ABSTRACT
Surfactin as a nonvolatile cyclic lipopeptide has attracted great attention over the past few decades. This work focused on identification, quantification, and tracing of surfactin in Moutai liquor by ultra-performance liquid chromatography coupled with triple quadrupole tandem mass spectrometer. Our results showed that surfactin was mainly produced in Daqu making stage and stacking fermentation stage, and it increased to 1421 ± 22 μg kg–1 in the liquor fermentation stage. In addition, five strains of Bacillus spp. (MT1-MT5) were isolated from Daqu to produce surfactin, and the highest surfactin production reached to 51 ± 1 mg L–1. Therefore, Bacillus spp. played an important role in the production of surfactin. This work detected surfactin in Chinese liquor, and analyzed the concentration during the liquor fermentation. This work would facilitate the research in nonvolatile compounds of distilled liquor and shed new light on flavor–omics in Chinese liquor.
Graphical Abstract
KEYWORDS:
Introduction
Chinese liquor is a traditional distilled alcoholic beverage with an approximate 2000-year history.[Citation1] Moutai liquor is the most famous Chinese liquor, likes whiskey in Scotland, and brandy in France.[Citation2,Citation3] Moutai liquor is primarily produced in Kweichow province in southwest China and plays a critical role in the Chinese liquor industry.[Citation4]
Chinese liquor has a unique flavor style with a full-bodied long-lasting aroma. Nonvolatile components play important roles in the perceived aroma and flavor. In order to study the characteristics of Chinese liquor, many works focused on detecting the nonvolatile compounds. For instance, a total of 268 nonvolatile compounds were detected in Chinese liquor by gas chromatography time-of-flight mass spectrometry (GC-TOFMS), including organic acids, alcohols, sugars, and benzene derivatives.[Citation5] However, peptides are rarely reported from Chinese liquor because they are often present in very low concentrations in this complex matrix. Currently, tripeptide and tetrapeptide were identified from sesame flavor-type liquor.[Citation6] In addition, lichenysin was also identified in strong-aroma type liquor,[Citation7] which could reduce the headspace concentration of off–flavors and selectively affect the volatility of aroma.[Citation8] Surfactin, as an efficient lipopeptide, has attracted great attention over the past few decades.[Citation9] However, surfactin in Chinese liquor remains poorly understood.
Surfactin as one of the lipopeptides is similar to lichenysin.[Citation10,Citation11] The first amino acid of surfactin is glutamic acid, different from glutamine in lichenysin.[Citation12,Citation13] Surfactin has antifungal and insecticidal properties and could promote plant growth, such as reduced significantly the Verticillium wilt incidence in tomato plant by 70.43 ± 7.08%.[Citation14,Citation15] Bacillus is considered as the major producer of surfactin.[Citation13] Moreover, Bacillus is also dominant in the fermentation of Chinese liquor, especially in Daqu fermentation process (account for 14.8%-18.2%).[Citation16] However, whether Bacillus plays an important role in producing surfactin during the liquor fermentation is still unclear.
This work systematically detected the concentrations of surfactin in Moutai liquor samples by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometer (UPLC–MS/MS). The contents of surfactin in Moutai liquor were also examined in the making process, to reveal the origin of surfactin. This work developed a qualitative and quantitative analysis for surfactin in Chinese liquor and traced its source during the Moutai liquor fermentation. In addition, Bacillus strains were isolated from Daqu and cultured to produce surfactin.
Materials and methods
Chemicals
Surfactin standard was purchased from Sigma Technology (St. Louis, Mo., USA). Formic acid was purchased from Acros Organics (NJ, USA). Methanol was purchased from Tedia (Ohio, USA). The surfactin standard dissolved in methanol was stored at −20°C until analysis.
Sample collection and reservation
Samples of liquor, Daqu, and fermented grains were all collected from Kweichow Moutai Co., Ltd. (Maotai, Renhuai, China). Liquor samples were stored at 4°C until analysis. Eight solid samples were from the same production cycle including wheat, initial of Daqu fermentation, the end of Daqu fermentation, mature Daqu, distilled grains, initial of stacking fermentation, end of stacking fermentation (initial of liquor fermentation) and the end of liquor fermentation. All solid samples were withdrawn, enclosed with aluminum foil paper, and stored in the refrigerator of −80°C after liquid nitrogen freezing until required for analysis.
Isolation of surfactin in Moutai liquor and solid samples
For the isolation of surfactin in liquor, the purification of analyte in liquor samples was optimized by solid-phase extraction (SPE) according to Zhang et al.[Citation7] First, surfactin was purified from a sample by using different concentrations of methanol. Second, surfactin was stayed on the SPE column and eluted with absolute methanol. Third, the elution fractions were collected and condensed. This work obtained 50 times concentrated sample by using 50 mL of liquor and 1 mL of methanol.
Before identification and quantification of surfactin in Moutai liquor, purification treatment should be performed at the optimized condition: 70% methanol phase was used to purify the liquor sample, and then the target components were eluted from SPE column by absolute methanol.
Ultrasound-assisted solid–liquid extraction (USLE) based on the ultrasonic bath, a common and simply operated device was used for the extraction of surfactin in solid samples. USLE of surfactin was carried out in a KQ 500B ultrasound bath operating at 40 kHz, and 500 W. The vacuum freeze-drying solid samples were powdered to a homogenous size by a mill and sized through a no. 40 mesh sieve. An aliquot of 10 g of powdered solid sample was extracted by ultrasonication with 20 mL of methanol in an ultrasonic clean bath at 500 W for 20 min and then diluted and refiltered (0.22 μm) for subsequent UPLC–MS/MS analysis.
Identification and quantification of surfactin in Moutai liquor and solid samples
After purification treatment, surfactin in different samples was identified and quantified according to the report with slight modifications.[Citation7] The main optimized conditions of UPLC – MS/MS were as bellow: injection volume 5 μL, source temperature 150°C, desolvation gas 1000 L h–1 at 500°C.
Screening and identification of surfactin-producing strains
Daqu (25 g) was dissolved in 100 mL physiological saline to mix thoroughly. One milliliter solution was inoculated into 250 mL Erlenmeyer flask containing 50 mL of Luria–Bertani (LB) broth (in g L–1: peptone 10, NaCl 10, and yeast extract 5) and cultured at 50°C and 200 rpm. After cultured for 24 h, the sample was diluted to 10−6 CFU/mL. Then, the diluted sample (100 μL) was inoculated into the blood plate and cultured at 37°C for 48 h. Because of the hemolysis, transparent circles would appear near the strains which could produce surfactin. Stains with transparent circles were inoculated to LB plates and cultured at 37°C until more single colonies were obtained.
The obtained strains were identified by 16s rRNA sequencing. Each PCR reaction was 25 μL volume containing 12.5 μL Green Taq Mix (Vazyme Biotech, Nanjing, China), 0.5 μL each primer (20 μmol/L), 1 μL DNA template, and 11 μL nuclease-free water. The PCR conditions were as follows: preheating at 95°C for 6 min, 40 cycles at 95°C for 1 min, 55°C for 30 s, 72°C for 2 min. The information of primers was shown in Table S1.
Microbial culture conditions and surfactin production analysis
The bacteria isolated from Daqu were aerobically inoculated into 250 mL Erlenmeyer flask containing 50 mL of LB broth and cultured at 37°C with 200 rpm shaking for 24 h. After cultivation, the fermentation broth was diluted with methanol and refiltered (0.2 μm) to remove bacterial cells; then, the surfactin contents were determined by UPLC–MS/MS as mentioned above.
Results and discussion
Effect of pre-treatment on surfactin determination
To identify surfactin in Moutai liquor, liquor samples were directly determined by UPLC–MS/MS with surfactin standard as the control. The retention time of standard C15 surfactin is 3.17 min by single ion monitoring reaction (SIR) with M/Z 1037 (Fig. S1A), compared to observing no significant signals without pretreatment (Fig. S1B). This result suggested that intrinsic complex components interfered the surfactin’s separation and ionization in Moutai liquor, so it was necessary to pre-treat the liquor samples. To purify the surfactin, the SPE column was washed by mobile phase, containing different concentrations of methanol (Fig. S1C, S1D). The optimizing detection of surfactin was obtained by 70% of the methanol phase (Fig. S1D). Furthermore, it indicated that 70% methanol could not elute the surfactin from SPE column, whereas absolute methanol could elute all of the surfactin (Fig. S2).
Identification of the surfactin in Moutai liquor
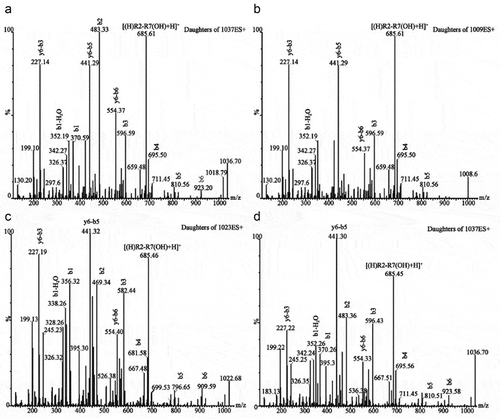
Surfactin’s homologues were identified by UPLC–MS/MS in Moutai liquor. The total ion chromatogram was shown in Fig. S1D. All the MS peak profiles were extracted (), and their [M + H]+ peaks at 1008.68, 1022.68, 1036.70 and corresponding [M + Na]+ peaks at 1030.68, 1044.68, and 1058.70 ( and ), which were classified to C13, C14, and C15 surfactin’s homologues.[Citation17] Moreover, three protonated surfactin’s homologues were fragmented by the collision–induced dissociation (CID) – MS/MS. Their amino acid sequences were glutamic acid–leucine–leucine–valine–aspartic acid–leucine–leucine, but varied in the fatty-acid moiety that was composed of β–hydroxy fatty acids of different lengths: C13 (m/z 1009), C14 (m/z 1023), and C15 (m/z 1037). This could be verified by the base fragment–ion, m/z 685 common to every precursor–ion because it was a product of cleavages between glutamic acid–leucine and fatty acids–leucine, with the net charge retained in the residual hexapeptide (leucine–leucine–valine–aspartic acid–leucine–leucine). Another abundant fragment was observed at m/z 441 () and was common to every species analyzed. It was the cleavage product of y6–b5, a residual tetrapeptide of leucine–leucine–valine–aspartic acid.[Citation17] However, the fragment ions of fatty acid in different precursor ions were different by 14 mass units (m.u.). For example, the fragment b1 (m/z 356.32) and its dehydrated form (m/z 338.26) from the precursor at m/z 1022.68 were 14 m.u. smaller than their equivalents (m/z 370.26 and 352.26) from the precursor ion at m/z 1036.7 ().
Table 1. Positive ESI MS spectrum of fractions isolated from Moutai liquor
Quantification of surfactin in Moutai liquor
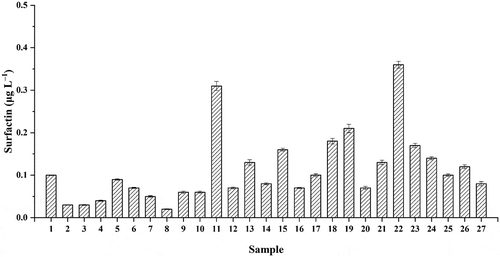
Twenty-seven commercial Moutai liquor samples with different storage times were collected to quantify the contents of surfactin (, Table S2). The calibration curve (y= 86218x–30064) was shown with strong linearity (R2= 0.999). All the RSDs were less than 10% in each experiment (, Table S2). These results indicated that the methods of pretreatment and quantification in Moutai liquor were reliable. Total surfactin concentrations in Moutai liquor were ranged from 0.02 to 0.36 μg L–1 (). Our work firstly identified and quantified the surfactin in Moutai liquor. It would facilitate the research in nonvolatile compounds of distilled liquors.
Tracking of surfactin during the Moutai liquor making process
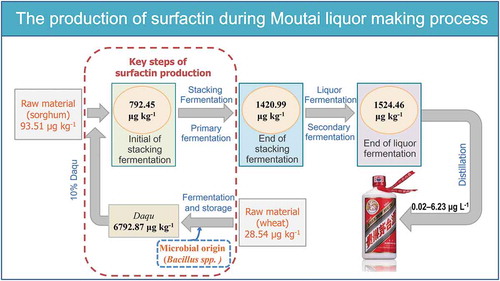
Moutai liquor production was a process of solid-state fermentation with a profiling of batch fermentation (). One fermentation cycle included stacking fermentation using Daqu as a starter culture and distilled grains in the last batch fermentation as a substrate. The subsequent liquor fermentation begined from the end of stacking fermentation and continued in a pit for obtaining fermented grains. The primary Moutai liquor and distilled grains were obtained by distilling the fermented grains. In the present study, surfactin was detected in the commercial Moutai liquor, distilled from the fermented grains. Thus, we believed that surfactin was originated from the fermentation process. Therefore, samples were collected to identify surfactin contents by USLE pre–treated and UPLC–MS/MS, including wheat, initial of Daqu fermentation, the end of Daqu fermentation, mature Daqu, distilled grains, initial of stacking fermentation, end of stacking fermentation (initial of liquor fermentation) and the end of liquor fermentation.
Figure 3. Scheme of Moutai liquor fermentation process (a) and surfactin contents throughout the fermentation process (b). ISF (Initial of stacking fermentation), ESF (End of stacking fermentation), ILF (Initial of liquor fermentation), ELF (End of liquor fermentation), DGS (Distilled grains)
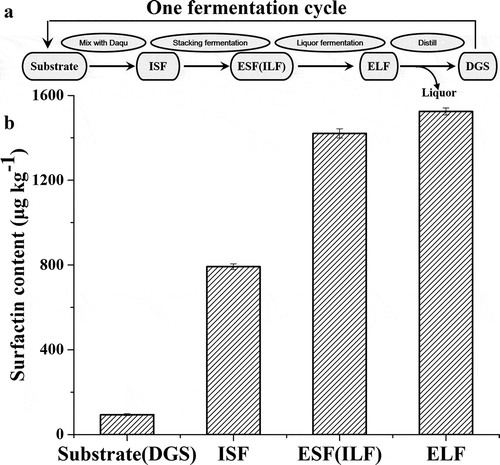
The content of surfactin was 94 ± 4 μg kg–1 in the substrate ( and ). After distilled grains mixed with Daqu (9:1), the surfactin content increased to 792 ± 13 μg kg–1, and then it increased to 1421 ± 22 μg kg–1 at the end of stacking fermentation. It slightly increased to 1524 ± 17 μg kg–1 at the end of liquor fermentation. It indicated that the surfactin was mainly originated from Daqu and stacking fermentation stage in Moutai liquor, which provided 45.8% and 41.2% of the total amount of surfactin in the liquor making process. The acidity was increased significantly after the liquor fermentation (), and this might limit the production of surfactin during the liquor fermentation stage.[Citation18]
Table 2. Process parameters and surfactin contents of samples in Moutai liquor making process
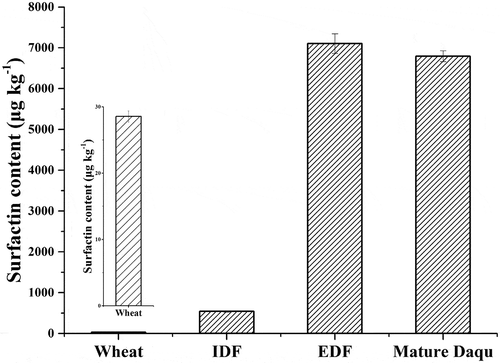
In addition, the concentration of surfactin was 6793 ± 133 μg kg–1 in Daqu. It indicated that Daqu was the direct contributor for surfactin. Daqu was made from grounded wheat and the last batch mature Daqu. We quantified the surfactin contents in wheat and samples during the Daqu making process ( and ). The content of surfactin in wheat was 29 ± 1 μg kg–1 and then increased to 541 ± 17 μg kg–1 after the grounded wheat was mixed with mature Daqu. Surfactin was little in wheat, confirmed that raw materials were not the main source of surfactin. Surfactin’s concentration increased significantly during the Daqu fermentation process and reached to 7104 ± 242 μg kg–1 at the end of fermentation, same as the end of storage process (6793 ± 133 μg kg–1 in mature Daqu). This indicated that surfactin in Daqu was mainly produced during Daqu preparation process and kept stable in Daqu storage process. Surfactin has the potential ability of biocontrol in Daqu, and can resist the invasion of foreign harmful microorganisms. For example, surfactin could inhibit the growth of Streptomyces sampsonii, a harmful microorganism which could disturb the growth of Saccharomyces cerevisiae and produce off-flavor compound of geosmin.[Citation19,Citation20]
Figure 4. Surfactin contents during the Daqu making process. IDF: initial of Daqu fermentation, EDF: end of Daqu fermentation
Considering the significant difference of surfactin’s concentration in Daqu making process and liquor making process, it concluded that surfactin was mainly produced in Daqu making stage and stacking fermentation stage, and then distilled into liquor in the distillation stage. Interestingly, Bacillaceae dominated the end of Daqu fermentation and stacking fermentation stage.[Citation21] Moreover, many researches also reported that Bacillus was the major producer of surfactin.[Citation22] Therefore, surfactin in Moutai liquor might be derived from Bacillus.
Microbial origin of surfactin in Moutai liquor
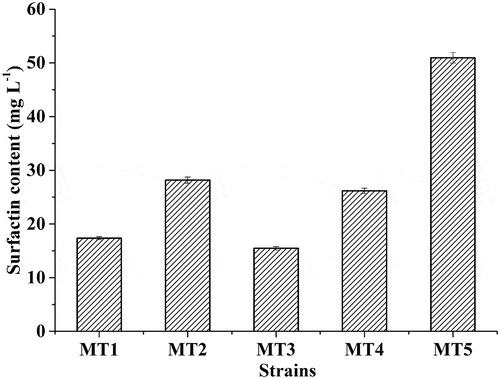
According to the transparent circles on the blood agar plates, 20 strains were isolated from samples at the end of Daqu fermentation. Moreover, we selected five strains (MT1-MT5) which could produce surfactin with the high levels (> 10 mg L–1) (). Among them, the highest surfactin production reached to 51 ± 1 mg L–1. The five strains were identified through aligning their 16s rRNA sequences by NCBI database. The strains of MT1, MT2, MT3, and MT4 were Bacillus subtilis, and the strains of MT5 were Bacillus amyloliquefaciens. The 16s rRNA sequences were deposited in GenBank, and the GenBank accession numbers were listed in Table S3. The previous study reported that Bacillus was considered as the producer of surfactin.[Citation22] For example, Bacillus megaterium, isolated from marine, could produce lipopeptides[Citation23]; B. subtilis could produce surfactin in the corn steep liquor media[Citation24]; B. amyloliquefaciens used distillers’ grains as a substrate to produce surfactin.[Citation25] Thus, the Bacillus spp. might play an important role in producing surfactin during Moutai liquor making process.
Previous study revealed notable enrichment of surfactin synthetic pathway in B. amyloliquefaciens MT45 from Moutai Daqu, including central carbon metabolism and fatty acid biosynthesis to provide sufficient precursors for surfactin.[Citation26] Therefore, MT1-MT5 strains might have similar characteristics to MT45 in gene expression and result in the overproduction of surfactin. In addition, microbial interactions might also improve the synthesis of surfactin. Previous study confirmed that B. amyloliquefaciens MT45 co-cultured with Bacillus strains which exhibited remarkable hydrolase activities in Daqu could increase the surfactin yield by 50%.[Citation25] It was because that Bacillus with high level of surfactin cannot simultaneously produce sufficient hydrolase to utilize the large molecular substrate in Daqu.[Citation25] At this time, other Bacillus with highly hydrolytic enzymes in this system could provide sufficient substrates to hyperproducer for excess synthesis of surfactin.[Citation25]
Bacillus with the ability of producing surfactin could also be isolated from other fermented foods which production process without distillation, such as Cheonggukjang (a Korean fermented soy food).[Citation27] However, the contents of surfactin in these fermented foods remain poorly understood and need to be proved by more accurate researches.
Conclusion
In this study, the surfactin in Moutai liquor was identified and quantified by UPLC–MS/MS. The concentrations of surfactin in Moutai liquor were different, potentially caused by raw material, fermentation conditions change. Furthermore, the surfactin was first traced in Moutai liquor making process. The surfactin in Moutai liquor was mainly produced by Bacillus spp. in Daqu making process and stacking fermentation stage, and then distilled into liquor in the distillation stage. It was an important breakthrough and innovation in Chinese liquor research, and facilitated advances in nonvolatile compounds of distilled liquors.
Supplemental Material
Download MS Word (2.7 MB)Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not–for–profit sectors. The authors are grateful to Dr. Yan Zhi, Na Yang, Bowen Wang from Jiangnan Univ. for their help in the preparation of this manuscript. The authors declare that there is no conflict of interest.
Supplementary Material
Supplemented data of this article can be accessed here.
References
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66(22), 5425–5432. DOI: 10.1021/acs.jafc.8b00692.
- Fan, W.; Shen, H.; Xu, Y. Quantification of Volatile Compounds in Chinese Soy Sauce Aroma Type Liquor by Stir Bar Sorptive Extraction and Gas Chromatography-mass Spectrometry. J. Sci. Food Agric. 2011, 91(7), 1187–1198. DOI: 10.1002/jsfa.4294.
- Xu, Y.; Ji, K. Moutai (Maotai): Production and Sensory Properties. Alcoholic Beverages. Woodhead Publishing Limited: UK, 2012.
- Zhang, J.; Tian, Z.; Ma, Y.; Shao, F.; Huang, J.; Wu, H.; Tian, L. Origin Identification of the Sauce-flavor Chinese Baijiu by Organic Acids, Trace Elements, and the Stable Carbon Isotope Ratio. J. Food Qual. 2019. DOI: 10.1155/2019/7525201.
- Fang, C.; Du, H.; Jia, W.; Xu, Y. Compositional Differences and Similarities between Typical Chinese Baijiu and Western Liquor as Revealed by Mass Spectrometry-based Metabolomics. Metabolites. 2019, 9(2), 1–18. DOI: 10.3390/metabo9010002.
- Wu, J.; Huo, J.; Huang, M.; Zhao, M.; Luo, X.; Sun, B. Structural Characterization of a Tetrapeptide from Sesame Flavor-type Baijiu and Its Preventive Effects against AAPH-induced Oxidative Stress in HepG2 Cells. J. Agric. Food Chem. 2017, 65(48), 10495–10504. DOI: 10.1021/acs.jafc.7b04815.
- Zhang, R.; Wu, Q.; Xu, Y.; Qian, M. C. Isolation, Identification, and Quantification of Lichenysin, a Novel Nonvolatile Compound in Chinese Distilled Spirits. J. Food Sci. 2014, 79(10), 1907–1915. DOI: 10.1111/1750-3841.12650.
- Zhang, R.; Wu, Q.; Xu, Y. Lichenysin, a Cyclooctapeptide Occurring in Chinese Liquor Jiannanchun Reduced the Headspace Concentration of Phenolic Off-flavors via Hydrogen-bond Interactions. J. Agric. Food Chem. 2014, 62(33), 8302–8307. DOI: 10.1021/jf502053g.
- Boka, B.; Manczinger, L.; Kecskemeti, A.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N. S.; Vagvolgyi, C.; Szekeres, A. Ion Trap Mass Spectrometry of Surfactins Produced by Bacillus Subtilis SZMC6179J Reveals Novel Fragmentation Features of Cyclic Lipopeptides. Rapid Commun. Mass Spectrom. 2016, 30(13), 1581–1590. DOI:10.1002/rcm.7592.
- Alajlani, M.; Shiekh, A.; Hasnain, S.; Brantner, A. Purification of Bioactive Lipopeptides Produced by Bacillus Subtilis Strain BIA. Chromatographia. 2016, 79(21–22), 1527–1532. DOI: 10.1007/s10337-016-3164-3.
- Deng, Q.; Wang, W.; Sun, L.; Wang, Y.; Liao, J.; Xu, D.; Liu, Y.; Ye, R.; Gooneratne, R. A Sensitive Method for Simultaneous Quantitative Determination of Surfactin and Iturin by LC-MS/MS. Anal. Bioanal. Chem. 2017, 409(1), 179–191. DOI: 10.1007/s00216-016-9984-z.
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a Crystalline Peptidelipid Surfactant Produced by Bacillus Subtilis: Isolation, Characterization and Its Inhibition of Fibrin Clot Formation. Biochem. Biophys. Res. Commun. 1968, 31(3), 488–494. DOI: 10.1016/0006-291x(68)90503-2.
- Grangemard, I.; Wallach, J.; Dana, R. M.; Peypoux, F. Lichenysin, a More Efficient Cation Chelator than Surfactin. Appl. Biochem. Biotechnol. 2001, 90, 199–210. DOI: 10.1385/ABAB:90:3:199.
- Panneerselvam, P.; Senapati, A.; Kumar, U.; Sharma, L.; Lepcha, P.; Prabhukarthikeyan, S. R.; Jahan, A.; Parameshwaran, C.; Govindharaj, G. P. P.; Lenka, S.; et al. Antagonistic and Plant-growth Promoting Novel Bacillus Species from Long-term Organic Farming Soils from Sikkim, India. 3 Biotech. 2019, 9(11). DOI: 10.1007/s13205-019-1938-7.
- Dhouib, H.; Zouari, I.; Ben Abdallah, D.; Belbahri, L.; Taktak, W.; Triki, M. A.; Tounsi, S. Potential of a Novel Endophytic Bacillus Velezensis in Tomato Growth Promotion and Protection against Verticillium Wilt Disease. Biol. Control. 2019, 139. DOI: 10.1016/j.biocontrol.2019.104092.
- Zhang, L.; Wu, C.; Ding, X.; Zheng, J.; Zhou, R. Characterisation of Microbial Communities in Chinese Liquor Fermentation Starters Daqu Using Nested PCR-DGGE. World J. Microbiol. Biotechnol. 2014, 30(12), 3055–3063. DOI:10.1007/s11274-014-1732-y.
- Korenblum, E.; de Araujo, L. V.; Guimarães, C. R.; de Souza, L. M.; Sassaki, G.; Abreu, F.; Nitschke, M.; Lins, U.; Freire, D. M. G.; Barreto-Bergter, E.; et al. Purification and Characterization of a Surfactin-like Molecule Produced by Bacillus Sp. H2O-1 and Its Antagonistic Effect against Sulfate Reducing Bacteria. BMC Microbiol. 2012, 12(252), 2–13. DOI: 10.1186/1471-2180-12-252.
- Cosby, W. M.; Vollenbroich, D.; Lee, O. H.; Zuber, P. Altered Srf Expression in Bacillus Subtilis Resulting from Changes in Culture pH Is Dependent on the spo0K Oligopeptide Permease and the comQX System of Extracellular Control. J. Bacteriol. 1998, 180(6), 1438–1445. DOI: 10.1128/JB.180.6.1438-1445.1998.
- Hai, D.; Hu, L.; Yan, X.; Xiaowei, D. Community of Environmental Streptomyces Related to Geosmin Development in Chinese Liquors. J. Agric. Food Chem. 2013, 61(6), 1343–1348. DOI: 10.1021/jf3040513.
- Zhi, Y.; Wu, Q.; Du, H.; Xu, Y. Biocontrol of Geosmin-producing Streptomyces Spp. By Two Bacillus Strains from Chinese Liquor. Int. J. Food Microbiol. 2016, 231, 1–9. DOI: 10.1016/j.ijfoodmicro.2016.04.021.
- Wang, L.; Wang, Y. Y.; Wang, D. Q.; Xu, J.; Yang, F.; Liu, G.; Zhang, D. Y.; Feng, Q.; Xiao, L.; Xue, W. B.; et al. Dynamic Changes in the Bacterial Community in Moutai Liquor Fermentation Process Characterized by Deep Sequencing. J. Inst. Brew. 2015, 121(4), 603–608. DOI: 10.1002/jib.259.
- Zanotto, A. W.; Valerio, A.; de Andrade, C. J.; Pastore, G. M. New Sustainable Alternatives to Reduce the Production Costs for Surfactin 50 Years after the Discovery. Appl. Microbiol. Biotechnol. 2019, 103(21–22), 8647–8656. DOI: 10.1007/s00253-019-10123-7.
- Ma, Y.; Kong, Q.; Qin, C.; Chen, Y.; Chen, Y.; Lv, R.; Zhou, G. Identification of Lipopeptides in Bacillus Megaterium by Two-step Ultrafiltration and LC-ESI-MS/MS. Amb Express. 2016, 6(79), 2–15. DOI: 10.1186/s13568-016-0252-6.
- Gudiña, E. J.; Fernandes, E. C.; Rodrigues, A. I.; Teixeira, J. A.; Rodrigues, L. R. Biosurfactant Production by Bacillus Subtilis Using Corn Steep Liquor as Culture Medium. Front. Microbiol. 2015, 6. DOI: 10.3389/fmicb.2015.00059.
- Zhi, Y.; Wu, Q.; Xu, Y. Production of Surfactin from Waste Distillers’ Grains by Co-culture Fermentation of Two Bacillus Amyloliquefaciens Strains. Bioresour. Technol. 2017, 235, 96–103. DOI: 10.1016/j.biortech.2017.03.090.
- Zhi, Y.; Wu, Q.; Xu, Y. Genome and Transcriptome Analysis of Surfactin Biosynthesis in Bacillus Amyloliquefaciens MT45. Journal. 2017, 7, 40976. DOI: 10.1038/srep40976.
- Lee, J. Y.; Shim, J. M.; Yao, Z.; Liu, X.; Lee, K. W.; Kim, H. J.; Ham, K. S.; Kim, J. H. Antimicrobial Activity of Bacillus Amyloliquefaciens EMD17 Isolated from Cheonggukjang and Potential Use as a Starter for Fermented Soy Foods. Food Sci. Biotechnol. 2016, 25(2), 525–532. DOI: 10.1007/s10068-016-0073-z.