 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study evaluated bioactive components and antioxidant properties of malted millet, soy residue “okara” and wheat flour. The flour extracts were screened for phenolic content, flavonoid content and total antioxidant capacity using standard methods. The antioxidant activities of the studied flours to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, reduce iron (111) chloride and inhibit lipid peroxidation and nitric oxide (NO) were determined spectrophotometrically and the effective concentration (IC50) of the extract required to inhibit DPPH, lipid peroxidation and nitric oxide radical formation by 50% was obtained using linear regression analysis. Results indicated that okara flour had a significantly (p < .05) highest flavonoid content following this trend: Okara flour (48.42mg/100g) > malted millet flour (34.73mg/100g) > wheat flour (19.02mg/100g). Malted millet flour showed significantly (p < .05) highest phenol content (38.36mg/100g) >okara flour (26.26mg/100g)> wheat flour (21.17mg/100g). In all these assays studied, the reference antioxidant (ascorbic acid) exhibited lowest IC50 values, while among the flour extracts, wheat flour displayed significantly (p < .05) lowest IC50 value in DPPH and nitric oxide radical assays. The lipid peroxidation assay showed that malted millet exhibited significantly (p < .05) lowest IC50 value (50.19µg/ml) indicating that malted millet demonstrated highest anti-lipid peroxidative functions. The methanolic extract of the studied flours exhibited potent antioxidant activities that increased with increasing amount of extract concentration and this validates their potential use in functional food application.
Introduction
Plant food materials possess bioactive compound such as carotenoids, phenolics flavonoids, and anthocyanins that exhibited their antioxidant properties through scavenging of free radicals.[Citation1,Citation2] These bioactive compounds are essential to human health because they exhibit several physiological activities such as antioxidant, anticarcinogenic and antimicrobial properties. Natural antioxidants such as flavonoids, tocopherols, and phenolic acids may inhibit lipid peroxidation in food and provide protection against oxidative damage from free radicals.[Citation3] Antioxidants are substances which protect cells against the damaging effects of reactive oxygen species (ROS) otherwise called, free radicals.[Citation4] Free radicals are highly reactive chemical substances such as superoxide, hydroxyl radical, singlet oxygen, etc., that are capable of attacking healthy cells of the body thereby causing cellular damage on protein, nucleic acid, and lipid.[Citation5] Epidemiological studies have established the mode of action of antioxidants to include scavenging of reactive oxygen (ROS) and nitrogen species (RNS), inhibition of the formation of ROS and RNS as well as the removal or repair of ROS and RNS induced damage, etc.[Citation6] Antioxidant compounds like phenolic acids, polyphenols, and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases.[Citation7] It has been scientifically accepted that the reactive oxygen species and free radicals generated during cellular metabolism or peroxidation of lipids and other biological molecules play important roles in the pathogenesis of chronic diseases such as coronary heart disease and cancer. Studies have shown that dietary antioxidants play an inhibitory role of combating the reactive oxygen species and free radicals thereby reducing the risk of these chronic diseases. Millets (Pennisetum glaucum) are small-seeded grains with different varieties belonging to the Poaceae (Gramineae) family and one of the most drought-resistant grains grown in over 40 countries predominantly in Africa and Asia as a staple food grain and source of feed and fodder, fuel and construction material.[Citation8,Citation9] There are four major types of millet which include Pearl millet (Pennisetum glaucum), Foxtail millet (Setaria italica), Proso millet or white millet (Panicum miliaceum), and Finger Millet (Eleusine coracana).[Citation10] According to[Citation11], the current global production statistics was 32 million tonnes, with India being the top producing countries, followed by Nigeria. This made it the sixth most important agricultural cereal crop annually cultivated as rain fed crop in arid and semi-arid areas of Africa and Indian sub-continent.[Citation12] Pearl millet (Pennisetum glaucum) produces the largest seeds and it is the variety most commonly used for human consumption[Citation13] It is an excellent antioxidant source, which can scavenge the free radicals and have reducing capabilities.[Citation14,Citation15] It is consumed as staple food in many homes, especially among the low-income populace predominantly in Northern Nigeria and some parts of West Africa countries such as Niger, Mali, and Burkina Faso.[Citation9] Nutritionally, millet is a rich energy source, fiber, B-vitamin and some micronutrients such as potassium, phosphorus, copper, magnesium, zinc, iron and manganese.[Citation16] Additionally, it contains several physiological functional components such as phytochemicals, which include dietary fiber and polyphenol compounds and antioxidants required for human health.[Citation17,Citation18] Extensive researches have shown that polyphenol compounds possess certain health-promoting properties such as prevention and reducing the incidence of degenerative diseases which, include cancer and cardiovascular diseases.[Citation19] Reports have also shown that there are several potential health benefits such as prevention of cancer and cardiovascular diseases, reduction of tumor incidence, lowering of blood pressure, etc., are found with consumption of millets and has contributed to its use in nutraceutical and functional food applications.[Citation8,Citation20,Citation21] However, the presence of various antinutrients such as phytic acids, tannin, poor digestibility of the protein and carbohydrates had been reported to have greatly limited its industrial food uses. Malting of millet has been reported to increase protein digestibility, solubility of starch and in vitro and bio accessibility of minerals such as calcium, iron, and zinc with significant reduction in phytic acid contents [Citation22,Citation23,Citation24] In these recent times, food processing by-products has been given considerable attention as a result of their functionality and potential as functional ingredients. It has been documented that these food processing wastes contain some physiological compounds such as dietary fiber, proteins, polysaccharides, antioxidants, phytochemicals that promote physiological health effects.[Citation25] Okara or soy residue is a low-value by product of soybean (Glycine max) obtained from tofu or soymilk processing, representing about 55% of total soybean. Soybean (Glycine max) has also been documented to possess certain physiological effects including cholesterol-lowering effect, anticarcinogenic effect, which was basically attributed to the antioxidant activity of the isoflavones.[Citation26] Okara has a high nutritive value due to its high quality protein, fat, carbohydrates, and dietary fiber. It contains isoflavones a class of phytoestrogens phytochemical, with high antioxidant and estrogenic activity that are also found in whole soybeans and other soy products.[Citation26,Citation27] Okara was considered as having little market value and was used as animal feed or as agro waste but very rich in dietary fiber and soy protein, which could be incorporated in functional food application due to its antioxidant potentials, hence this study.
Wheat (Triticum aestivum L) is the most extensively grown cereal crop in the world, covering about 237 million hectares annually, and accounting for a total of 420 million tonnes.[Citation28] Wheat flour contains essential nutrients such as carbohydrate, minerals, fats, and proteins. It is an important crop of choice used basically in the production of bread and baked products such as cakes, biscuits and other confectioneries. It has very high levels of a class of proteins known as gluten (8 – 14%). To this end, this study is aimed at determining the bioactive components and antioxidant properties of soy residue (okara) and malted millet as well as wheat flour for possible utilization in functional food applications.
Materials and methods
Chemicals
All organic solvents and chemicals used in this study were of analytical grade from Sigma, Poole, UK and BDH Laboratory Supplies, UK. All other reagents were of analytical grade.
Source of raw materials
The Wheat Flour (Triticum aestivum) used was commercial baker’s grade wheat flour milled by Nigeria Flour Mills (Market brand), Soybean (Glycine max) TGX 15 variety, millet (Pennisetum glaucum) were purchased from a local market in Lagos.
Millet flour preparation
Malting of millet was carried out according to the procedure described by[Citation29] with necessary modifications. Millet grains (Pennisetum glaucum) were sorted, washed and steeped in tap water (1:3, w/v) for 24 h at room temperature. The water was changed after every 6-h interval to avoid fermentation. The steeped grains were spread on perforated trays lined with muslin cloth and germinated at 34 ± 2ºC for 24 h. It was moistened and allowed to sprout for 48 h. The sprouted grains were washed and then spread uniformly on a stainless steel tray and dried in a cabinet dryer (Mitchell Dryers Ltd. Denton Holmes Carlisle, CA2 SDU, England) maintained at 60°C for 5–6 h. The dried malts were cleaned to remove the vegetative parts (rootlets and shoots). The grains were thereafter milled in a disc attrition mill to obtain fine-malted flour which were packaged in an air tight polyethylene film for further analyses.
Preparation of soybean residue (okara) flour
The soybean seeds (Glycine max), TGX 15 variety were sorted, cleaned and washed by floatation to remove all the foreign materials, damaged grains and debris. The cleaned beans were blanched in hot water for 30 min at 100°C and dehulled. The dehulled cotyledons were washed with hot (100°C) water twice and wet milled using 5 l of water to 1 kg of beans. The slurry obtained was mixed and filtered through a muslin cloth to remove the milk and recover the pulpy residue called okara. The fresh pulpy okara was dried using cabinet dryer (Mitchell Dryers Ltd. Denton Holmes Carlisle, CA2 SDU, England) at a temperature of 60°C for 12 h. The dried okara was milled in a disc attrition mill to obtain the flour and sieved through 0.25 mm pore sized sieve. Okara flour was then packaged hermetically in polyethylene film for further analyses.
Preparation of sample extracts
The flour sample (15 g) was weighed into a beaker containing 200 ml of methanol, placed and shaken in a mechanical shaker for 24 h. After shaking, it was then filtered and evaporated to dryness under reduced pressure in a rotary evaporator. The concentrated extract was re-dissolved with methanol to a concentration of 10 mg/ml and stored in the refrigerator until analysis. All the analyses were carried out in triplicates. The extraction yield (%) was calculated as follows:
Antioxidant components assay
Estimation of total phenolic content
The amount of total phenol content was determined by Folin-Ciocalteau colorimetric method[Citation30] using gallic acid as a standard following the method as described by.[Citation31] A 0.5 ml sample of extract and 0.1 ml of Folin- Ciocalteu reagent (0.5 N) were mixed and incubated at room temperature for 15 min. After this, 2.5 ml sodium carbonate solution (7.5% w/v) was added and further incubated for 30 min at room temperature. The absorbance of the solution was measured on a UV-visible spectrophotometer (Milton Roy Spectronic 601, USA) at 760 nm. The concentration of total phenol was expressed as gallic acid equivalent (GAE) (mg/g of dry mass) which is a commonly used reference value.
Total flavonoid content estimation
Total soluble flavonoid of the extract was determined using aluminum chloride colorimetric method using ascorbic acid as standard.[Citation32] 1 ml of sample solution (100μg/ml) was mixed with 3 ml of methanol, 0.2 ml of 10% Aluminum chloride, 0.2 ml of 1 M potassium acetate and 5.6 ml of distilled water. The resulting mixture was incubated at room temperature for 30 min and the absorbance of the reaction mixture was measured on a UV-visible spectrophotometer (Milton Roy Spectronic 601, USA) at 415 nm. The calibration curve was prepared by preparing ascorbic acid solutions at various concentrations in methanol.
Total antioxidant capacity determination
The total antioxidant capacity of the extracts was determined using the method of Prieto et al.[Citation33] A sample of the extract (0.3 ml) was mixed with 3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in a boiling water bath at 95° C for 90 min. After the samples had cooled to room temperature, the absorbance of the aqueous solution of each was measured at 695 nm. The total antioxidant capacity was expressed as equivalent of ascorbic acid.
DPPH radical scavenging activity assay
The free-radical scavenging activity of the extract was determined spectrophotometrically. The ability of a compound to decolorize DPPH free radical signifies the radical scavenging activity of the tested compound. This assay was based on the scavenging of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical which was estimated according to the procedure described by [Citation34,Citation35] An aliquot of 0.5 ml of sample extract in ethanol (95%) at different concentrations (20, 40, 60, 80, 100μg/ml) was mixed with 2.0 ml of reagent solution (0.004 g of DPPH in 100 ml methanol). The control contained only DPPH solution in place of the sample while methanol was used as the blank. The mixture was vigorously shaken and left to stand at room temperature. After 30 min the decrease in absorbance of test mixture (due to quenching of DPPH free radicals) was read at 517 nm on a UV-visible spectrophotometer (Milton Roy Spectronic 601, USA). The scavenging effect was calculated using the expression:
where A0 is the absorption of the blank sample and A1 is the absorption of the extract
Ascorbic acid was used as standard.
Effective concentration (IC50) is defined as concentration of sample required to scavenge DPPH radical by 50%. This was obtained by linear regression analysis. The higher the antioxidant activity, the lower is the value of IC50.
Reducing power assay
The ability of methanolic extracts to reduce iron (III) to iron (II) was assessed by the method of.[Citation36] The dried extract (20–100 μg) in 1 ml of the corresponding solvent was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (K3Fe(CN)6; 10 g/l), and then the mixture was incubated at 50°C for 30 min. After incubation, 2.5 ml of TCA (100 g/l) was added and the mixture was centrifuged at 1650 rpm for 10 min. Finally, 2.5 ml of the supernatant solution were mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (1 g/l) and the absorbance was measured at 700 nm on a UV-visible spectrophotometer (Milton Roy Spectronic 601, USA). High absorbance indicates high reducing power.
In vitro lipid peroxidation assay
Lipid peroxidation was induced by Fe2+−ascorbate system in liver homogenate and estimated as thiobarbituric acid reacting substances (TBARS) by the method of [Citation37] as described by.[Citation35] Freshly excised rat liver was sliced and processed to obtain 10% homogenate in cold 150 mMKCl−Tris−HCl buffer. The reaction mixture contained liver homogenate, Tris−HCl buffer (20 mM pH 7.0), FeCl2 (2 mM), ascorbic acid (10 mM), and 0.5 ml plant extract (25 − 100 µg/ml) in a final volume of 1 ml. The reaction mixture was incubated at 37°C for 1 h. Lipid peroxidation was measured as malondialdehyde (MDA) equivalent using trichloroacetic acid (TCA), thiobarbituric acid (TBA) and HCl (TBA-TCA reagent: 0.375% w/v TBA; 15% w/v TCA and 0.25 N HCl). The incubated reaction mixture was mixed with 2 mL of TBA-TCA reagent and heated in a boiling water bath for 15 min. After cooling, the flocculent precipitate was removed by centrifugation at 10,000 g for 5 min. Finally, malondialdehyde concentration in the supernatant fraction was determined spectrophotometrically at 535 nm. The concentrations of extract that would cause 50% inhibition of the production of thiobarbituric acid reactive substances (IC50 values) were calculated. Ascorbic acid was used as standard. Inhibition of lipid peroxidation (%) by the extract was calculated using the formula
Where
C = absorbance value of the fully oxidized control.
E = absorbance in the presence of extract as (A535 + TBA) – (A535–TBA).
Nitric oxide scavenging activity assay
The compound sodium nitroprusside is known to decompose in aqueous solution at physiological pH (7.2) producing nitric ions (NO•). Under aerobic condition, NO• reacts with oxygen to produce stable products (nitrate and nitrite), which can be determined using Griess reagent. The absorbance of the chromophore that formed during diazotization of the nitrite with sulfanilamide and subsequent coupling with Naphthylethylene diamine dihydrochloride can be immediately read at 550 nm.
Four-milliliter sample of flour extract or standard solution of different concentrations (20, 40, 60, 80, 100 μg/ml) were taken in different test tubes and 1 ml of Sodium nitroprusside (5 Mm in phosphate buffered saline) solution was added into the test tubes. They were incubated for 2 h at 30°C to complete the reaction. A 2 ml sample was withdrawn from the mixture and mixed with 1.2 ml of Griess reagent (1% Sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride in 2% H3PO4). The absorbance of the chromophore formed during diazotization of nitrite with sulfanilamide and its subsequent coupling with napthylethylenediamine was measured at 550 nm on a UV-visible spectrophotometer (Milton Roy Spectronic 601, USA) as modified by.[Citation38] Ascorbic acid was used as standard and inhibition of nitric oxide radical was calculated using the expression: .
where A0 is the absorbance of the Control and A1 is the absorbance of the extract or standard.
Statistical analysis
Analyses were performed in triplicates. The results are presented as mean ± standard deviation and data were subjected to one-Way analysis of variance (ANOVA) using IBM SPSS Statistics Version 20. Means were separated using Duncan’s New Multiple Range Test and significance was accepted at p < .05 and IC50 values were calculated using linear regression analysis on MS Excel 2010.
Results and discussions
Antioxidants are known as reducing agents that terminate the chain reaction through removing free radical intermediates and inhibit excessive oxidation reactions.[Citation39] Antioxidants offer resistance against oxidative stress by scavenging the free radicals, inhibiting the lipid peroxidation and by many other mechanisms and thus prevent the disease initiation and progression.
Extraction yield
The extraction yields of the methanolic extracts of the flours were: wheat flour (30.4%), malted millet (29.73%) and okara flour (38.33%). The variations in yield from methanolic extracts of wheat, malted millet, and okara flour might be explained by the polarity of extracted component, solubility of endogenous compounds present in extracted materials, sample particle size and greater amount of extractable material present [Citation40,Citation41] The result obtained by wheat and malted millet flours was similar to the findings of [Citation42] who reported extraction yield of apricot varieties extracted with 70% methanol.
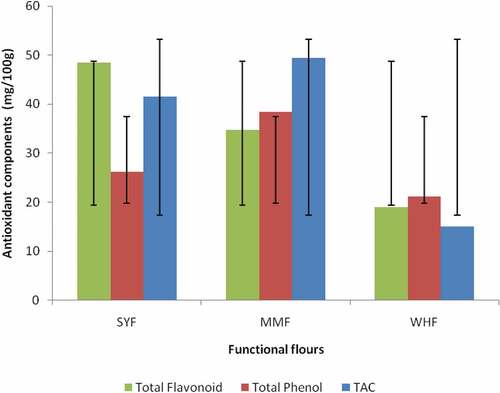
The antioxidant components of the functional flour are depicted in . Results showed that okara flour had a significantly (p < .05) highest flavonoid content (48.42mg/100g), followed by malted millet flour (34.73mg/100g), and wheat flour had the least value (19.02mg/100g). Malted millet flour showed a significantly (p < .05) highest (TPC) phenol content (38.36mg/100g) than malted millet flour (26.26mg/100g) and wheat flour had the least value (21.17mg/100g). The significant highest total phenol content of malted millet flour might probably be due to hydrolysis of condensed procyanidins that occurred during the malting process.[Citation43] The obtained result in this study is in line with the findings of.[Citation29] The total antioxidant capacities of plant food are from the cumulative capacity of all the food bioactive components like flavonoids, vitamin C, and other phenolic contents and their optimized interactions to scavenge free radicals.[Citation44] The mean total antioxidant capacity of okara, malted millet and wheat flours differ significantly (p < .05) in the order: malted millet flour>okara flour > wheat flour at 49.37 ± 0.47, 41.49 ± 0.94 and 15.12 ± 0.25 mg/100g, respectively (). This shows that malted millet flour contain significantly highest total antioxidant capacity than other flours.
Figure 1. Antioxidant components of functional flours
The antioxidant activities of phenolic compounds and flavonoids in biological systems have already been established based on their abilities to act as scavengers of singlet oxygen and free radicals.[Citation45] Polyphenols are the biggest group of phytochemicals that have been found in plant-based foods and have been linked to several health benefits. Flavonoids are antioxidants and free radical scavengers which prevent oxidative cell damage and have strong anticancer activity.[Citation46] Phenolic compounds have health benefits as they improve human health against diabetes, cardiovascular diseases, and associated diseases because of their high antioxidant properties.[Citation47] They are also radical scavengers with the ability to exhibit inhibitory effects on mutagenesis and carcinogenesis of various nuero-degenerative diseases and cancers in humans.[Citation48] In addition, they possess antioxidant, antidiabetic, antimicrobial and neuroprotective activities. This study presented okara as having highest flavonoid content than other flour samples and this might probably be attributed to its significant isoflavone concentration as reported by.[Citation49] Isoflavones are biologically active compounds occurring naturally in a variety of plants, with relatively high levels found in soybeans. This study is similar to the findings of[Citation44] who studied the antioxidant capacity of pumpkin or beet to be greater than those of onion, tomato and broccoli or carrot.
Antioxidant capacity of plant materials is determined by a mixture of different antioxidant assays having different mechanisms, synergistic interactions and bioavailability of different types of polyphenols present and above all, no single assay is conclusively applicable to all of them.[Citation19] Therefore, it is necessary to combine more than one method in order to determine in vitro the antioxidant properties of food materials. In this study, the antioxidant activities of the flours were measured through different assays: DPPH, reducing power, lipid peroxidation inhibitory and nitric oxide radical scavenging activity. The DPPH method is commonly used for determination of free radical scavenging activity of antioxidant.[Citation50] DPPH (1,1-diphenyl-2-picrylhydrazyl) is a very stable organic free radical and presents the ability of accepting an electron or hydrogen radical. The ability of a compound to decolorize DPPH free radical signifies the radical scavenging activity of the tested compound. The capacity of wheat, malted millet, and okara flour extracts to scavenge the stable DPPH radical is shown in . The standard which was gallic acid and the flour extracts differed significantly (p < .05) within each other. Wheat flour extract exhibited highest DPPH free radical scavenging activity (87.08%), followed by malted millet flour (83.50%) and least activity in okara flour (81.21%). The extracts showed a concentration dependent inhibition of the DPPH radicals and highest scavenging activity was observed in gallic acid. The percentage inhibition increases as the concentration increased from 20 to 100µg/ml. The greatest activity was obtained at a higher concentration of 100µg/ml in the flour extracts. This study is in agreement with the findings of [Citation50] who reported that radical scavenging activity increased with increasing percentage of the free radical inhibition. The results obtained indicated that the flour extracts are capable of scavenging the free radicals and prevent the initiation of free radicals by stabilizing them to participate in any deleterious reactions. A stronger radical quenching agent generally resulted in a lower IC50 value. In the same vein, the ability of methanolic extracts of the flours to reduce iron (III) to iron (II) is depicted in . Significant reduction (p < .05) was observed in the reducing power of the flour extracts when compared with the reference ascorbic acid. The reducing power of the flour extracts at 100µg/ml concentration ranged between 0.63% and 0.25% with maximum reducing power obtained in the reference antioxidant- ascorbic acid (0.63%), followed by okara and wheat flour extracts having 0.28% while malted millet flour extract recorded the minimum reducing power (0.25%). At the highest concentration (100µg/ml) of the sample extracts, SYF (0.28%) and WHF (0.28%) were not significantly different (p > .05) but differed significantly with MMF (0.25%). High absorbance indicated high reducing power. In this assay, the presence of reductant (i.e., antioxidants) causes the reduction of the Fe3+/ferricyanide complex to the ferrous form (Fe2+). The reducing power is associated with antioxidant activity and may serve as a significant reflection of the antioxidant property [Citation51], impyling that antioxidants are reducing agents. Obtained results confirmed earlier findings of [Citation2] on reducing power of pepper. Also,[Citation52] reported higher chelating activity on Fe2+ for wheat flours extracts than for buckwheat flours extracts. Some phenolic compounds such as flavonoids and phenolic acids have been reported to exhibit antioxidant activity through their reductive capacity in a Fe3+-Fe2+ system.[Citation53] It was observed that the results followed a trend similar to the DPPH radical scavenging activity of the flour extracts and was compared with known antioxidant ascorbic acid. All the flour extracts displayed a concentration-dependent effect from 20 to 100µg/ml, and maximum percentage inhibition recorded at 100µg/ml. The total antioxidant potential of a food can be determined by its capacity to prevent lipid peroxidation in an in vitro system. The lipid peroxidation inhibition of the flour extracts was shown in . The flour extracts significantly reduced the accumulation of lipid peroxides in a concentration-dependent manner. Ascorbic acid was used as a reference antioxidant and the percentage inhibition increases as the concentration increased from 20 to 100µg/ml. At 100µg/ml concentration, there was a significant difference (p < .05) in all the samples, though the reference antioxidant showed the highest inhibition (94.97%), followed by malted millet flour (92.82%), okara flour (86.71%) and the least value obtained in wheat flour (84.71%). This result agrees with the report of [Citation54] Oboh and Adefegha (2010) on the inhibition of Fe2+- induced lipid peroxidation by wheat biscuit. Lipid peroxidation is free radical processes involving a source of secondary free radical, which can further act as second messenger or can directly react with other biomolecule, thereby enhancing biochemical lesions. However, antioxidants are important inhibitors of lipid peroxidation not only as a defense mechanism of living cells against oxidative damage but also for food preservation.[Citation55] depicts the scavenging activity of nitric oxide radical by the flour extracts. The percentage inhibition increases as the concentration increased from 20 to 100µg/ml. Ascorbic acid was used as a reference antioxidant and showed 85.94% inhibition at 100µg/ml concentration. The flour extracts showed a concentration-dependent inhibition and maximum percentage inhibition recorded at 100µg/ml concentration. At the highest concentration of the sample extracts, the nitric oxide radical scavenging activity displayed the highest percentage inhibition (85.94%) by reference compound (ascorbic acid), which varied significantly (p < .05) with wheat flour (72.18%) and malted millet flour (71.61%). The least value was recorded by okara flour extract (68.44%). No significant difference (p > .05) was observed between wheat and malted millet flour extracts.
Table 1. Reducing power of okara, malted millet and wheat flour extracts which was estimated by potassium ferricyanide method
Figure 2. Scavenging of DPPH radical by the Methanolic extracts of okara, malted millet and wheat flour
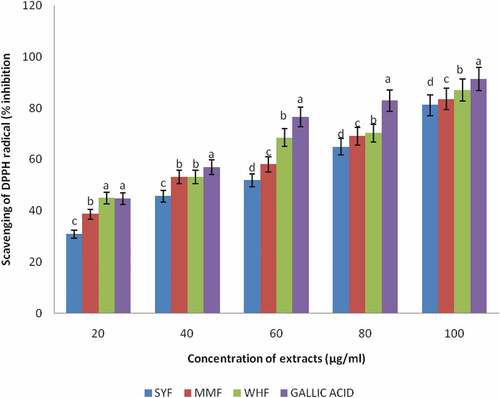
Figure 3. Scavenging of Lipid Peroxidation by Methanolic extracts of okara, malted millet and wheat flour
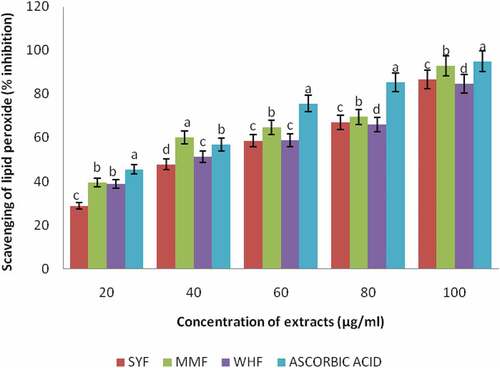
Figure 4. Scavenging of Nitric oxide by Methanolic extracts of selected okara, malted millet, and wheat flour
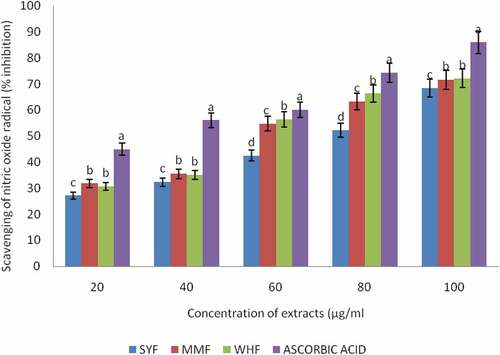
Nitric oxide was generated from sodium nitroprusside, which in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitric oxide. The result evidenced the inhibitory effect of flour extracts on nitric oxide radical generated from sodium nitroprusside at physiological pH, generated from sodium nitroprusside at physiological pH, which is an indication of its scavenging property. Incubation of solutions of sodium nitroprusside in phosphate buffer saline at 30°C C for 2 h resulted in nitrite production, which is reduced by the tested methanolic extracts of malted millet, wheat flour, and okara in this descending order. This probably might be attributed to the antioxidant activities in the extract, which compete with oxygen to react with nitric oxide thereby inhibiting the generation of nitrite.
The Effective concentration (IC50) of the flour extracts in different antioxidant assays is depicted in . IC50 is defined as the concentration of the extract required to inhibit DPPH, lipid peroxidation and Nitric oxide radical formation by 50%. It is obtained from the % inhibition versus concentration plot of the extract using linear regression analysis. It is a measure of the antioxidant activity of plant materials. The higher the antioxidant activity, the lower is the value of IC50. Significant difference (p < .05) existed in the IC50 obtained in DPPH assay of wheat flour extract and other flour extracts studied. The IC50 values showed that okara, malted millet and wheat flour extracts scavenged 50% DPPH radicals at the respective lowest sample concentrations in the following order: 58.27µg/ml, 54.31µg/ml and 51.26µg/ml while reference standard gallic was found to be 46.78µg/ml. In lipid peroxidation assay, the IC50 was found to be 54.90, 50.19 and 54.71 µg/ml for the extracts, respectively, and ascorbic acid which is the standard reference antioxidant as 45.75± µg/ml. Interestingly, the IC50 of malted millet flour extract was the lowest (50.19µg/ml) and differed significantly (p < .05) among the flour extracts indicating that malted millet demonstrated highest anti-lipid peroxidative functions. In nitric oxide (NO) radical assay, the IC50 values ranged from 71.40µg/ml, 62.60µg/ml and 61.35µg/ml while the reference standard ascorbic acid was 51.66µg/ml. On the overall, wheat flour displayed significant lowest IC50 values (51.26 and 61.35µg/ml) in DPPH and nitric oxide radical assays, respectively, while on the other hand malted millet flour extract exhibited significant lowest IC50 value (50.19µg/ml) in lipid peroxidation. The strong antioxidant activities of malted millet, okara and wheat flour could be attributed to their significant polyphenolic and flavonoid contents.
Table 2. IC50 (Effective concentration) of okara, malted millet and wheat flour extracts in different antioxidant assays
Conclusion
The results of the study showed that methanolic extract of malted millet, okara and wheat flours possess appreciable amount of bioactive compounds; total phenolic content and total flavonoid content but significantly higher in malted millet and okara flour. Antioxidant activity was measured by DPPH radical scavenging activity, reducing power, lipid peroxidation and nitric oxide radical inhibition. These exhibited potent total antioxidant activity increased with increasing amount of extract concentration compared favorably with the standard (Ascorbic acid) at different concentrations studied and could be attributed to their significant phenolic and flavonoid contents. The outcome of this study revealed that malted millet and okara flours can be considered as food source of natural bioactive compounds with antioxidant properties as was exhibited by their radical scavenging ability. Also, malted millet and okara flours with their antioxidant characteristics as quality criteria, could serve as good dietary source of natural antioxidants and may be considered potential functional ingredients and consequently would contribute to development of value-added functional food products. In addition, the antioxidant potentials displayed by okara flour could be used to explore its economic valorization.
Acknowledgments
The first author is grateful to members of staff of Baking and Milling division of Food Technology department FIIRO as well as Mr Adenekan of Biochemistry Department of Lagos State University Teaching Hospital for their assistance and support during the research work.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study does not involve any human or animal testing. The paper is a part of the PhD thesis of Ibidapo Olubunmi.
References
- Sun, J.; Chu, Y.; Wu, X.; Liu, R. H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. DOI: 10.1021/jf0207530.
- Oboh, G.; Rocha, J. B. T. Polyphenols in Red (Capsicum Annum Variety aviculare) and Their Protective Effect on Some Pro-oxidants Induced Lipid Peroxidation in Brain and Liver. Eur. Food Res. Technol. 2007, 225(2007), 239–247. DOI: 10.1007/s00217-006-0410-1.
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 1–10. Article ID 7616791. DOI: 10.1155/2017/7616791.
- Lim, Y. Y.; Lim, T. T.; Tee, J. J. Antioxidant Properties of Guava Fruit: Comparison with Some Local Fruits. Sunway Acad. J. 2006, 3, 9–20.
- Kumar, S.;. Free Radicals and Antioxidants: Human and Food System. Adv. Appl. Sci. Res. 2011, 2(1), 129–135.
- Lim, H. S.; Park, S. H.; Ghafoor, K.; Hwang, S. Y.; Park, J. Quality and Antioxidant Properties of Bread Containing Turmeric (Curcuma Longa L.) Cultivated in South Korea. Food Chem. 2011, 124(4), 1577–1582. DOI: 10.1016/j.foodchem.2010.08.016.
- Ibrahim, U. K.; Salleh, R. M.; Maqsood-ul-Haque, S. N. S. Bread Towards Functional Food: An Overview. Int. J. Food Eng. 2015. DOI: 10.18178/ijfe.1.1.39-43.
- Saleh, A. S. M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. DOI: 10.1111/1541-4337.12012.
- Maiti, C. K.; Sen, S.; Paul, A. K.; Acharya, K., FAO. Food and Agriculture Organization of the United Nations. Cultivation Area of Millets. FAO Area employed and cultivation Rome: FAO 2007. 2007. .
- Yang, X.; Wan, Z.; Perry, L.; Lu, H.; Wang, Q.; Zhao, C.; Li, J.; Xie, F.; Yu, J.; Cui, T. Early Millet Use in Northern China. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 3726–3730. DOI: 10.1073/pnas.1115430109.
- FAO. Food and Agriculture Organization. Economic and Social Department: The Statistical Division. Statistics Division 2012. 2012. http://faostat.fao.org/site/567/DesktopDefault. aspx? PageID=567.(accessed Sep 29, 2012).
- Food and Agriculture Organization of the United Nations. FAO. 2014. http://faostat.fao.org/default.aspx
- ICRISAT (International Crops Research Institute for the Semi-Arid Tropics). Annual Report. 2007. http://test1.icrisat.org/Publications/EBooksOnlinePublications/Annulreport2007.pdf
- Muthulisi, S.; Taylor, J. R. N.; de Milliano, W. A. J.; Duodu, K. G. Occurrence and Location of Tannins in Finger Millet Grain and Antioxidant Activity of Different Grain Types. Cereal Chem. 2007, 84(2), 169–174. DOI: 10.1094/CCHEM-84-2-0169.
- Odusola, K. B.; Ilesanmi, F. F.; Akinloye, O. A. Assessment of Nutritional Composition and Antioxidant Ability of Pearl Millet (Pennisetum glaucum). Am. J. Res. Commun. 2013, 1(6), 262–272.
- NIN- National Institute of Nutrition. Indian Foods Nutritional Value. 2003
- Devi, P. B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N. G.; Priyadarisini, V. B. Health Benefits of finger Millet (Eleusine Coracana L.) Polyphenols and Dietary fiber: A Review. J. Food Sci.Technol. 2011,DOI: 10.1007/s13197-011-0584-9
- Dykes, L. A.; Rooney, L. W. Sorghum and Millet Phenols and Antioxidants. J. Cereal Sci. 2006, 44, 236–251. DOI: 10.1016/j.jcs.2006.06.007.
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. DOI: 10.1080/1040869059096.
- Gupta, N.; Srivastava, A. K.; Pandey, V. N. Biodiversity and Nutraceutical Quality of Some Indian Millets. Proc. National Academy Sci. 2012, 82(2), 265–273.
- Truswell, A. S.;. Cereal Grain and Coronary Heart Disease. Eur. J. Clin. Nutr. 2002, 56(1), 1–4. DOI: 10.1038/sj.ejcn.1601283.
- Coulibaly, A.; Chen, J. Evaluation of Energetic Compounds, Antioxidant Capacity, Some Vitamins and Minerals, Phytase and Amylase Activity during Germination of Foxtail Millet. Am. J. Food Technol. 2011, 6(1), 40–51. DOI: 10.3923/ajft.2011.40.51.
- Krishnan, R.; Dharmaraj, U.; Malleshi, N. G. Influence of Decortication, Popping and Malting on Bioaccessibility of Calcium, Iron and Zinc in finger Millet. LWT Food Sci. Technol. 2012, 48, 169–174. DOI: 10.1016/j.lwt.2012.03.003.
- Ahmed, A. I.; Abdalla, A. A.; El Tinay, A. H. Effect of Traditional Processing on Chemical Composition and Mineral Content of Two Cultivars of Pearl Millet (Pennisetum glaucum). J. Appl. Sci. Res. 2009, 5(12), 2271–2276.
- Fărcaș Anca, C.; Socaci, S. A.; Tofană, M.; Mudura, E.; Salanță, L. The Content in Bioactive Compounds of Different Brewers’ Spent Grain Aqueous Extracts. Bull. UASVM Food Sci. Technol. 2016, 73(2), /2016 ISSN-L 2344-2344; Print ISSN 2344-2344; Electronic ISSN 2344-5300. DOI: 10.15835/buasvmcn-fst:12356.
- Rüfer, C. E.; Kulling, S. E. Antioxidant Activity of Isoflavones and Their Major Metabolites Using Different in Vitro Assays. J. Agric. Food Chem. 2006, 54(8), 2926–2931. DOI: 10.1021/jf053112o.
- Setchell, K. D.;. Phytoestrogens: The Biochemistry, Physiology, and Implications for Human Health of Soy Isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333–1346. DOI: 10.1093/ajcn/68.6.1333S.
- Olabanji, O. G.; Omeje, M. U.; Mohammed, I.; Ndahi, W. B.; Nkema, I. Wheat. In Cereal Crops of Nigeria: Principles of Production and Utilization, Xxii, 337; Idem, N.U.A. And F.A. Showemimo., Eds.; Ade Commercial Press, Zaria, Nigeria, 2007; pp 230–249.
- Adebiyi, J. A.; Obadina, A. O.; Adebo, O. A.; Kayitesi, E. Comparison of Nutritional Quality and Sensory Acceptability of Biscuits Obtained from Native, Fermented, and Malted Pearl Millet (Pennisetum glaucum) Flour. Food Chem. 2017, 232, 210–217. DOI: 10.1016/j.foodchem.2017.04.020.
- McDonald, S.; Prenzler, P. D.; Antolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001, 73, 73−84. DOI: 10.1016/S0308-8146(00)00288-0.
- Slinkard, K.; Singleton, V. L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49−55.
- Chang, C.; Yang, M.; Wen, H. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178−182.
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337−341. DOI: 10.1006/abio.1999.4019.
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential oil”. Phytother. Res. 2000, 14, 323−328. DOI: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q.
- Oyesola, O.; Oshodi, T.; Ogundele, O.; Micah, C.; Adenekan, S. Toxicity Screening and in Vitro Antioxidant Activities of Aqueous Extracts of Morinda Lucida and Saccharum Officinarum Leaves. Biokemistri. 2013, 25(2), 72–78.
- Yildrim, A.; Mavi, A.; Kara, A. A. Determination of Antioxidant and Antimicrobial Activities of Rumexcrispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. DOI: 10.1021/jf0103572.
- Buege, J.; Aust, D. S. Microsomal Lipid Peroxidation. In Methods in Enzymology; Fleisscher, S., Packer, L., Eds.; Academic Press: New York, 1978; Vol. 52, pp 302−311.
- Alisi, C. S.; Onyeze, G. O. C. Nitric Oxide Scavenging Ability of Ethyl Acetate Fraction of Methanolic Leaf Extracts of Chromolaenaodorata (Linn.). Afr. J. Biochem. Res. 2008, 2, 145−50.
- Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free- Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules. 2011, 16, 10104–10122. DOI: 10.3390/molecules161210104.
- Shabbir, G.; Anwar, F.; Sultana, B.; Khalid, Z. M.; Afzal, M.; Khan, M. Q.; Ashrafuzzaman, M. Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonixregia (Bojer Ex hook.) raf.]. Molecu. 2011, 16, 7302–7319. DOI: 10.3390/molecules16097302.
- Do, Q. D.; Angkawijaya, A. E.; Tran-Nguyen, P. L.; Huynh, L. H.; Soetaredjo, F. E.; Ismadiji, S.; Ju, Y. Effect of Extraction Solvent on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity of Limnophilaarmatica. J. Food Drug Ana. 2014, 22, 296–302. DOI: 10.1016/j.jfda.2013.11.001.
- Mujtaba, A.; Masud, T.; Ahmad, A.; Naqvi, S. M. S.; Qazalbash, M. A.; Levin, R. E. Effect of Solvents on Extraction Yield, Total Flavonoid, Total Phenolic Contents, DPPH Scavenging Activity and Antibacterial Potential of Three Apricot Cultivars. Transylvanian Rev. 2016, XXIV(10),1662–1676, Special Issue.
- Taylor, J. R. N.; Duodu, K. G. Effects of Processing Sorghum and Millets on Their Phenolic Phytochemicals and the Implications of This to the Health- Enhancing Properties of Sorghum and Millet Food and Beverage Products. J. Sci. Food Agric. 2015, 95, 225–227. DOI: 10.1002/jsfa.6713.
- Pellegrini, N.; Serafini, M.; Colombi, B.; DelRio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays. J. Nutr. 2003, 33, 2812–2819. DOI: 10.1093/jn/133.9.2812.
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. DOI: 10.1016/S1360-1385(97)01018-2.
- Okwu, D. E.;. Phytochemical and Vitamin Contents of Indigenous Species of South Eastern Nigeria. J. Sustainable Agric. Environ. 2004, 6, 30–34.
- Nicoletti, I.; Daniela, M. D.; De Rossi, A.; Taddei, F.; D’Egidio, M. G.; Corradini, D. Identification and Quantification of Soluble Free, Soluble Conjugated, and Insoluble Bound Phenolic Acids in Durum Wheat (Triticum Turgidum L Var durum) and Derived Products by RP-HPLC on a Semi Micro Separation Scale. J. Agric. Food Chem. 2013, 61, 11800–11807. DOI: 10.1021/jf403568c.
- Tanaka, M.; Kuei, C. W.; Nagashima, Y.; Taguchi, T. Application of Antioxidative Maillard Reaction Products from Histidine and Glucose to Sardine Products. Nippon Suisan Gakkaishi. 1998, 54, 1409–1414. DOI: 10.2331/suisan.54.1409.
- Vineet, K.; Rani, A.; Lulua, H. Investigations of Amino Acids Profile, Fatty Acids Composition, Isoflavones Content and Antioxidative Properties in Soy Okara. Asian J. Chem. 2016, 28(4), 903–906. DOI: 10.14233/ajchem.2016.19548.
- Bhaskar, R.; Rajeswari, V.; Sathish, K. T. In Vitro Antioxidant Studies in Leaves of Annona Species. Indian J. Exp. Biol. 2007, 4, 480−485.
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and Involvement of Aqueous Reducing Compounds in Oxidative Defense Systems of Various Senescing Leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. DOI: 10.1021/jf00055a012.
- Sedej, I. J.; Sakaa, M. B.; Misan, A. A.; Mandia, A. I. Antioxidant Activity of Wheat and Buckwheat Flours. Proc. Nat. Sci. Matica. 2010, 118, 59–68.
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of Extraction Solvent Mixtures on Antioxidant Activity Evaluation and Their Extraction Capacity and Selectivity for Free Phenolic Compounds in Barley (Hordeum Vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. DOI: DOI: 10.1021/jf061087w.
- Oboh, G.; Adefegha, S. A. Inhibitory Properties of Phenolic – Enriched Plantain- Wheat Biscuits on Fe2+ - Induced Lipid Peroxidation in Rat’s Brain - in Vitro. Adv. Food Sci. 2010, 32, 162–169.
- Vinesh, K.; Tyagi, D. Phytochemical Screening and Free-radical Scavenging Activity of Bergenia Stracheyi. J. Pharmacogn. Phytochem. 2013, 2(2), 175–180.
