Falcinelli et al.[Citation9], the sprouts of four Italian pomegranate varieties contained high antioxidant activity. These studies provided the basis for the feasibility of developing pomegranate sprout industry in China.
In this study, we analysed the physiologically active components, nutrient elements, antioxidant activities, seed germination rate and the types and contents of fatty acids in seed oil of different pomegranate varieties as well as the differences in nutrient distribution between seeds and sprouts. The nutritional values and characteristics of seed and sprouts are expected to provide a reference for the targeted development and application of their health benefits.
Materials and Methods
plant material and chemicals
Pomegranate seed (Tunisia soft seed, Taishanhong, and Qingpiruanzi) and the weight of 100 seeds per determined after cleaning and drying (Tunisia soft seed = 1.657 g; Taishanhong = 1.866 g; Qingpiruanzi = 2.675 g). Seed water content of the three using three replications. After the seeds are naturally air dried, they were stored at −80°C until further use. According to Falcinelli et al.[Citation9], the ready-to-eat stage of sprouts was assumed to be a stage between the complete cotyledon expansion and just before the emission of the first true leaf. Biologically active ingredients such as crude protein, soluble sugar, and crude fat contents and water contents of pomegranate seeds and sprout powders were determined using the procedures of Kruger et al.[Citation10], Chow et al.[Citation11], Horwitz et al.[Citation12], respectively.
Pomegranate oil content
Five grams of pomegranate seed powder was treated with petroleum ether at a 1:10 ratio, placed in the Scirntz SB-5200D acoustic magnetic extractor and extracted by ultrasonication for 40 min. The excess waste liquid was evaporated at 70°C with rotation, incubated at 80°C and evaporated to a constant weight. The extraction rate of pomegranate seed oil (PSO) was calculated. PSO content was conducted using three replications.
Total phenolic content determination
The Folin-phenol method was used to determine the total phenolic content (TP).[Citation13] First, 0.5 g of fine powder of pomegranate seeds and fresh pomegranate sprout of the studied three cultivars (Tunisia soft seed, Taishanhong and Qingpiruanzi) was individually immersed in 60% ethanol at a 1:40 ratio (material to liquid) and heated under reflux for 5 min. After cooling, the mixture was centrifuged at 3000 rpm for 15 min. Then, 0.5 mL of the phenolic extract was mixed with 2.5 mL of Folin-Ciocalteu reagent and 2 mL of 7.5% sodium carbonate (Na2CO3). The mixture was incubated in a water bath at 50°C for 5 min and then cooled to room temperature, and the absorbance was read at 760 nm by (BECKMAN DU-800®). Reactions were conducted in triplicate, and the results are reported as gallic acid equivalents (GAE) per gram of pomegranate seeds and sprouts.
Total flavonoid content determination
Total flavonoid (TF) content was determined using the method of Fernandes et al. after slight modifications.[Citation14] First, 0.5 g of seeds and sprouts powder of the studied three cultivars was placed in grinding pot, added 4 mL of 70% ethanol, and diluted the mixture to 10 mL with water. Then, the vessel was sealed with plastic wrap, heated at 70°C in water bath for 45 min, and allowed to stand for 5 min. One millilitre of this solution was diluted to 5 mL with 70% ethanol, and then 0.3 mL of 5% NaNO2 was added and mixed. Then, the mixture was incubated for 6 min, 4mL of 4% NaOH was added, and the mixture was diluted to 10 mL with distilled water. After a 10-min incubation, the absorbance was measured at a wavelength of 510 nm by spectrophotometer (BECKMAN DU-800®); with 70% ethanol served as a control. Reactions were conducted in triplicate, and the results are reported as equivalents (RE) per gram of pomegranate seeds and sprouts.
Vitamin C(VC) content determination
Extraction solution: Two grams of pomegranate seed and sprout powder of the studied three cultivates was extracted by adding 5 mL of 1% HCl, and diluted to 25 mL. Then, 0.2 mL of the extract was added to 0.4 mL of 10% HCl, and 9.4 mL of distilled water was added. The absorbance was measured at 243 nm using distilled water as a control.
For preparation of the alkali treatment solution, 0.2 mL of extract solution mentioned (above), 2 mL of distilled water, and 0.8 mL of 1 M NaOH solution were mixed and after 15 min, 0.8 mL of 10% HCl was added to a total volume of 10 mL. The absorbance was read at 243 nm by (BECKMAN DU-800®). Making ascorbic acid standard solution, according to the difference in value between the first reading and the second reading, the concentration of VC can be calculated. Reactions were conducted in triplicate.
Antioxidant activity measurements
ABTS scavenging ability
The ABTS content was determined using Miller after slight modifications.[Citation15] The ABTS working solution was prepared as follows: 7.4 mmol/L ABTS and 2.6 mmol/L K2S2O8 were mixed at a 1:1 volume. After a 12-h reaction in the dark, the solution was filtered and diluted to an absorbance of 0.700 ± 0.005 at 734 nm as monitored using a Beckman Coulter Du-800 spectrophotometer. Next, 0.8 mL of working with 0.2 mL of the sample solution and shaken for 10 s; the reaction was incubated for 6 min, the absorbance was measured 734 nm using (BECKMAN DU-800®), and the value was recorded as A. Then, 0.8 mL of working solution was mixed with 0.2 mL of 95% ethanol, shaken for 10 s, and incubated for 6 min, and the absorbance at 734 nm was recorded as A0. Reactions were conducted in triplicate, and the results are reported as Trolox equivalents (TE) per gram of pomegranate seed flour and pomegranate sprout.
DPPH radical scavenging capacity estimation
The DPPH content was measured using Thaipong et al. method.[Citation16]Twenty-four milligrams of DPPH in 100 mL of methanol to ensure an absorbance reading of 1.1 ± 0.01. One hundred fifty microliters of the phenolic extract mixed with 2850 μl of DPPH solution, and after 24 h, the absorbance was measured 517 nm using spectrophotometer (BECKMAN DU-800®).
FRAP reduction ability
The FRAP content was measured using Thaipong et al. method.[Citation16] The FRAP working solution: one hundred millilitres of 30 mmol/L acetic acid buffer, 10 mL of 10 mmol sodium acetate buffer, and 10 mL of FeCl3-6H2O solution were mixed to obtained. 150 µl of phenolic extract was mixed with 2850 μl of FRAP working solution and heated at 37°C for 30 min in the dark, and the absorbance was recorded 593nm using spectrophotometer (BECKMAN DU-800®). Reactions were conducted in triplicate, and the results are reported as Trolox equivalents (TE) per gram of pomegranate seed flour.
Sprouting
March 2018, 4 biological replicates of 100 seeds of each cultivar were placed in petty cotton in Petri dishes. Seeds were incubated at 28°C in the dark, water levels were regularly quantified daily. When the sprout reached 0.3 cm, it was recorded as a successful germination. If seed germination did not change for three consecutive days, the time and of the last seed germination were considered as germination time (GT) and germination percentage (GP), respectively. When the number of germinated seeds reached the highest peak during germination, the number of germinated seeds accounted for the germination percentage of the number of total seeds used and is recorded as germination potential (G). On the last day of germination, the root length, dry weight, and fresh weight of all sprouts were determined.
Fatty acid contents determination
Fatty acid methyl esters (FAMEs) used in the analysis were prepared using the KOH-methanol method according to Zhang et al.[Citation17] One hundred milligrams of oil was dissolved in 2.5 mL of ether n-heptane solution (volumetric ratio 2:1 V/V), 2.5 mL of 0.8mol/L KOH–methanol solution, and 2.5 mL of a methanol solution Distilled water (17.5 mL) was added and mixed for 10 min. Two millilitres of the supernatant liquid was used, and the mixture was rotated and then centrifuged for 10 min. Then, the supernatant was used and subjected to GC-MS analysis.
GC-MS analysis
Trace ISQ-LT GC-MS was used for the fatty acid analysis. Helium was used as the carrier gas at a flow rate of 1.0 mL/min through a DB-5MS column (30 m × 0.25 mm × 0.25 μm). The initial oven temperature was 80°C. This temperature was maintained for 2 min, increased at a rate of 20°C/min until it reached 220°C, then increased again at a rate of 10°C/min to a final temperature of 300°C, at which it was maintained for 3 min. MS spectra were obtained at an ionization energy of 70 m/z 33–450, an interface temperature of 250°C, and an ion source temperature of 230°C.
Mineral content determination
The concentrations of macro elements (calcium, magnesium, and sodium) and trace elements (iron, zinc, manganese, and copper) in and sprouts were determined using Horwitz method.[Citation13] Mineral contents were measured using an inductively coupled plasma PerkinElmer-Optima 8300 instrument.
Statistical analysis
The data were analysed by one-way analysis of variance (ANOVA) and sample means were compared by Tukey’s test. P < 0.05 was considered significant in all cases. The JASP software was used for statistical analysis.
Results and discussion
Sprout and root growth
The germination percentage (GP) and germination potential (G) represent the viability and vitality of seed quality. The germination percentage of Tunisia soft seed and Taishanhong exceeded 80%, while it was as low as 23.33% for Qingpiruanzi (). This observed difference may be due to the hardness of the Qingpiruanzi seed coat and thus the poor seed shell permeability, resulting in observed lower germination. Among the studied three varieties, ‘Tunisia soft seed’ and ‘Qingpiruanzi’ seeds exhibited the highest and lowest germination potential, respectively, indicating that the former exhibited the best seed vigour than the later.
Table 1. Germination percentage (GP), Germination time (GT), Germination potential (G), and root growth coefficient of different varieties of pomegranate seeds
Significant differences (P <0.05) observed in the mean germination time of the studied varieties (). The germination time required for Qingpiruanzi, Taishanhong, and Tunisia soft seed to germinate was 15, 22, and 23 days, respectively. The root length and fresh and dry weight of the three varieties ranged from 1.84–2.97 cm, 5.88–6.99 mg, and 0.48–0.78 mg, respectively, with Taishanhong performing better than Tunisia soft seed and Qingpiruanzi. The germination percent is an important reference indicator for the development and utilization of a pomegranate sprout variety. Based on these results, Tunisia soft seed and Taishanhong seed deemed a good choice.
The water content of the three cultivar's seeds and sprouts ranged from 24.12 mg/g to 36.71mg/g and 42.58 mg/g to 56.92 mg/g, respectively, which was predictably higher in the sprouts than seeds (). The oil content of the three cultivar's seeds and sprouts varied between 164.44 mg/g to 183.52 mg/g and 11.27 mg/g to 14.09 mg/g, respectively, which was substantially higher in the seeds than the sprouts (). Crude fat contents ranged between [117.52 -218.63 mg/g] among the cultivars’ seed and were not present in their respective sprouts (). The soluble sugar content of pomegranate seeds and sprouts ranged from 8.29 mg/g to 14.06 mg/g and 16.93mg/g to 25.81 mg/g, respectively, with two-fold greater concentration in the sprouts compared to seeds (). The protein content of the seeds was 102.05–161.37 mg/g, a value that was much higher than that of sprouts (14.71–21.46 mg/g). The protein content before seed germination was 6.94–8.33-fold higher than that after germination. The order of the protein content was Taishanhong seed> ‘Tunisia soft seed’ seed> Qingpiruanzi seed> Taishanhong sprout> ‘Tunisia soft seed’ sprout> Qingpiruanzi sprout, with seed showing greater contents than their respective sprouts.
Table 2. Comparison of biologically active components between pomegranate seeds and sprouts
Flavonoid content of seeds ranged from 0.51 mg/g to 0.68 mg/g, while ranged in sprouts from 1.97 mg/g to 2.48 mg/g. Flavonoid contents in pomegranate sprout approximately 3.6-fold greater than those in seeds. The total phenolic content of the pomegranate sprout extracts (14.68–16.29mg/g) was more than 4-fold greater than that of the seed extracts (3.88–4.96 mg/g). Both values were greater than the total phenolic content of ‘Orlando orange’ seeds (63.349 mg/100 g) studied by Al et al.[Citation18] but lower than contents recorded in strawberry (14.8–23.7 mg GAE/g)and apple (11.9–22.1 mg GAE/g).[Citation19] Braga et al.[Citation20] studied the antioxidant properties of different food waste slag, including total phenol contents of grape residue (768.56 ± 116.35 mg GAE/g, peanut skin (404.40 ± 13.22 mg GAE/g) and mango (160.25 mg GAE/g, and the highest total contents were observed in peanut skin (2.44 mg QE/g), while the contents in grape residue (1.76 mg QE/g) and mango (1.70 mg QE/g) were not significantly different.
The VC content was high in pomegranate sprouts, reaching 29.1–32.8 mg/100 g, a value that was approximately 10-fold higher than that of seeds () and greater than that of some Spanish pomegranate varieties, even the juice.[Citation14] According to Guo et al.[Citation21], after germination, the contents of total phenolic compounds, total flavonoids, and VC content increased significantly and were 4.5-fold, 6.8-fold, and 38-fold higher, respectively, than those in the original mung beans. Several studies have shown that sprouts have more phenolic substances and antioxidant capacity than seeds[Citation22–Citation24]; pomegranate is no exception.
Phenolic compounds not only are the most abundant hydrophilic antioxidants and the most active compounds in the diet[Citation25] but also stimulate cell defence and help prevent oxidative damage.[Citation26] Pomegranate sprouts have high nutritional and health value as a developable dietary antioxidant.
Types and contents of fatty acids
Types and contents of fatty acids contained 12, 10, and 14 different types Tunisia soft seed, Taishanhong, and Qingpiruanzi, respectively (). The sum of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) was 8.43–10.03, 4.95–8.36, and 79.32–83.01%, respectively, in PSO. Among the saturated fatty acids, the highest content was observed in Taishanhong seed (10.03%), and the lowest in Tunisia soft seed (8.43%). Among the monosaturated fatty acids, the highest content was recorded in Qingpiruanzi seed (8.36%), and the content in Tunisia soft seed was only 4.95%. Among the polyunsaturated fatty acids, Tunisia soft seed had the highest content (83.01%), and the lowest content was observed in Taishanhong seed (79.32%). The most abundant fatty acid in PSO was unsaturated fatty acids. Unsaturated fatty acids are indispensable fatty acids in the human body. PA was the most important unsaturated fatty acid in PSO, accounting for 71.85–77.78% of the total oil content and 82.27–88.42% of the total unsaturated fatty acid content. This observed range was greater than that reported by Kyralan[Citation27], who studied the fatty acid composition in PSO from 15 pomegranate varieties in ‘Tunisia soft seed’ seed (PA: 70.42–76.17%), and less than the 83.4% reported for a variety in Georgia.[Citation28] Among the studied three varieties, ‘Tunisia soft seed’ seed oil had the highest proportion of PA. In addition, PSO contains essential fatty acids such as Stearoleic acid (C18:1) and linoleic acid W6 (C18:2). Among these essential fatty acids, the lowst linoleic content in ‘Tunisia soft seed’ was 4.92%, and the highest content was observed in Taishanhong seeds (7.43%).
Table 3. Types and contents of fatty acids in different varieties of pomegranate oil
The treatment and prevention of diseases is a frequently studied topic. The consumption of fruits is safe and effective and provides a sufficient amount of nutrients to prevent diseases. Similar to other fruits, pomegranates harbour different ingredients that have different functions and have been used to treat some diseases. Oral administration of PSO to mice improved insulin resistance and obesity caused by a high-fat diet.[Citation29,Citation30] In a 12-week cycle of feeding on high-fat diets, mice treated with PSO had significantly less fat and improved insulin sensitivity.[Citation31]
Pomegranate seeds have a certain bitterness and astringency due to the influence of their woody texture, but PSO in seeds is very beneficial to human health. The use of capsules or edible oils has great developmental potential in health care. PSO not only paves the way for waste utilization but also moves towards the development of value-added products.
Minerals
'Tunisia soft seed’, Taishanhong, and Qingpiruanzi seeds and sprouts contain large amounts of macro and trace elements required by the human body (). Ca content in the seeds and sprouts the highest among all mineral. In particular, Ca content in ‘Tunisia soft sprout’ reached 238.25 mg/100 g; this value is higher than reported in other study using pomegranate seeds (20.15–55.21 mg/100g) in Turkey.[Citation32] Mn concentration generally was very low in seed and was not detected in sprouts. Pomegranate seeds and pomegranate sprouts are also rich in sodium and magnesium. Fe content in (8.56–13 mg/100g) was lower than to that in apple (14.46 mg/100g).[Citation33] In general, the mineral elements of pomegranate sprouts are more abundant than seeds.
Table 4. Mineral contents (mg/100g of dry matter) in pomegranate seed and sprout powders
Antioxidant activity
Antioxidant activity is mainly manifested as the inhibition of the oxidative degradation of lipids, the free radical scavenging capacity, the inhibition of pro-oxidants (such as chelation transition metals) and the reducing power. Although many methods have been employed to determine the antioxidant capacity, there is no single evaluation antioxidant available. Therefore, we evaluated the antioxidant capacity of pomegranate seeds and sprouts by determining the concentrations of ABTS free radicals, DPPH inhibition ability, and FRAP reduction (Fe3+-TPTZ) ability.[Citation16,Citation17]
As shows, ABTS inhibitation rate of pomegranate seeds was 1.61–2.53 μmol TE/g; however, it was lower than that reported from 4 other Chinese varieties (7.8–15.81 μmol TE/g).[Citation34] The pomegranate sprout ABTS inhibition rate was 3.77–4.78 μmol TE/g. ABTS inhibition rate of pomegranate sprouts was significantly greater than that of seeds. The order of ABTS inhibition rate among the three varieties was Qipiruanzi sprout> 'Tunisia soft seed' sprout> Taishanhong sprout> Qingpiruanzi seed> ‘Tunisia soft seed’ seed> Taishanhong seed. Sritongtae et al.[Citation35] studied the effects of chemical constituents and antioxidant activities of rice bean sprouts and found that germination significantly increased the free radical scavenging activity and antioxidant capacity and inhibited toxic free radicals due to the high antioxidant activity.
Figure 1. Antioxidant activities measured in the seeds and sprouts of three pomegranate (Tunisia soft Taishanhong, and Qingpiruanzi) using the ABTS, DPPH, and FRAP tests (μmol TE−1 DW)
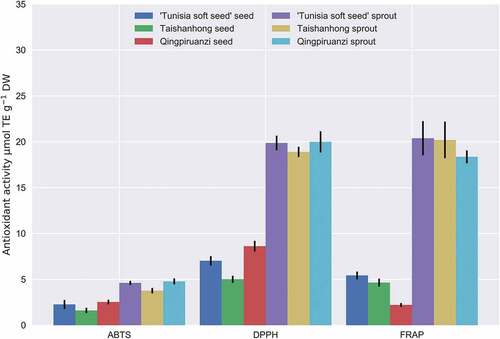
Among the three pomegranate varieties, the DPPH clearance rate of seeds was 5.01–8.61 μmol TE/g, and 18.90–19.99 μmol TE/g for sprouts. Qingpiruanzi sprouts exhibited the strongest DPPH free radical scavenging ability. The DPPH clearance rate of sprouts was 2.32–3.77-fold higher than that of seeds. In grape seed extracts, DPPH not only has high antioxidant also inhibits the activity of different bacterial and fungal strains.[Citation36]
The lowest FRAP reducing of pomegranate seeds was observed for Qingpiruanzi (2.21 μmol TE/g), and the highest for Tunisia soft seed (5.43 μmol TE/g). This range was greater than that reported for small seeds (2.73 μmol TE/g) and American sunflower seeds (0.08 μmol TE/g).[Citation37] Compare other seeds, pomegranate seeds shows greater antioxidant activity. Additionally, FRAP reducing of pomegranate sprouts was generally higher than that of seeds. FRAP reducing ability of ‘Tunisia soft seed’ seeds was more than 2 times higher than that of Qingpiruanzi seeds; however, FRAP reducing ability of Qingpiruanzi sprouts (18.36 μmol TE/g) was still much higher than that of ‘Tunisia soft seed’ seeds (5.43 μmol TE/g).
Although pomegranate seeds had a certain antioxidant capacity, it was far less than that of sprouts. ABTS inhibition rate, DPPH free radical scavenging ability, and FRAP reducing ability of the different varieties of pomegranate seeds and sprouts significantly differed. Thus, the genotype, growth conditions, and climate may affect the antioxidant capacity of pomegranate.
We noticed that the literature[Citation34,Citation38] reported that different methods (concentration of antioxidants, volume of extract) and different extraction materials and methods, will lead to greatly different results. If you compare other literature based on only one experimental result, the reference value is poor. Therefore, using the same experimental method, the comparison results of multiple pomegranate varieties have higher credibility. In addition, to find the best way to extract material, a unified experimental method is very important.
The correlation between TP contents and ABTS inhibition rate was 0.946, between TP contents and DPPH clearance was 0.977, and between TP contents and FRAP reducing power was 0.985 (). Our results were higher than the values observed (r = 0.839–0.863) of 44 traditional Brazilian fruit pulps by Stafussa et al.[Citation39], while our results were lower than values (r = 0.769–0.991) reported by Liu et al.[Citation40], different from the values observed (r = −0.945 to −0.968) of cereal sprouts and grasses by Niroula et al.[Citation41] Despite the differences in value in correlation reported in the literature, a commonality amongst them is that the total antioxidant capacity and TP contents exhibited a high correlation, and the higher the total phenol content was, the stronger the corresponding antioxidant capacity.
Figure 2. Correlations with total phenolic contents (TPC), ABTS, DPPH, and FRAP in seeds and sprouts
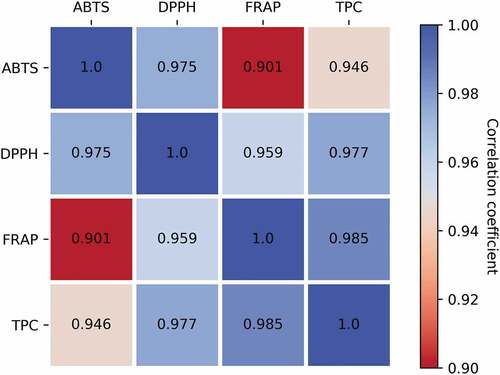
As shown in the present study, pomegranate sprouts are rich in various nutrients and have a higher antioxidant capacity than their seeds counterpart. As a high-quality natural food, the value of pomegranate sprouts has not been fully developed and future research is required to facilitate its adoption and use.
Conclusion
The contents of biologically active components, antioxidant activity, concentrations of mineral elements, and the types and contents of fatty acids in pomegranate oils from different pomegranate varieties were substantially different. Germination results showed increase in water content, soluble sugar, total phenolic, flavonoid, vitamin C and a decrease in protein and oil content. Pomegranate sprouts have higher antioxidant capacity than seeds. Based on the germination rate and total phenol and flavonoid contents, ‘Tunisia soft seed’ possessed the best antioxidant activity. Pomegranate seeds are rich in oils and fats, including a large amount of PA, which has a very high health value. This study contributes to the identification and development of natural functional foods.
Additional information
Funding
References
- Yuan, Z.; Fang, Y.; Zhang, T.; Zhang, J.; Han, F.; Liu, C.; Liu, M.; Xiao, W.; Zhang, W.; Zhang, M.; et al. The Pomegranate (Punica Granatum L.) Genome Provides Insights into Fruit Quality and Ovule Developmental Biology. Plant Biotechnol. J. 2017, 16, 1363–1374. DOI: 10.1111/12875.
- Kanatt, S. R.; Chander, R.; Sharma, A. Antioxidant and Antimicrobial Activity of Pomegranate Peel Extract Improves the Shelf Life of Chicken Products. J. Food Sci. Techno. 2010, 45, 216–222. DOI: 10.1111/j1365-2621.2009.02124x.
- Ambigaipalan, P.; Camargo, A. C.; Shahidi, F. Identification Of Phenolic Antioxidants And Bioactives Of Pomegranate Seeds Following Juice Extraction Using HPLC-DAD-ESI-MS(n). Food Chem. 2017, 221, 1883–1894. DOI: 10.1016/2016.10.058.
- Mirmiran, P.; Fazeli, M. R.; Asghari, G.; Shafiee, A.; Azizi, F. Effect of Pomegranate Seed Oil on Hyperlipidaemic Subjects: A -Controlled Clinical Trial. J. Nutri. 2010, 104, 402–406. DOI: 10.1017/S0007114510000504.
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Cell. Long. 2015, 2015, 1–19. DOI: 10.1155/2015/938475.
- Tehranifar, A.; Zarei, M.; Nemati, Z. Investigation of Physico-Chemical Properties and Antioxidant Activity of Twenty Iranian Pomegranate (Punica Granatum L.) Cultivars. 2010, 126, 180–185. DOI: 10.1016/2010.07.001.
- Yu, M.; Liu, H.; Shi, A.; Liu, L.; Wang, Q. Preparation of Resveratrol-Enriched and Poor Allergic Protein Peanut Sprout from Ultrasound Treated Peanut Seeds. 2016, 28, 334. DOI: 10.1016/2015.08.008.
- Vinokur, Y.; Rodov, V.; Horev, B.; Goldman, G.; Aharoni, N. Pomegranate Sprouts, Preparations Derived Therefrom and Compositions Comprising Same: US, US 8501257 B2P] (2013).
- Falcinelli, B.; Marconi, O.; Maranghi, S.; LuttsAdolfo, S.; Rosati, F. F.; Benincasa, P. Effect of Genotype on the Sprouting of Pomegranate (Punica Granatum L.) Seeds as a Source of Phenolic Compounds from Juice Industry By-Products. Plant Foods Hum. Nutri. 2017, 72, 432–438. DOI: 10.1007/s11130-017-0645-y.
- Kruger, N. J.;. The Bradford Method for Protein Quantitation. The Protein Protocols Handbook; Humana Press: Totowa, NJ, 2009; pp 17–24.
- Chow, P. S.; Landhäusser, S. M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol. 2004, 24, 1129–1136. DOI: 10.1093//24.10.1129.
- Horwitz, W.; Horwitz, W.; Horwitz, W.; Kane, P. F.; Cunniff, P. Official Methods of Analysis of the AOAC International. Trends Food Sci. Tech. 2005, 6, 382. DOI: 10.1007/978-1-4615-8389-9_3.
- Liu, Y.; Fang, S.; Zhou, M.; Shang, X.; Yang, W.; Fu, X. Geographic Variation in Water-Soluble Polysaccharide Content and Antioxidant Activities of Cyclocarya Paliurus Leaves. Prod. 2018, 121, 180–186. DOI: 10.1016/2018.05.017.
- Fernandes, L.; Pereira, J. A.; Lopéz-Cortés, I.; Salazar, D. M.; G-Álvarez, J.; Ramalhosa, E. Composition and Antioxidant Activity of Several Pomegranate (Punica Granatum, L.) Cultivars Grown in Spain. Food Res. Technol. 2017, 4, 1–16. DOI: 10.1007/s00217-017-2884-4.
- Miller, N. J.; Sampson, J.; Candeias, L. P.; Bramley, P. M.; Riceevans, C. A. Antioxidant Activities of Carotenes and Xanthophylls. FEBS Lett. 1996, 384, 240–252. DOI: 10.1016/0014-5793(96)00323-7.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneroszevallos, L.; Byrne, D. H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Comp. Anal. 2012, 19, 669–675. DOI: 10.1016/2006.01.003.
- Zhang, Z.; Luo, Y.; Wang, X.; Yu, F. Fruit Spray of 24-Epibrassinolide and Fruit Shade Alter Pericarp Photosynthesis Activity and Seed Lipid Accumulation in Styrax Tonkinensis. J. Plant Growth Regul. 2018, 37, 1066–1084. DOI: 10.1007/s00344-017-9769-4.
- F J, A.; Özcan, M. M.; Uslu, N.; Ghafoor, K. The Effect of Drying Temperatures on Antioxidant Activity, Phenolic Compounds, Fatty Acid Composition and Tocopherol Contents in Citrus Seed and Oils. J. Food Sci. Technol. 2018, 55(2), 1–8. DOI: 10.1007/s13197-017-2895-y.
- Kahkonen, M.; Hopia, A.; Vuorela, H. Antioxidant Activity of Plants Extracts Containing Phenolic Compounds. Agric. Food Chem. 1999, 47(10), 3954–3962. DOI: 10.1021/jf990146.
- Braga, G. C.; Melo, P. S.; Bergamaschi, K. B.; Tiveron, A. P.; Massarioli, A. P.; Alencar, S. M. Extraction Yield, Antioxidant Activity Andphenolics from Grape, Mango and Peanut Agro-Industrial By-Products. Rural. 2016, 46, 1498–1504. DOI: 10.1590/0103-8478cr20150531.
- Guo, X.; Li, T.; Tang, K.; Liu, R. H. Effect of Germination on Phytochemical Profiles and Antioxidant Activity of Mung Bean Sprouts (Vigna Radiata). J. Agric. Food Chem. 2012, 60, 11050. DOI: 10.1021/jf304443u.
- Huang, X.; Cai, W.; Baojun, X. Kinetic Changes of Nutrients and Antioxidant Capacities of Germinated Soybean (Glycine Max L.) And Mung Bean (Vigna Radiata L.) With Germination Time. Food Chem. 2014, 143, 268–276. DOI: 10.1016/2013.07.080.
- Kim, S-J. I.; Zaidul, S. M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of Phenolic Compositions between Common and Tartary Buckwheat (Fagopyrum) Sprouts. Food Chem. 2008, 110, 814–820. DOI: 10.1016/2008.02.050.
- Wang, H.; Wang, J.; Guo, X.; Brennan, C. S.; Li, T.; Fu, X.; Liu, R. H. Effect of Germination on Lignan Biosynthesis, and Antioxidant and Antiproliferative Activities in Flaxseed (Linum Usitatissimum L.). Food Chem. 2016, 205, 170–177. DOI: 10.1016/2016.03.001.
- Blokhina, O.; Virolainen, E.; Fagerstedt, K. V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. DOI: 10.1093//mcf118.
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry Dietary Fiber Functionalized with Phenolic Antioxidants from Olives. Interactions between Polysaccharides and Phenolic Compounds. Food Chem. 2019, 280, 310–320. DOI: 10.1016/2018.12.057.
- Kýralan, M.; Gölükcü, M.; Tokgöz, H. Oil and Conjugated Linolenic Acid Contents of Seeds from Important Pomegranate Cultivars (Punica Granatum L.) Grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 1029. DOI: 10.1007/s11746-009-1436-x.
- Pande, G.; Akoh, C. C. Antioxidant Capacity And Lipid Characterization Of Six Georgia-Grown Pomegranate Cultivars. J. Agric. Food Chem. 2009, 57, 9427. DOI: 10.1021/jf901880p.
- McFarlin, B. K.; Strohacker, K. A.;Kueht, M. L. Pomegranate Seed Oil Consumption during a Period of High-Fat Feeding Reduces Weight Gain and Reduces Type 2 Diabetes Risk in CD-1 Mice. J. Nutri. 2008, 102, 54–59. DOI: 10.1017/S0007114508159001.
- Vroegrijk, I. O.; van Diepen, J. A.; Van, D. B. S.; Westbroek, I.; Keizer, H.; Gambelli, L. Pomegranate Seed Oil, a Rich Source of Punicic Acid, Prevents Diet-Induced Obesity and Insulin Resistance in Mice. Food Chem. Toxicol. . J. . . . Biol. Res. Assoc. 2011, 49, 1426–1430. DOI: 10.1016/2011.03.037.
- Jelodar, G.; Mohsen, M.; Shahram, S. Effect of Walnut Leaf, Coriander and Pomegranate on Blood Glucose and Histopathology of Pancreas of Alloxan Induced Diabetic Rats. J. . Comp. 2007, 4, 299–305. DOI: 10.1016/2006.07.013.
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of High Hydrostatic Pressure (HHP) on Bioaccessibility, as Well as Antioxidant Activity, Mineral and Starch Contents in Granny Smith Apple. Food Chem. 2011, 128(2), 520–529. DOI: 10.1016/2011.03.074.
- Okatan, V.; Çolak, A. M.; Güçlü, S. F.; Gündoğdu, M. The Comparison of Antioxidant Compounds and Mineral Content in Some Pomegranate (Punica Granatum L.) Genotypes Grown in the East of Turkey. Acta Sci. Pol. . Cultus. 2018, 17, 201–211. DOI: 10.24326/2018.4.18.
- Jing, P.; Ye, T.; Shi, H.; Sheng, Y.; Slavin, M.; Gao, B.; Liu, L.; Yu, L. Antioxidant Properties and Phytochemical Composition of China-Grown Pomegranate Seeds. Food Chem.. 2012, 132(3), 1457–1464. DOI: 10.1016/2011.12.002.
- Sritongtae, B.; Sangsukiam, T.; M R, M.; M R, M.; Duangmal, K. Effect of Acid Pretreatment and the Germination Period on the Composition and Antioxidant Activity of Rice Bean (Vigna Umbellata). Food Chem. 2017, 227, 280–288. DOI: 10.1016/2017.01.103.
- Madhavan, V.; Jennifer Emelda, E.; Santhanakrishnan, T. Effect of Solvents on Phytochemicals, Antioxidant and Antimicrobial Activity of Grape Seed Extract (Vitis Vinifera). J. Free Radicals Antioxid. 2016, 143, 440–445. DOI: 10.1016/2007.02.010.
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J. M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. DOI: 10.1021/jf803011r.
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M. S.; Conti, G. O.; Sadrabad, E. K. Antioxidant Activity and Total Phenolic Content of Ethanolic Extract of Pomegranate Peels, Juice and Seeds. Food Chem. Toxicol. 2018, 14, 108–111. DOI: 10.1016/2018.02.023.
- Stafussa, A. P.; Maciel, G. M.; Rampazzo, V.; Bona, E.; Makara, C. N.; Junior, B. D.;Haminiuk, C. W. I. Bioactive Compounds of 44 Traditional and Exotic Brazilian Fruit Pulps: Phenolic Compounds and Antioxidant Activity. J. Food Prop. 2018, 21, 106–118. DOI: 10.1080/10942912.2017.1409761.
- Liu, Y.; Chen, P.; Zhou, M.; Wang, T.; Fang, S.; Shang, X.; Fu, X. Geographic Variation in the Chemical Composition and Antioxidant Properties of Phenolic Compounds from Cyclocarya Paliurus (Batal) Iljinskaja Leaves. Molecules. 2018, 23, 2440. DOI: 10.3390/molecules23102440.
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total Phenolic Contents and Antioxidant Activity Profile of Selected Cereal Sprouts and Grasses. J. Food Prop. 2019, 22, 427–437. DOI: 10.1080/10942912.2019.1588297.
