ABSTRACT
The aim of this study was to characterize Caramuri Pouteria elegans (A.DC.) Baehni, an exotic fruit of the Amazonian biome to highlight it is a new source nutritional potential. The nutritional (vitamin A, B, C, and E) and volatile composition of the fruits was determined by physical [mass, diameter (longitudinal and transverse), yield] and chemical characterization (pH, acidity, soluble solids, proximate and mineral compositions) using headspace solid-phase microextraction (HS-SPME) by gas chromatography-mass spectrometry (GCMS). The amount of vitamin C, potassium, and magnesium detected per 100 g of sample was 143.08 mg, 97.50 mg, and 14.75 mg, respectively. Volatile profile by HS-SPME revealed 87 peaks, however, only 26 peaks were noted due to the small peak area (<1.0). Arithmetic and Kovats retention indexes were calculated for compound(s) identification. The percentage of volatiles identification was 88.46%. The majority compound was α-Pinene with 21.77%. The study reveals that Caramuri is a great source of vitamins and minerals, especially vitamin C.
Introduction
Brazil has numerous native and exotic fruit species, which are either unexplored or underexploited, but now, are receiving attention as sources of various bioactive compounds.[Citation1] Amazon Biome has the highest biodiversity found on this planet, with some highly promising fruits which have immense nutritional potential and are abundant sources of vitamins, minerals, energy and dietary fiber.[Citation2]
Its flora biodiversity comprises innumerable fruit species with high biological value. The nutritional composition (vitamins, minerals, and volatile compounds) of these fruits is equal to or much higher than that of any commercial fruit. However, studies aimed at characterization of these fruits are inadequate to present their effective use in natura or in food formulations. Studies on this would, in turn, highlight the nutritional potential of the fruits and also aid in biome preservation besides enhancing the socio-economic aspect.
The richness of nutrients in Brazilian exotic fruits is one of the main factors that lead to the growing interest in the consumption of fruits and their products.[Citation3] Epidemiological studies have indicated that frequent ingestion of fruits may lower the effects of oxidative stress and hence, reduce the risk of various diseases.[Citation4] This protective effect is attributed to the presence of a variety of antioxidants, such as vitamin C, phenolics, and carotenoids, which are quantifiable by various methods that evaluate the antioxidant capacity of fruits.[Citation5] Plants synthesize cellulose, proteins, carbohydrates, and several other important substances through primary metabolism to perform main vital functions. In addition to this, they also carry out secondary metabolism, to produce low molecular weight substances that are responsible for various functions that are not always well defined but also, not less important.[Citation6] Determination of these compounds can be carried out by the solid-phase microextraction (HS-SPME) technique that integrates sampling, extraction, and concentration in a single step. The technique is practically solvent free and uses a small fused silica fiber, covered with a polymeric film, adapted in a device similar to a syringe.[Citation7] Amongst the numerous fruit species in the Amazon, fruits of the Sapotaceae family deserve special mention, especially Caramuri Pouteria elegans (A.DC.) Baehni, which are tasty and are consumed in natura or in the form of liqueurs, candies, and juices. Flowering and fruiting of Caramuri occur between the months of February and October, that is approximately 9 months until the optimum harvest point.[Citation8,Citation9]
The physical, chemical and nutritional characterization of the native fruit species aids in their use as a new raw material in the food industry. They act as a source of aromatic compounds and nutrients with high biological value, which are expressed by the high levels of vitamins, minerals, and volatiles.[Citation6] In view of the above, the present work was aimed at the chemical, nutritional, and volatile characterization of the Caramuri Pouteria elegans (A.DC.) Baehni fruit, in the hypothesis that with the information acquired it can be considered as a new option of source of nutrients for the diet.
Materials and methods
Plant material and evaluation of fruit
Ripe fruits were harvested between the period September 2018 to February 2019, from INPA- National Institute for Research in the Amazon, Campus in Manaus, Brazil (latitude 3º092ʹ820”S, 59º994ʹ046”O Longitude). Fruits were harvested at random in the morning and were divided into five equal batches which represented replicates. The fruits were frozen in liquid nitrogen and stored in a freezer −80ºC till further use.
Physical and chemical characterization
After harvesting, 40 fruits from each development stage were immediately characterized on the basis of their fresh weight (g) and diameter (longitudinal, LD; transverse, TD (cm)) by using a semi-analytical balance (Mettler PC 2000 – Sao Paulo, Brazil)) and a digital caliper (Leetools 150 mm). Colour of the peel (L *, a *, b *) was determined at different points using a color measuring spectrophotometer (HunterLab ColorQUEST II Sphere) – Sao Paulo, Brazil.
Total soluble solids (TSS) of the pulp were measured with a digital refractometer ‘Palette’ PR-100 (ATAGO U.S.A., Inc.), with an automatic temperature compensation at 25°C (Association of official agricultural chemists[Citation10] and the results were expressed as °Brix. Titratable acidity was expressed as 100 g−1 pulp in natura, considering citric acid as the predominant acid. The pH of the pulp was measured with a pH meter, Tec-3P-MP (TECNAL – Sao Paulo, Brazil) according to the methods described Association of official agricultural chemists.[Citation10] All the experiments were repeated 6 times independently.
Amount/content of humidity, ether extracts, proteins, fibers, and ashes was determined by following the methods described by the Association of official agricultural chemists.[Citation10] Similarly, Vitamin A, C, and E content was measured by high-performance liquid chromatography (HPLC) following the methodology described by the Association of official agricultural chemists.[Citation10]
The mineral composition of the samples was evaluated using nitric acid digestion. To 0.5 g of the sample taken in a digestion tube, 6 mL of HNO3 was added and the tube was kept in a digester block. The temperature was gradually increased until it reached 210ºC with the extract turning colorless. For the determination of calcium, copper, iron, sodium, potassium, zinc, manganese, and magnesium, an atomic absorption spectrophotometer, SpectrAA 220 FS (Varian, 2000 – Kyoto, Japan) was used. Specific features such as wavelength, slit, and mixing of gases were calibrated separately for each element. To construct the calibration curve, ready-to-use calibration standards (AAS standards, Merck) were procured and diluted with deionized water. All the experiments were performed in triplicates.[Citation11]
Volatile compounds
Volatile compounds were extracted by the HS-SPME technique. Pulp (sample, 1 g) was transferred to a 10 mL glass vial (suitable for volatile retention) which was continuously stirred at 50°C for 15 min. The 50/30 μm DVB/CAR/PDMS fiber (divinylbenzene/carboxen/polydimethylsiloxane) was used to separate the volatile compounds present in the sample. The fiber was packed at a temperature of 270°C for 1 h prior to use. The pre-conditioning time for the analytes was 25 min. The fiber was exposed to the headspace of glass vial containing the sample at 50°C for 15 min, the syringe was immediately taken to the CG-MS injector, wherein, the volatile compounds were desorbed at 250°C for 2 min resulting in a splitless injection.
A spectrometer, CGMS-2010 Plus (Shimadzu) Tokyo, Japan with a mass selective detector model QP2010 Plus was used to detect the volatile compounds. A capillary column of fused silica (30 m × 0.25 mm and 0.25 μm thick) with 5% of diphenyl-, 95% polydimethylsiloxane polymer (DB5) acting as a stationary phase. For best separation, temperature gradient was established in the column initiating from 60ºC, with an increase of 3ºC per min until the maximum temperature of 270ºC was attained. The carrier gas was helium and the flow rate was adjusted to 1.8 mL min−1 for splitless injection with initial pressure of 100 KPa in the column. The conditions adjusted in the mass spectrometer (MS) were: mass selective detector operating by electronic impact and impact energy of 70 eV; scanning speed of 1000 m/z s−1; scan interval of 0.5 fragments/sec and filter for mass of the detected fragments being 29 Da and 600 Da. Each component was identified by comparing its mass spectra with already existing information present in the spectrometer databases (Willey229.lib and FFSC1.3.Lib) and the component identification book by Adams.[Citation12] For comparing and calculating the indices, standards of the saturated alkanes (C8-C20) (Sigma-Aldrich) were used, and confirmed in many compounds by their relative retention indices. Only peaks with percentage of area above ‘1ʹ were considered.
Statistical analysis
Significant differences between means were determined using Tukey’s honestly significant difference test (HSD), at 5% probability level (p < .05).[Citation13]
Results and discussion
Physical characteristics of the fruits in terms of mass (g) and longitudinal and transverse were observed to be 21.69 g, and 16.08 × 9.71 mm, respectively. Additionally, a significant yield percentage 44.08%, was noted (). With respect to the color of the fruit, yellow color aspects for peel and pulp, elucidated from the data for the coordinates L * a * and b * were 69.82 and 63.33, 11.81 and 75.71, 2.28 and 23.45, respectively. Physical characteristics of the fruit are important as these provide information related to color, size, pulp yield, and various other important variables which can decide its fate in the food industry. According to Costa et al,[Citation14] external quality is also very important as it is directly related to the quality attributes of the agricultural products. In general terms, ‘external quality of fruits and vegetables’ relates to the color, texture, size, shape, yield, absence of defects, amongst others.
For nutritional characterization, three fruit fractions (peel, pulp, and seed) were considered (). It was observed that seeds presented better results than the peel and pulp fractions in terms of ash, lipid and protein data/contents. However, the data of ºBrix (14.01) and vitamins from the fruit pulp showed significant values of vitamin C 143.07 mg/100 g. These values were much higher than those found in already known and marketed fruits like lemon, banana, and orange among many others. While comparing Caramuri’s vitamin C data with other exotic tropical fruits of the Brazilian flora, we noticed similar values with Murici (148 mg/100 g).[Citation3] However, vitamin C content in Caramuri is higher than that in açaí, cajá, jambolão, puçá coroa-de-frade, puçá preto, umbu, and uvaia, where the content is 84.0 mg/100 g, 26.5 mg/100 g, 112.0 mg/100 g, 41.1 mg/100 g, 28.9 mg/100 g, 18.4 mg/100 g, and 39.4 mg/100 g, respectively, of the fresh fruit matter.[Citation3]
Table 1. Nutritional values of caramuri fruit (Pouteria elegans (A.DC.) Baehni) in the peel, pulp, and seed fractions
On evaluating the mineral content in the fruit (peel, pulp, and seed) (), the highest values for potassium and magnesium per 100 g of sample were recorded in the peel (151.22 mg and 21.37 mg, respectively) followed by the pulp (97.50 mg and 14.75 mg, respectively). In the seed sample (100 g), minerals with high proportions were potassium (59.91 mg), manganese (26.81 mg), and iron (21.06 mg). Silva et al[Citation6] studied the availability of minerals in a Brazilian native species and observed low contents for all the minerals analyzed. Concentration of nutrients/minerals in the fruit is closely linked to the availability of those nutrients in the soil and this may have a direct effect on the protein content as they constitute amino acids.[Citation15] In human body, minerals perform several essential functions like regulating enzyme activities, maintaining osmotic pressure, facilitating nutrient transfer, constituting extracellular body tissues e.g. bones and teeth, assisting in growth and immune function, and cognitive organ development.[Citation16]
Table 2. Mineral composition of caramuri fruit (peel, pulp, and seed)
The volatile profile of Caramuri illustrated in (as described in ), wherein, 26 compounds detected by gas chromatography are presented. The percentage of volatiles identification was 88.46%, additionally, three peaks (11.53%) were not identified on the basis of retention time, mass spectra, arithmetic and Kovats retention index.
Table 3. Volatile compounds of caramuri (Pouteria elegans (A.DC.) Baehni) obtained by HS-SPME
Figure 2. Caramuri fruit (Pouteria elegans (A.DC.) Baehni) chromatograms by volatile compounds obtained by HS-SPME
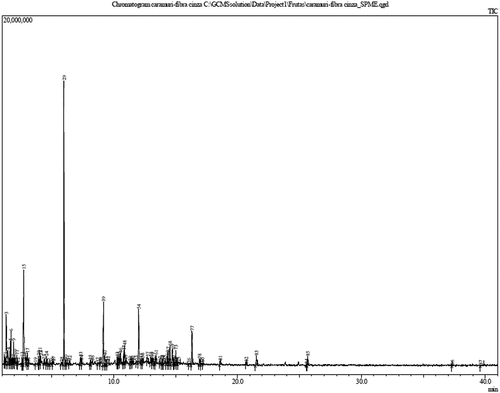
Compounds that were detected and identified are as follows: ketones (ethyl acetate, hexanedione), aldehyde (hexanal, 2-Methylbutanal, and pentanal), alkane (nonane, tridecane, and dodecane), isopropylbutanoate (ester), monoterpenes (pinene, carene, limomene, citronellene, gurjunene, funebrene, linalool and ocimene). It was observed that pinene had the highest percentage of peak area (21.77%), followed by isopentenyl formate (9.79%), limonene (6.57%), and undecane (5.72%). Terpenes specially the α-pinene can show functionally act as antioxidants and help in protecting lipids, blood, and other bodily fluids from the free radical attack by reactive oxygen species, hydroxyl groups, peroxides, and superperoxide radicals (Bendich & Olson, 1989). The majority volatile composition of the aforementioned compound further underscores the benefits arising from fruit consumption. According to Belitz,[Citation17] aldehydes, along with alcohols, esters, alkanes, and monoterpenes, can be synthesized by the degradation of unsaturated fatty acids. The synthesis occurs during fruit metabolism, mainly in the ripening phase, where breakdown of molecules such as, sugars and lipids takes place. Hence, the synthesis of secondary compounds such as volatile compounds occurs. These compounds are released from plant tissues as a part of the plant’s defense system, which can directly repel the microorganisms and animals or help in attracting the plant’s natural predators like herbivores.[Citation18] Other poorly established functions for most of the isoprenics derivatives involve thermal protection effects, protection from oxidative damage, photorespiration at high temperatures and low concentrations of O2, allelopathy, and photoprotection. Generally, volatile compounds are released from plant tissues only after cell disruption, following which the previously compartmentalized enzymes and their substrates, come in contact with each other.[Citation19]
Several quality parameters can be directly associated with the acceptance of a fruit. Amongst them, volatile compounds, besides playing an important role in attracting dispersers and perpetuators of their species in the wild, are also the determinants for the consumer’s sensorial attraction/acceptance. A large amount of chemical groups comprise these volatile compounds, e.g. monoterpenes, sesquiterpenes, aldehydes, esters, alcohols, fatty acids, hydrocarbons, and Shikimate derivatives.[Citation20,Citation21] The aroma determines the quality of the product and its market price.[Citation22] In the present work, it is important to note the importance of the knowledge of the biochemical routes of generation of the volatile compounds, which are largely responsible for the aroma. According to Tieman et. al.[Citation23] the volatile organic compounds are synthesized from several precursors, including amino acids, lipids, and carotenoids; however, while some of the synthetic routes are known, for most volatile compounds these metabolic steps remain unknown.
Conclusion
Caramuri is a good source of vitamins with bioactive functions, especially Vitamin C and minerals, highlighting the potassium that has a primordial function in cell metabolism. Among the analyzed portions the pulp stands out as a potential regulator of metabolic disturbances. Corroborating with the data obtained, the volatile profile was composed mainly of monoterpene constituents, especially alpha-pinene, which also has antioxidant and antimicrobial functions. Therefore, the fruit becomes an alternative for the enrichment of diets.
Acknowledgments
The authors thank the CAPES and the Amazon Research Foundation (FAPEAM) for financial support, Process 062.01725/2014 - PAPAC and 062.00682/2015 Universal.
Additional information
Funding
References
- Bailão, E. F. L. C.; Devilla, I. A.; Conceição, E. C. D.; Borges, L. L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16(10), 23760–23783. DOI: 10.3390/ijms161023760.
- Aguiar, J. P. L.; Yuyama, K.; Yuyama, L. K. Frutas Da Amazônia E Potencialidades Nutricionais. Seção 10 Frutas E Potencialidades. In Tratado De Alimentação, Nutrição & Dietoterapia; Editora Payá: São Paulo, 2016; pp 1163–1175.
- Rufino, M. D. S. M.; Alves, R. E.; de Brito, E. S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. DOI: 10.1016/j.foodchem.2010.01.037.
- Crowe, F. L.; Roddam, A. W.; Key, T. J.; Appleby, P. N.; Overvad, K.; Jakobsen, M. U.; Tjønneland, A.; Hansen, L.; Boeing, H.; Weikert, C.; et al. Fruit and Vegetable Intake and Mortality from Ischaemic Heart Disease: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) - Heart Study. Eur. Heart J. 2011, 32(10), 1235–1243. DOI: 10.1093/eurheartj/ehq465.
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A. A. Nutrition and Oxidative Stress: A Systematic Review of Human Studies. Int. J. Food Sci. Nutr. 2012, 64(3), 312–326. DOI: 10.3109/09637486.2012.738651.
- Silva, E. P.; de Abreu, W. C.; Gonçalves, O. A.; Damiani, C.; Vilas Boas, E. V. D. B. Characterization of Chemical and Mineral Composition of Marolo (annona Crassiflora Mart) during Physiological Development. Food Sci. Technol (Campinas). 2017, 37(1), 13–18. DOI: 10.1590/1678-457x.0107.
- Theodoridis, G.; Koster, E. H. M.; de Jong, G. J. Solid-phase Microextraction for the Analysis of Biological Samples. J. Chromatogr. B. 2000, 745(1), 49–82. DOI: 10.1016/S0378-4347(00)00203-6.
- Cavalcante, P. B.;. Frutas Comestíveis Da Amazônia; Companhia Souza Cruz indústria e comércio: Belém, 1988; pp 279.
- Yuyama, L. K. O.; Yuyama, K.; Aguiar, J. P. L.; Alencar, F. H.; Nagahama, D.; Marinho, H. A. Fruteiras Da Amazônia: Potencial Nutricional; Editora INPA: Manaus, 2013; pp 106.
- Association of Official Agricultural Chemists – AOAC. Official Methods of the Association of the Agricultural Chemists; AOAC: Washington, DC, USA, 2010.
- Instituto Adolfo Lutz. IAL. Normas Analíticas do Instituto Adolfo Lutz. Análise deágua e alimento, 5 ed.; IAL: Sao Paulo, 2008; Vol. 1, pp 533.
- Adams, R. P.;. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry; Allured Publishing Corporation: Carol Stream, USA, 2007; pp 804.
- Ferreira, D. F. Sisvar: Sistema de análise de variância. Versão 5.3; Ufla: Lavras, 2010.
- Costa, C.; Antonucci, F.; Pallottino, F.; Aguzzi, J.; Sun, D. W.; Menesatti, P. Shape Analysis of Agricultural Products: A Review of Recent Research Advances and Potential Application to Computer Vision. Food Bioprocess. Technol. 2011, 4(5), 673–692. DOI: 10.1007/s11947-011-0556-0.
- Spindula, M. C.; Campanharo, M.; Rocha, V. S.; Monnerat, P. H.; Favarato, L. F. Composição mineral de grãos de trigo submetidos a doses de sulfato de amônio e trinexapac-etil. Pesquisa Agropecuaria Tropical, Goiânia. 2010, 40(4), 513–520. DOI: 10.1590/S1983-40632010000400014.
- Roesler, R.; Catharino, R. R.; Malta, L. G.; Eberlin, M. N.; Pastore, G. Antioxidant Activity of Caryocar Brasiliense (pequi) and Characterization of Components by Electrospray Ionization Mass Spectrometry. Food Chem. 2008, 110(3), 711–717. DOI: 10.1016/j.foodchem.2008.02.048.
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Aroma Compounds. In Food Chemistry; Springer; Ed.; Springer-Verlag Berlin Heidelberg: Berlin, 2009; pp 340–402.
- Dudareva, N.; Negre, F. Pratical Applications of Research into the Regulation of Plant Volatile Emission. Curr. Opin. Plant Biol. 2005, 8, 113–118. DOI: 10.1016/j.pbi.2004.11.007.
- Baldwin, E. A.;. Fruit Flavor, Volatile Metabolism and Consumer Perceptions. In Fruit Quality and Its Biological Basis; Knee, M., Ed.; CRC Press LLC: Boca Raton, FL, 2002; pp 89–106.
- Lalel, H. J. D.; Singh, Z.; Tan, S. C. Glycosidically-bound Aroma Volatile Compounds in the Skin and Pulp of ‘kensington Pride’ Mango Fruit at Different Stages of Maturity. Postharvest. Biol. Technol. 2003, 29(2), 205–218. DOI: 10.1016/S0925-5214(02)00250-8.
- Munafo, J. P., Jr.; Didzbalis, J.; Schnell, R. J.; Schieberle, P.; Steinhaus, M. Characterization of the Major Aroma-active Compounds in Mango (mangifera Indica L.) Cultivars Haden, White Alfonso, Praya Sowoy, Royal Special, and Malindi by Application of a Comparative Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2014, 62(20), 4544–4551. DOI: 10.1021/jf5008743.
- Augusto, F.; Lopes, A. L.; Zini, C. A. Sampling and Sample Preparation for Analysis of Aromas and Fragrances. Trends Anal. Chem. 2003, 22, 160‐168. DOI: 10.1016/S0165-9936(03)00304-2.
- Tieman, D. M.; Zeigler, M.; Schmelz, E. A.; Taylor, M. G.; Bliss, P.; Kirst, M.; Klee, H. J. Identification of Loci Affecting Flavour Volatile Emissions in Tomato Fruits. J. Exp. Bot. 2006, 57, 887–896. DOI: 10.1093/jxb/erj074.

