ABSTRACT
This study was designed to investigate clenbuterol, salbutamol, and ractopamine concentrations in fresh meat products. A total of 801 samples of ribs, loin, and internal organs of pigs, cattle, and lamb were collected from retail and wholesale markets in nine districts of Jilin Province, Northeast China. The three β-agonists were analyzed by LC-MS/MS. Results showed that 11.36% of the samples contained prohibited β-agonists. Detection rates of β-agonists decreased in the following order: Clenbuterol (6.37%) > Ractopamine (2.62%) > Salbutamol (2.37%). The β-agonists levels were higher for pork than for beef and lamb. The detection rate of meat products decreased in the following order: pork loin (28.72%) > pork ribs (24.72%) > pork internal organs (20.24%) > beef loin (6.67%) > beef internal organs (6.19%) > beef ribs (5.00%) > lamb internal organs (3.45%) > lamb ribs (3.41%) > lamb loin (3.26%). Only one β-agonists was detected per sample. On the surface of our research, the amount β-agonists of added to fresh meat products in Jilin Province of China is relatively safe.
Introduction
Meat constituted a major part of the human diet, which contribute to human’s required nutrients, protein, fat, various inorganic salts, and vitamins.[Citation1] Farm animal farming is the main source of human access to meat. Clenbuterol, salbutamol, and ractopamine are several common β-agonists that are often used to promote or improve feed efficiency and achieve higher muscle to fat ratios in farm animals.[Citation2,Citation3]
Consumption of meat products containing β-agonists may pose a potential risk to consumer health.[Citation4] Since 1990, numerous incidences of acute food poisoning resulting from the consumption of β-agonists contaminated meat have been reported.[Citation4–Citation7] Ingestion of foods containing β-agonists can have serious adverse effects, such as food poisoning[Citation4–Citation9], cardiovascular and central nervous diseases.[Citation10,Citation11] In addition, studies of surface long-acting β-agonists may increase the risk of acute attacks of fatal and non-fatal asthma.[Citation11] In many countries, including European Union countries[Citation12] and China[Citation13], these drugs have been banned due to their potential health risks to the human body.
Currently, meat is the main route of exposure of β-agonists to the human body.[Citation14] Ensuring the safety of fresh meat products remains a huge challenge as illegal veterinary drugs such as β-agonists are detected in meat. Therefore, detecting β-agonists in fresh meat products is important to ensure food safety.
Various analytical methods based on LC-MS[Citation14–Citation20], GC-MS[Citation21,Citation22], internal extractive electrospray ionization-mass spectrometry (iEESI-MS)[Citation23,Citation24], capillary electrophoresis with electrochemical detection[Citation25] and immunoassay (EIA)[Citation16,Citation26–Citation28] are available for the detection of β-agonists. Since LC-MS/MS can rapidly and accurately quantify β-agonists in meat products[Citation29], we used LC-MS/MS to detect β-agonists in meat products. Clenbuterol, ractopamine, and salbutamol were selected as target analytes because they are the most commonly used β-agonists.[Citation9]
Jilin Province, located in the central part of Northeast China and the geographical center of Northeast Asia, has a temperate continental monsoon climate with a permanent population of about 27.53 million. Due to the long and cold winters in northern China, people in Jilin Province need to eat meat to keep out the cold. In 2017, the consumption of meat products in Jilin Province was 19.5 kg per capita, including 16.7 kg of pork, 0.9 kg of beef, 0.4 kg of mutton and 1.5 kg of other.[Citation30] Therefore, the purpose of this study was to determine the levels of three β-agonists, clenbuterol, ractopamine, and salbutamol, in 801 samples from pig, beef, and mutton by LC-MS/MS.
Materials and methods
Chemicals and materials
Standard chemicals (three substances) were purchased from Dr. Ehrenstofer (Augsburg, Germany). Clenbuterol, ractopamine, and salbutamol were dissolved in 50% methanol to a concentration of 100 mg/L. Standard solutions were stored at −20°C when not in use. Mixture of standards for calibration curves and recovery tests were performed using these stock solutions. β-Glucoronidase/arylsulfatase, methanol (HPLC grade) and isopropanol (HPLC grade for liquid chromatography) were provided by Merck (Darmstadt, Germany). Acetonitrile (HPLC grade) was obtained from Labscan (Madrid, Spain) and dichloromethane was provided by BDH (Barcelona, Spain). Ethyl acetate (HPLC grade), Sodium acetate (HPLC grade), Acetic acid (HPLC grade), Perchloric acid (ACS reagent, 70%), Sodium hydroxide (ACS reagent, ≥97.0%), Ammonia solution, Sodium chloride, and Formic acid were provided by Sigma (Madrid, Spain). Sample extraction and clean-up were carried out with Oasis MCX-small SPE cartridges (60 mg/3 mL) from Waters (Massachusetts, USA).
Sample preparation
Sampling was performed from June 2017 to July 2018 in nine cities in Jilin Province, Northeast China. A total of 801 samples of ribs, loin, and internal organs of pigs, cattle, and lamb were collected from retail and wholesale markets in nine cities of Jilin Province (Table S1). All samples were harvested at various aquaculture farms from different cities and were transported to markets through intermediaries. We purchased and transported these samples to our laboratory within 6 h. The edible tissues (muscle and skin) were chopped and kept frozen in plastic bags at a set storage temperature (−20°C).
Sample extraction was performed according to standard procedures within 3 days after sample collection.[Citation31] Eight milliliters of sodium acetate buffer (0.2 M, pH 5.2) and 50 μL of β-glucuronidase/arylsulfatase were added to a 2.00 g aliquot of homogenized sample. Enzymatic hydrolysis of conjugated metabolites was carried out at 37°C for 12 h. After hydrolysis, the mixture was shaken horizontally for 15 min and then centrifuged (5000 rpm, 10 min). Add 4 mL of the supernatant to 5 mL of perchloric acid (0.1 M) and mix well, then adjust the pH to 1 with perchloric acid. After centrifuging (5000 rpm, 10 min) the mixture transfer all the supernatant (about 10 mL) to a 50 mL centrifuge drum and adjust the pH to 11 with sodium hydroxide solution. Add 10 mL of saturated sodium chloride solution, 6 mL of isopropanol and 4 mL of ethyl acetate, mix well and centrifuge (5000 rpm, 10 min).
All organic phases were transferred and dried in a TurvoVap (Zymark) under a nitrogen stream at 40°C water bath. After a conditioning step with 5 mL of sodium acetate buffer (0.2 M, pH 5.2), the mixture was applied to the Oasis MCX-small SPE cartridges placed on a vacuum manifold device (waters). Subsequently, the cartridge was washed with 2 mL water, 2 mL Aqueous formic acid (2%) and 2 mL methanol and dried with strong vacuum for 5 min. Finally, the last washing step was performed with 2 mL methanol and elution was carried out with 4 mL formic acid/methanol with 5% of ammonia. The eluate was evaporated to dryness with nitrogen at 40°C water bath. The residue was dissolved in 200 μL of mobile phase and a 20 μL aliquot was injected in the LC-MS/MS system.
LC-MS/MS analysis
Instrumental analysis was performed using an UltiMate 3000 HPLC system with a TSQ Quantis tandem quadrupole mass spectrometer (Thermo Fisher Scientific, Massachusetts, USA). An X-SELECT C18 column (2.1 mm × 150 mm, 3.5 μm; Waters, Dublin, Ireland) was used for separation. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Chromatographic separation was performed in gradient mode: 0–2 min, 0.2 mL/min 4% B; 3–8 min, 0.2 mL/min increase to 80% B; 9–21 min, 0.2 mL/min linear decrease to 23% B; 22–24 min, 0.2 mL/min increase to 95% B; 25–25.5 min, 0.2 mL/min decrease to 4% B. The column temperature was maintained at 30°C, and the flow rate was set to 0.2 mL/min. The MS instrument was operated in the electrospray ionization (ESI) mode with positive using multiple reaction monitoring (MRM). The precursor ion, two product ions, collision energy, and cone voltage for each target compound are listed in . LC-MS/MS chromatogram at MRL concentration for three β-agonists was presented in Figure S1. The capillary voltages were ESI positive (3 kV), and the capillary temperature was set at 350°C. The source and desolvation temperatures were set at 120°C and 400°C, respectively. The cone and desolvation gas (nitrogen) flow rates were 50 and 650 L/h, respectively. The collision gas (argon) was maintained at a pressure of 3 × 10−3 mbar in the collision cell. The signal acquisition was performed by multiple reaction monitoring mode (MRM) with eight transitions.
Table 1. LC-MS/MS parameter for three targeted β-agonists
Method evaluation
The validation procedure was performed taking into consideration the requirements outlined in the CODEX guidelines (CAC/GL 16 and 71)[Citation32,Citation33] to evaluate the performance of the analytical method. The validation of the level of each β-agonists substance was performed based on the Chinese MRLs in foodstuff of animal origin. Matrix-match calibration curves were constructed using blank meat samples spiked with standard solutions. Representative matrices, namely ribs, loin, and internal organs of pigs, cattle, and sheep have previously been shown to be free of target analytes. All ribs, loin, and internal organs samples were screened based on ribs, loin, and internal organs of pigs, cattle and sheep calibration curves. The precision and accuracy were expressed as recoveries and coefficient variations (CVs). Recovery experiments were performed by incorporating five concentrations of internal standards (0.0, 0.5, 1.0, 2.0 and 5.0 ppb) in blank meat samples, using five replicate samples on one day for each concentration level.[Citation34] The concentration levels with the signal-to-noise (S/N > 2.5) were defined as lower limit of quantification (LLOD) and S/N > 9 were defined as lower limit of quantification (LLOQ). The LLOQ and higher limit of quantification (HLOQ) were assessed on the basis of quadruple determinations of meat samples ranging from 0.3 to 30 ng/mL and 5–500 ng/mL.
Statistical analysis
Data arrangement, descriptive statistical analysis (geometric mean, mean, and range) and chi-square test were performed using the GraphPad prism 7 (GraphPad Software, California, USA).
Results and discussion
Analytical performance
The concentrations of three β-agonists contained in foodstuff of animal origin were determined using LC-MS/MS. Table S2 provides the matrix calibration curves for each part of the pig, cattle, and sheep. summarizes target analytes, LLOQ, recoveries, and CVs in pigs, cattle, and sheep. The LLOQ was calculated based on a minimal acceptable S/N ratio that was sufficiently low to detect veterinary drugs at levels close to their MRLs. Linearities, expressed as the square of the correlation coefficients, were greater than 0.98. The average recoveries of veterinary drugs were in the range of 54–114% with CVs of 3–15%, indicating that the accuracy and precision were favorable and met the requirements for such analysis. Recovery experiment results for certain compounds (Clenbuterol, Ractopamine, and Salbutamol) were satisfactory according to the CODEX guidelines as they were higher than the 90% recovery criteria. Therefore, they were acceptable for a study aimed at detecting β-agonists residues. This screening method has the higher sensitivity comparing with the previous methods using liquid chromatography analysis to determine β-agonists residues in meat products.[Citation35,Citation36]
Table 2. Recovery and coefficients of variation of target β-agonists in pigs, cattle, and sheep
Occurrence of β-agonists residues in meat products
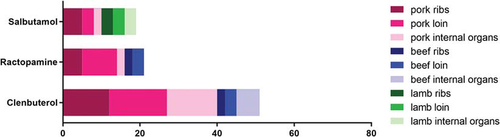
The occurrence patterns of β-agonists in meat products from pigs, cattle, and sheep are shown in . All three β-agonists were detected. The total detection rate of β-agonists in meat samples was 11.36% (91 of 801 samples). Clenbuterol were detected in the most (51 samples at 6.37%), followed by Ractopamine (21 samples at 2.62%), Salbutamol (19 samples at 2.37%). As a result, there are still cases of illegal addition of β-agonists to animal feed in Jilin Province, China. Despite the zero-tolerance policy of β-agonists residues in meat products between China and the European Union, illegally used β-agonists are still present in animal feed in many countries.[Citation37–Citation39]
Table 3. Occurrence patterns of β-agonists for meat products from pigs, cattle, and sheep in Jilin Province, Northeast China
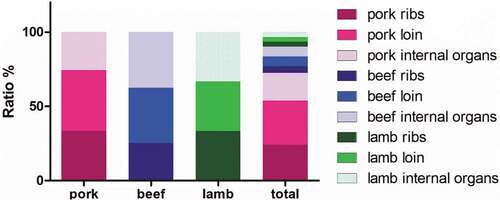
The numbers of detected β-agonists are shown according to meat type in . The detection frequency of pigs, cattle, and lamb was significantly different (p < .05). The detection rate in pork was 24.72% (66/267), which was higher than that (5.99%, 16/267) in beef and (3.37%, 9/267) in lamb. Among all the samples (.), Pork loin had the highest proportion (29.67%), followed by pork ribs (26%). Clenbuterol was the most commonly detected β-agonists in meat products. However, clenbuterol was less frequently detected in lamb than in beef, and it was not detected in lamb samples. In contrast, salbutamol was detected more frequently in lamb than in beef. In this study, only one β-agonists was detected per sample. However, three β-agonists were detected in pork and beef. This situation may be due to different medication habits of different farms. In addition, β-agonists have different metabolic velocities in different animals, and there are differences in the residual state of tissues and organs in various parts of the body.[Citation40,Citation41] Although the use of β-agonists can promote animal growth, irrational use of drugs can also cause damage to animals.[Citation40,Citation42] When using a β-agonists, the drug metabolism is significantly easier to master than using multiple β-agonists at the same time, and the withdrawal time is also easy to control, which helps to keep the β-agonists’ residues in a safe range.
Meat samples were collected from nine cities in Jilin Province, Northeast China. There were no significant differences of detection rate among sampling sites (p > .05). Thus, these results indicated that meat products were evenly distributed in wholesale and retail markets due to the process of transporting meat to market through intermediaries and auctions. Most meat products were mixed according to auction procedures and intermediary levels. Additional investigations are needed to monitor regional variance at the supply and farming stage.
The Range, Geometric mean and Mean ± SD of β-agonists detection for each ribs, loin and internal organs of the pig, cattle, and lamb were shown in –. The mean concentrations of β-agonists in total samples were the highest for Clenbuterol (0.85 μg/kg), followed by Ractopamine (0.81 μg/kg) and Salbutamol (0.61 μg/kg) being the lowest. Although ractopamine is limited to 50 μg/kg in muscle and 150 μg/kg in liver in the United States[Citation43], it is prohibited in China. Ingesting foods containing β-agonists increases the public’s health risks, especially for athletes, which can lead to positive stimulant testing.[Citation44–Citation46]
Table 4. β-agonists concentrations (μg/kg, on fresh weight basis) in pork meat collected from Jilin Province, China
Table 5. β-agonists concentrations (μg/kg, on fresh weight basis) in beef meat collected from Jilin Province, China
Table 6. β-agonists concentrations (μg/kg, on fresh weight basis) in lamb meat collected from Jilin Province, China
Limitation of study
Our study had some limitations. First, sampling time difference at the retail market may have affected the residue level of the β-agonists during the monitoring study. We evaluated the residues of β-agonists in meat samples at the retail distribution stage; however, the β-agonists concentration varies by storage period and conditions in the surroundings. Then, all samples were collected from June to July of each study year, which means that we could not account for seasonal factors affecting the residue concentration in meat samples. Indeed, our results may underestimate the actual β-agonists concentrations during summer because β-agonists are actively metabolized in meat tissue at higher temperatures. In general, most drugs are absorbed and excreted faster in warm water. Thus, temperature appears to be an important factor affecting the reported dietary effects of drug disposition.[Citation47] To identify definitive trends with statistically significant results by monitoring, further studies are required, taking into account various meat species-specific factors such as use pattern and amount of β-agonists as well as withdrawal period.
Conclusion
This is the first representative study monitoring β-agonists concentrations in Jilin Province, Northeast China. Clenbuterol (6.37%) was major substances found in meat products from Jilin Province markets. Three β-agonist (clenbuterol, salbutamol, and ractopamine) residues are detected but at very low levels. Due to the β-agonists levels were higher for pork than for beef and lamb, it is recommended that intensive national inspections are needed to be strictly enforced in maintain β-agonists residues remain within safe levels for human consumption. The results of this study can be used to provide a status of β-agonists addition and to assess human exposure to β-agonists in food products.
Conflict of interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Cashman, K. D.; Hayes, A. Red Meat’s Role in Addressing ‘Nutrients of Public Health Concern’. Meat Sci. 2017, 132, 196–203. DOI: 10.1016/j.meatsci.2017.04.011.
- Wang, L.-Q.; Zeng, Z.-L.; Su, Y.-J.; Zhang, G.-K.; Zhong, X.-L.; Liang, Z.-P.; He, L.-M. Matrix Effects in Analysis of β-Agonists with LC-MS/MS: Influence of Analyte Concentration, Sample Source, and SPE Type. J. Agric. Food Chem. 2012, 60(25), 6359–6363. DOI: 10.1021/jf301440u.
- Hanrahan, J. P. Beta-Agonists and Their Effects on Animal Growth and Carcass Quality; Elsevier Applied Science: London, 1987; pp 1–196.
- Brambilla, G.; Cenci, T.; Franconi, F.; Galarini, R.; Macrì, A.; Rondoni, F.; Strozzi, M.; Loizzo, A. Clinical and Pharmacological Profile in a Clenbuterol Epidemic Poisoning of Contaminated Beef Meat in Italy. Toxicol. Lett. 2000, 114(1), 47–53.
- Pulce, C.; Lamaison, D.; Keck, G.; Bostvironnois, C.; Nicolas, J.; Descotes, J. Collective Human Food Poisonings by Clenbuterol Residues in Veal Liver. Vet Hum. Toxicol. 1991, 33(5), 480–481.
- Salleras, L.; Domínguez, A.; Mata, E.; Taberner, J. L.; Moro, I.; Salvà, P. Epidemiologic Study of an Outbreak of Clenbuterol Poisoning in Catalonia, Spain. Public Health Rep. 1995, 110(3), 338–342.
- Brambilla, G.; Loizzo A.; Fontana L.; Strozzi M.; Guarino A.; Soprano V. Food Poisoning following Consumption of Clenbuterol-Treated Veal in Italy. Jama. 1997, 278(8), 635.
- Ramos, F.; Baeta, M. L.; Reis, J.; Silveira, M. I. N. Evaluation of the Illegal Use of Clenbuterol in Portuguese Cattle Farms from Drinking Water, Urine, Hair and Feed Samples. Food Addit. Contam Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26(6), 814–820. DOI: 10.1080/02652030902729908.
- Zhang, Z.; Yan, H.; Cui, F.; Yun, H.; Chang, X.; Li, J.; Liu, X.; Yang, L.; Hu, Q. Analysis of Multiple β-Agonist and β-Blocker Residues in Porcine Muscle Using Improved QuEChERS Method and UHPLC-LTQ Orbitrap Mass Spectrometry. Food Anal. Methods. 2016, 9(4), 915–924. DOI: 10.1007/s12161-015-0238-z.
- Barbosa, J.; Cruz, C.; Martins, J.; Silva, J. M.; Neves, C.; Alves, C.; Ramos, F.; Da Silveira, M. I. N. Food Poisoning by Clenbuterol in Portugal. Food Addit. Contam. 2005, 22(6), 563–566. DOI: 10.1080/02652030500135102.
- Salpeter, S. R.; Buckley, N. S.; Ormiston, T. M.; Salpeter, E. E. Meta-Analysis: Effect of Long-Acting Beta-Agonists on Severe Asthma Exacerbations and Asthma-Related Deaths. Ann. Intern. Med. 2006, 144(12), 904–912.
- Union, The Council of the European. Council Directive 96/22/EC of 29 April 1996 Concerning the Prohibition on the Use in Stockfarming of Certain Substances Having a Hormonal or Thyrostatic Action and of ß-Agonists, and Repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC; Official Journal of the European Communities, Editor, 1996.
- Ministry of Agriculture of the People’s Republic of China. Agriculture Law of the People’s Republic of China 2002, 2002.
- Mastrianni, K. R.; Metavarayuth, K.; Brewer, W. E.; Wang, Q. Analysis of 10 Beta-Agonists in Pork Meat Using Automated Dispersive Pipette Extraction and LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1084, 64–68. DOI: 10.1016/j.jchromb.2018.03.026.
- Shao, B.; Jia, X.; Zhang, J.; Meng, J.; Wu, Y.; Duan, H.; Tu, X. Multi-Residual Analysis of 16 β-agonists in Pig Liver, Kidney and Muscle by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2009, 114(3), 1115–1121. DOI: 10.1016/j.foodchem.2008.10.063.
- Shelver, W. L.; Thorson, J. F.; Hammer, C. J.; Smith, D. J. Depletion of Urinary Zilpaterol Residues in Horses as Measured by ELISA and UPLC-MS/MS. J. Agric. Food Chem. 2010, 58(7), 4077–4083. DOI: 10.1021/jf904253t.
- Xu, Z.; Hu, Y.; Hu, Y.; Li, G. Investigation of Ractopamine Molecularly Imprinted Stir Bar Sorptive Extraction and Its Application for Trace Analysis of β2-agonists in Complex Samples. J. Chromatogr. A. 2010, 1217(22), 3612–3618. DOI: 10.1016/j.chroma.2010.03.046.
- Morales-Trejo, F.; León, S. V.-Y.; Escobar-Medina, A.; Gutiérrez-Tolentino, R. Application of High-Performance Liquid chromatography–UV Detection to Quantification of Clenbuterol in Bovine Liver Samples. J. Food Drug Anal. 2013, 21(4), 414–420. DOI: 10.1016/j.jfda.2013.09.009.
- Wang, G.; Zhao, J.; Peng, T.; Chen, D.; Xi, C.; Wang, X.; Zhang, J. Matrix Effects in the Determination of Beta-Receptor Agonists in Animal-Derived Foodstuffs by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry with Immunoaffinity Solid-Phase Extraction. J. Sep. Sci. 2013, 36(4), 796–802. DOI: 10.1002/jssc.201200661.
- Xiong, L.; Gao, Y.-Q.; Li, W.-H.; Yang, X.-L.; Shimo, S. P. Simple and Sensitive Monitoring of Beta2-Agonist Residues in Meat by Liquid Chromatography-Tandem Mass Spectrometry Using a QuEChERS with Preconcentration as the Sample Treatment. Meat Sci. 2015, 105, 96–107. DOI: 10.1016/j.meatsci.2015.03.013.
- Gallo, P.; Brambilla, G.; Neri, B.; Fiori, M.; Testa, C.; Serpe, L. Purification of Clenbuterol-Like β2-agonist Drugs of New Generation from Bovine Urine and Hair by α1-acid Glycoprotein Affinity Chromatography and Determination by Gas Chromatography–Mass Spectrometry. Anal. Chim. Acta. 2007, 587(1), 67–74. DOI: 10.1016/j.aca.2007.01.034.
- Daniele DC.; Veronica M.; Marco P.; Marco V. Simultaneous etermination of β2-agonists in Human Urine by Fast-Gas Chromatography/Mass Spectrometry: Method Validation and Clinical Application. Chromatogr: Biomed. 2009, 24, 358–366.
- Lu, H.; Zhang, H.; Zhu, T.; Xiao, Y.; Xie, S.; Gu, H.; Cui, M.; Luo, L. Metabolic Effects of Clenbuterol and Salbutamol on Pork Meat Studied Using Internal Extractive Electrospray Ionization Mass Spectrometry. Sci. Rep. 2017, 7(1), 5136. DOI: 10.1038/s41598-017-05496-6.
- Xu, J.; Xu, S.; Xiao, Y.; Chingin, K.; Lu, H.; Yan, R.; Chen, H. Quantitative Determination of Bulk Molecular Concentrations of β-Agonists in Pork Tissue Samples by Direct Internal Extractive Electrospray Ionization-Mass Spectrometry. Anal. Chem. 2017, 89(21), 11252–11258. DOI: 10.1021/acs.analchem.7b00517.
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of Beta-Agonists in Pig Feed, Pig Urine and Pig Liver Using Capillary Electrophoresis with Electrochemical Detection. Meat Sci. 2010, 85(2), 302–305. DOI: 10.1016/j.meatsci.2010.01.018.
- Pu, C.; Pu, C.; Yang, H.; Zhao, D.; Deng, A.-P. Development of a Polyclonal Indirect ELISA with Sub-Ng G−1 Sensitivity for the Analysis of Clenbuterol in Milk, Animal Feed, and Liver Samples and a Small Survey of Residues in Retail Animal Products AU - He, L. Food Addit. Contam. 2009, 26(8), 1153–1161. DOI: 10.1080/02652030902906142.
- Zhang, F.; Chen, F.; Yang, T. Development of a Sensitive Monoclonal Antibody-Based ELISA for the Detection of Clenbuterol in Animal Tissues AU - Ren, Xiangfu. Food Agric. Immunol. 2009, 20(4), 333–344. DOI: 10.1080/09540100903365852.
- Yuan, Y.; Zhao, Y.; Wu, K.; Yang, H.; Zhao, K.; Li, J.; Deng, A. A Sensitive and Group-Specific Monoclonal Antibody-Based Indirect Competitive ELISA for the Determination of Salbutamol in Swine Meat and Liver Samples. Anal. Methods. 2017, 9(39), 5806–5815. DOI: 10.1039/C7AY01628J.
- Garcia, P.; Paris, A.-C.; Gil, J.; Popot, M.-A.; Bonnaire, Y. Analysis of Beta-Agonists by HPLC/ESI-MS(n) in Horse Doping Control. Biomed. Chromatogr. 2011, 25(1–2), 147–154. DOI: 10.1002/bmc.1562.
- National Bureau of Statistics. China Statistical Yearbook 2018; China Statistics Press, 2018.
- GB/T 22286-2008, Determination of β-agonists Residues in Foodstuff of Animal origin-Liquid Chromatography with Tandem-Mass Spectrometric Method; Standards Press of China: Beijing, China, 2008.
- Guidelines for the Design and Implementation of National Regulatory Food Safety Assurance Programmes Associated with the Use of Veterinary Drugs in Food Producing Animals, CAC/GL 71-2009(Rev.2-2014); Codex Alimentarius Commission(CAC); 2014.
- Kang, H.-S.; Lee, S.-B.; Shin, D.; Jeong, J.; Hong, J.-H.; Rhee, G.-S. Occurrence of Veterinary Drug Residues in Farmed Fishery Products in South Korea. Food Control. 2018, 85, 57–65. DOI: 10.1016/j.foodcont.2017.09.019.
- Blanca, J.; Muñoz, P.; Morgado, M.; Méndez, N.; Aranda, A.; Reuvers, T.; Hooghuis, H. Determination of Clenbuterol, Ractopamine and Zilpaterol in Liver and Urine by Liquid Chromatography Tandem Mass Spectrometry. Anal. Chim. Acta. 2005, 529(1), 199–205. DOI: 10.1016/j.aca.2004.09.061.
- Wang, L.; Zeng, Z.; Wang, X.; Yang, J.; Chen, Z.; & He, L. Multi-Residue Analysis of 9 β-agonists in Animal Muscles by LC-MS/MS Based on a New Polymer Cartridge for Sample Cleanup. Journal of separation science. 2013, 36(11), 1843-1852.
- Montes, A. N.; Granja, R. H. M. M.; Reche, K. V. G.; Giannotti, F. M.; de Souza J. K. G.; Ferrari, S. P. G.; Dos S. D.; Wanschel A. C. B. A.; Salerno, A. G. Laboratory Validation of an LC-MS/MS Method for the Detection of Ractopamine, Clenbuterol and Salbutamol in Bovine and Swine Muscle at Sub-Gkg(−1) Regulatory Limits. Food Addit. Contam. Part A Chem. Anal. Control Exposure Risk Assess. 2017, 34(5), 785–792.
- Li, T.; Cao, J.; Li, Z.; Wang, X.; He, P. Broad Screening and Identification of β-agonists in Feed and Animal Body Fluid and Tissues Using Ultra-High Performance Liquid Chromatography-Quadrupole-Orbitrap High Resolution Mass Spectrometry Combined with Spectra Library Search. Food Chem. 2016, 192, 188–196. DOI: 10.1016/j.foodchem.2015.06.104.
- Alvey, J. C.; Centner, T. J.; Stelzleni, A. M. Beta Agonists in Livestock Feed: Status, Health Concerns, and International Trade. J. Anim. Sci. 2014, 92(9), 4234–4240. DOI: 10.2527/jas.2014-7932.
- Feddern, V.; Aroeira, C. N.; Molognoni, L.; Gressler, V.; Daguer, H.; Dalla Costa, O. A.; Contreras Castillo C. J.; de Lima, G. J. M. M. Ractopamine Analysis in Pig Kidney, Liver and Lungs: A Validation of the Method Scope Extension Using QuEChERS as A Sample Preparation Step. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1091, 79–86. DOI: 10.1016/j.jchromb.2018.05.033.
- Ito, A.; Ohnuki, Y.; Suita, K.; Ishikawa, M.; Mototani, Y.; Shiozawa, K.; Kawamura, N.; Yagisawa, Y.; Nariyama, M.; Umeki, D.; et al. Role of Beta-Adrenergic Signaling in Masseter Muscle. Plos One. 2019, 14(4), 25. DOI: 10.1371/journal.pone.0215539.
- Zhao, Z.; Gu, X.; Li, J.; Li, J.; Xue, M.; Yang, X.; Gao, Y.; Qin, Y. Residue Distribution and Depletion of Ractopamine in Goat Tissues after Exposure to Growth-Promoting Dose. J. Anal. Toxicol. 2019, 43(2), 134–137. DOI: 10.1093/jat/bky067.
- Kearns, C. F.; McKeever, K. H. Clenbuterol and the Horse Revisited. Vet. J. 2009, 182(3), 384–391. DOI: 10.1016/j.tvjl.2008.08.021.
- U.S. Food and Drug Administration. Tolerances for Residues of New Animal Drugs in Food, 2003.
- UEFA. Clenbuterol Warning for Players; 2017. https://www.uefa.com/insideuefa/protecting-the-game/anti-doping/news/newsid=1617199.html.
- Barry, A. R.; Graham, M. M. Case Report and Review of Clenbuterol Cardiac Toxicity. J. Cardiol. Cases. 2013, 8(4), 131–133. DOI: 10.1016/j.jccase.2013.07.004.
- Woolum, J.; Mancuso, N.; Rutter, P. W.; Baum, R. A.; Akpunonu, P. Chomping at the Bit: A Descriptive Report on Pediatric Clenbuterol Ingestion. J. Pharm. Pract. (January 2019) DOI: 10.1177/0897190018823114.
- Luzzana, U.; Serrini, G.; Moretti, V. M.; Maggi, G. L.; Valfrè, F.; Polidori, P. Effect of Temperature and Diet Composition on Residue Depletion of Oxytetracycline in Cultured Channel Catfish. Analyst. 1994, 119(12), 2757–2759.