ABSTRACT
Sesame oil processing produces a growing number of low-value byproducts, which has become a major economic problem in the sesame oil industry. This review focuses on the preparation of biologically active peptides in sesame oil processing byproducts and the significance of the biologically active value of sesame peptides. Firstly, the origin of sesame peptides is described, and a personal point of view about the differences in sesame peptides in the byproducts of sesame processing produced by the different oil processing processes are proposed. Secondly, the preparation method of sesame peptide reported so far are studied. Finally, the possible biological activities of sesame peptides are described, and then a reference for future research works will be produced.
Introduction
As an traditional oil crop from ancient time, sesame is recognized as one of the four largest edible oil crops in China along with soybeans, peanuts and rape.[Citation1] Beatrice A[Citation2] proposes that sesame is a high-potential crop and is listed by the International Plant Genetic Resources Institute (IPGRI) as a crop that has been neglected and underutilized. Nowadays, sesame has become the dominant oil crop, and its processed products are also essential for every family, such as sesame oil[Citation3] and sesame paste .[Citation4]
The processing byproducts are mainly materials produced by the processing chain complying with relevant laws and regulations .[Citation5] In a sense, processing byproducts can be viewed as products with independent markets. Since the fat content of sesame is about 45–50%, [Citation6] apart from direct consumption, sesame is used primarily for processing oil, so the main byproduct is sesame meal after sesame oil processing .[Citation7] According to the statistics of the Food and Agriculture Organization of the United Nations in 2018, the global sesame cultivation area was 1.17 × 10 ^7 hectares, and the yield was 5.53 × 10 ^6 tons .[Citation8] Therefore, a large amount of sesame processing byproducts are produced every year worldwide. These sesame processing byproducts are generally used as animal feed and fertilizer for crop cultivation. However, rich protein resources in sesame meal have been ignored by people all the time .[Citation9] If the protein resources in sesame meal are fully utilized, such as making protein supplements, [Citation10] not only the processing cost of sesame products can be reduced, but the economic sustainable development of sesame processing industry can also be improved.
Bioactive peptides are polypeptides with a variety of biological functions, which play a significant role in human body on physiological regulatory .[Citation11] They are available in all kinds of eggs, milk, meat, fish and plants .[Citation12–14] Bioactive peptide is inactive in the sequence of its parent protein and has specific biological activity upon releasing under certain conditions. Bioactive peptides can be divided into two types, one is endogenously active peptide, which is released from the parent protein during gastrointestinal digestion. The other is exogenously active peptide, which is synthesized by enzymatic hydrolysis or artificial synthesis during food processing. They usually contain 2–20 amino acid residues per molecule, but it may contain more than 20 amino acids in some cases .[Citation15] Bioactive peptide after digestion can be absorbed through the intestine before it enters the blood circulation intact and exerts a systemic effect or produces a local effect in the gastrointestinal tract.
It has been found that peptides can exhibit various biological activities according to the order of amino acids, such as binding to minerals, [Citation16] participating in immunomodulation in-vivo, [Citation17] bacteriostatic, [Citation18] anti-oxidation, [Citation19] thrombolytic, [Citation20] cholesterol lowering[Citation21] and blood pressure lowering effects .[Citation22] Therefore, bioactive peptides, as one of the natural products, has considerable market potential. They can not only be used as food additives, but also be used to develop new functional food and nutritional health products suitable for different populations. Statistics shows that the circulation of polypeptide-related products in the market exceeds 40 billion dollars annually .[Citation23] Therefore, the development of related polypeptide products can meet the needs of consumers, increasing economic benefits, and further expanding consumers’ understanding of peptide biological functions.
This paper mainly provides a comprehensive review of bioactive peptides prepared from the byproduct of sesame oil processing and their preparation methods. On the one hand, the main methods for preparing sesame peptide from sesame processing by-products are described. On the other hand, the biological activity function of sesame peptide is comprehensively analyzed to provide researchers with preliminary research countermeasures for sesame peptide.
Origin of sesame bioactive peptide
As we all know, protein is the material basis of life and an important component of all cells and tissues in the human body. The most basic component unit of protein is the amino acid, which is a substance with a certain spatial structure formed by coiling and folding polypeptide chains composed of amino acids in a “dehydration condensation” mode. Studies have shown that proteins have a wide range of biological activitie. It is used as enzymes, hormones, antigens, and antibodies to participate in complex biological metabolism and disease prevention in the human body .[Citation24] However, some scholars believe that many proteins in organisms contain amino acid sequences with specific structures and specific functions, and these amino acid sequences with specific structural sequences may be one of many functions expressed by proteins and have the characteristic of single-point amplification of this function .[Citation25]
In 1902, Bayliss and Starling[Citation26] from the University of London of Medicine discovered polypeptide substances for the first time in the human body. Due to their far-reaching influence, the Nobel Prize Committee awarded them the Nobel Prize in Physiology. Thus, polypeptide substances formally appeared in scientific research and laid the foundation for long-term development thereafter. Now it can be basically confirmed that the biological function of protein is the decisive function of certain amino acid compositions. Only when these amino acid compositions are released can they show the functional characteristics. It is also true that bioactive peptides are defined as a class of peptide compounds with a relative molecular mass of less than 6000 Da, which is beneficial to the life activities of living organisms. They usually contain 2–20 amino acid residues per molecule, but in some cases may contain more than 20 amino acids. After digestion, bioactive peptides can be absorbed through the intestinal tract, entering blood circulation completely and exerting systemic effect, or producing a local effect in the gastrointestinal tract.
In recent years, many researchers have made significant research on polypeptide substances isolated from sesame protein. Some people extract sesame protein isolates from sesame seed, but most researchers extract sesame protein isolates from sesame meal (sesame cake, sesame residue), a byproduct of sesame processing, and then using sesame protein to prepare sesame polypeptide with biological activity. Interestingly, there are mainly three traditional sesame oil extraction technologies, namely, pressing method, water substitution method and solvent extraction method. In addition, the fact that sesame is roasted at high-temperature before oil processing is also the main reason for the change of its byproducts. At present, according to the existing literature, there is no difference between sesame protein and sesame peptide isolates from sesame processing byproducts produced by different oil processing technologies, which may be a serious impact on sesame active peptide.
Preparation method of sesame active peptide
At present, there are many ways to produce bioactive peptides, which can be subdivided into in-vivo production and in-vitro preparation. Gastrointestinal tract digestion is the crucial way to produce peptides in the body. Macromolecular proteins are decomposed into small molecular polypeptides through gastrointestinal tract digestion, which are then absorbed and utilized by the body. However, there are various methods of preparation outside the body, which can be roughly divided into protease enzymolysis, fermentation, simulated gastrointestinal digestion and other methods .[Citation27,Citation28] According to the existing literature reports, the methods for preparing sesame active peptides are mostly prepared outside the body, and methods for preparing sesame active peptides in the body have not been reported.
Preparation of sesame peptides by proteolysis
Currently, the rapid advance of protease preparation has played a key role in promoting the preparation technology of bioactive peptides, and has also strengthened the position of enzymatic hydrolysis in peptide preparation technology. In addition, multi-enzyme complex hydrolysis and immobilized enzyme hydrolysis technology have been developed from single-enzyme hydrolysis technology, [Citation29,Citation30] so that the prepared peptide has richer functional activity.
Enzymatic hydrolysis by single enzyme
Proteases are a set of peptides that can hydrolyze proteins. However, different kinds of proteases act on different peptide chain sites in proteins, so the products of the same protein hydrolysis by different proteases have different amino acid sequences and polypeptides with different molecular weight, which may show different biological activities.
Li Feng-xia et al.[Citation31] hydrolyze sesame protein by trypsin, papain, AS1.398 enzyme and Alcalase enzyme, and finally find that AS1.398 has the best hydrolysis effect on sesame protein, and the degree of protein hydrolysis could reach 23.64% after 4 hours. Similarly, Hou li-xia et al.[Citation32] hydrolyze sesame protein with papain to prepare sesame peptide. The optimum technological conditions are determined as follows: substrate concentration 4%, enzyme dosage 1500 U/g, pH value adjusting to 9.0, hydrolysis at 60°C for 3 h, under which the degree of hydrolysis of sesame protein is 14.98%. Zhao Shiguang et al.[Citation33] take high-temperature sesame meal as raw material, and hydrolyze sesame meal with pepsin, Alcalase alkaline protease, papain and neutral protease, respectively. Finally, sesame polypeptide is prepared with Alcalase enzyme, with the highest yield of 3.51%. Xiao Yang et al.[Citation34] extract sesame protein from sesame, then hydrolyze sesame protein with Alcalase enzyme, pepsin, papain, trypsin and AS1398 neutral protease. Finally, papain is selected to prepare sesame antioxidant peptide. Under the optimal process conditions, the inhibition rate of enzymatic hydrolyzate on pyrogallol oxidation is 35.6%. According to relevant reports, it is confirmed that alkaline protease, papain, neutral protease, trypsin, etc. can perform good enzymolysis on sesame protein.
Interestingly, Lu Xin et al.[Citation35] use the approximately ideal solution sequencing method to sequence the hydrolyzates with polypeptide yield and antioxidant activity as parameters see the . The sum of the distances between the optimal vectors and the hydrolyzates from bromelain, papain, neutral protease, trypsin, alkaline protease 2709 and Alcalase protease is 2.4349, 2.8805, 2.378, 1.363, 1.2923 and 1.2973 respectively. According to this, protease suitable for preparing sesame antioxidant peptide is alkaline protease 2709, Alcalase protease, trypsin, neutral protease, bromelain and papain from high to low. In addition, since the optimum pH for pepsin is 2–5, but the isoelectric point (PI) of sesame protein is 4–5, the solubility of sesame protein decreases under the condition of suitable pH for pepsin, which is not conducive to enzymatic hydrolysis.
Table 1. TOPSIS algorithm sequencing of antioxidant peptides prepared by enzymatic hydrolysis of sesame protein with different proteases
Multi-enzyme compound enzymatic hydrolysis
Enzymes have strict selectivity to the substrates they operate on. An enzyme can only act on a substance with similar molecular structure to promote certain chemical reactions and produce certain reaction products. This selective action is called enzyme specificity. Owing to the specificity of the enzyme, the range of action of the enzyme is limited, and the hydrolysis efficiency is correspondingly reduced. If two or more proteases are used in a compound ratio, [Citation36] the specificity of different enzymes to different protein sequences may play a synergistic role, improving enzymolysis efficiency and increasing yield.
Tang Zhang-hui et al.[Citation37] carry out multi-enzyme step by step enzymatic hydrolysis of sesame protein, and finally select three enzymes to carry out step by step enzymatic hydrolysis. The optimal enzymatic hydrolysis process selections are as follows: add 0.5% alkaline protease on first, and then add 0.6% neutral protease, finally add 1.5% papain, and carry out enzymatic hydrolysis under limited conditions. The result shows that the total nitrogen recovery rate is 63.14%, the trichloroacetic acid soluble nitrogen index is 55.11%, and the peptide conversion rate is 48.99%. Chen Yi-yong et al.[Citation38] use papain and flavourzyme to hydrolyze sesame protein step by step, with a hydrolysis degree of 55.76%, significantly higher than that of single papain (46.27%).
From these we can see that multi-enzyme hydrolysis of sesame protein can increase the degree of hydrolysis to a certain extent, thereby increasing the production rate of peptides.Interestingly, other studies have found that the hydrolyzate obtained by multi-enzyme composite hydrolysis technology is remarkably higher in biological activity than that obtained by single-enzyme hydrolysis.
Enzymatic hydrolysis of immobilized enzyme
Immobilized enzyme technology is a technology that uses physical or chemical means to block the free enzyme from solid materials or restrict it to a certain area for active and unique catalysis, and can be recycled for a long time .[Citation39] Immobilization of enzymes has become a major research direction in the field of enzyme application.
Gao Mingxia[Citation40] and others immobilize papain and neutral protease with sodium alginate as carrier and glutaraldehyde as a cross-linking agent. The best conditions for hydrolyzing sesame protein are solid-liquid ratio of 1: 25, enzyme dosage of 5 g/L, at 60°C, pH of 6.0 for 7 h. Under these conditions, the amino nitrogen content in the obtained product is 51.28%. Liang Yujie[Citation41] immobilize Alcalase alkaline protease with self-made magnetic chitosan microspheres as a carrier, and enzymatically hydrolyze sesame cake protein with the immobilized enzyme. Through the analysis of the test data that obtaining the mathematical model for preparing ACE inhibitory peptide, and gaining the optimal technological conditions as follows: the immobilized enzyme dosage is 7105.03 u/g, the temperature is 59°C, the pH is 10.96, and the enzymolysis time is 4.3 h. Under these conditions, the short peptide production rate and ACE inhibition rate are 86.35% and 65.5%, respectively.
Immobilized enzyme has the advantages of high stability, easy separation from reactants, continuous use and the like, and has industrial advantages, but at present, little research has been done in the preparation of sesame active peptides, which deserves attention in later research.
Preparation of peptides by microbial fermentation
The principle of preparing bioactive peptides by fermentation is to use strains that can produce protease to secrete protease to hydrolyze sesame protein. Comparing with enzymatic method, it has the advantages of short production cycle and low production cost, thus becoming a simple and effective method to separate and purify BAP. However, due to safety problems, the types of strains that can produce protease and can be approved by the Food and Drug Administration (FDA) are limited, mainly Aspergillus oryzae, Aspergillus niger, Bacillus subtilis, Bacillus licheniformis and so on, thus limiting its rapid development and application to a certain extent.
Dong Ying[Citation42] ferments sesame meal with Aspergillus sojae 2128 and find that the antioxidant activity of the ferment extract is significantly higiher than that of sesame lignan, but does not determine the specific composition of the antioxidant substance. In addition, in subsequent studies by others, it is find that sesame peptides are present in the product after fermentation. Chen Xue-hong et al.[Citation43] use Bacillus subtilis to ferment sesame meal, and find that the best fermentation conditions are: temperature 32°C, time 48 h, pH 7.0, and the obtain fermentation broth has the highest concentration of sesame peptide, 3.585 mg/mL. According to the 1:1 inoculation ratio of Aspergillus niger and Saccharomyces cerevisiae, Qian Senhe et al.[Citation44] conducted fermentation experiments on sesame meal under certain conditions. When the inoculum amount is 19.69%, the polypeptide content in the product after 36.11 hours of fermentation reaches 7.12%.
In conclusion, microbial fermentation is a simple and effective method to prepare bioactive peptides. Although this method is currently limited to a certain extent, some ferment active peptides such as glutathione, lactic acid bacteria peptide and other products have been industrialized. Therefore, the development prospect of this method is still broad.
Main biological activity of sesame peptide
Antioxidant sesame peptides
Antioxidant system mainly includes enzyme and non-enzyme system in airframe. Various enzyme activities are enhanced to remove excess oxygen free radicals in to exert an efficacious antioxidant effect when the antioxidant peptide enters the body in some way. Studies have shown that natural antioxidant peptides can reduce the formation of free radicals in the body to play an antioxidant role, and food-borne peptides are safe and popular, so finding effective natural antioxidant peptides is one of the research hotspots in the field of food science. The functional peptide prepared by the function-oriented controlled by the enzymatic hydrolysis technology has the characteristics of clear functional factors and remarkable efficacy. The functional peptide can be utilized as a dietary protein supplement or a bioactive food component .[Citation45] It is a key direction for the development of functional foods.
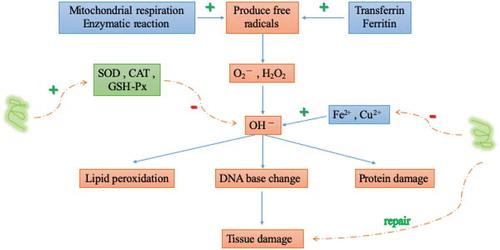
Superoxide dismutase, an extremely critical antioxidant enzyme, can remove O2− and transform into H2O2 to reduce or even block lipid peroxidation, protecting the body from injured in the body .[Citation46] They work together in order to carry out intracellular antioxidants, maintain the balance of oxygen free radicals in the body, protect the cell membrane, and ensure the integrity of cell structure and function .[Citation47,Citation48] The principal sources of free radicals in the body and the damage results and the antioxidant defense mechanism of free radical damage are shown in .
The most dynamic research field on the biological activity of sesame peptide is its antioxidant activity. The polypeptide prepared by Shao Yuanlong et al. using alkaline protease shows strong antioxidant activity, the DPPH radical scavenging rate of 0.2 g/L sesame peptide was 98.55%, 0.02% sesame peptide could obviously inhibit lard peroxidation, and 0.08% sesame peptide could inhibit the oxidation of frozen cooked meat emulsion by 80% .[Citation49] Enzymatic hydrolyzates can effectively inhibit the oxidation of lard, linoleum acid and other peroxidation systems. The increase in the amount or concentration of enzymatic hydrolyzates will lead to enhanced oxidation resistance. In lecithin protein system, the antioxidant effect of enzymatic hydrolyzate is similar to that of vitamin C .[Citation50] Xu Fazhi et al. separates and extractes 3 small peptides from ferment sesame meal, which are identified as tri-peptide, tetra-peptide and hexa-peptide respectively, and determines their antioxidant activities in vitro and in vivo respectively .[Citation51] The results show that the three small peptides have strong antioxidant activity, and the lower the molecular weight of the peptides, the stronger the antioxidant activity. In addition, studies on in-vitro antioxidant activities of sesame peptides obtained by hydrolyzing sesame protein isolating with different proteases show that sesame peptides have potent scavenging effects on superoxide anion radical, hydroxyl radical, alkoxy radical and DPPH radical .[Citation52,Citation53]
Antihypertension abilities
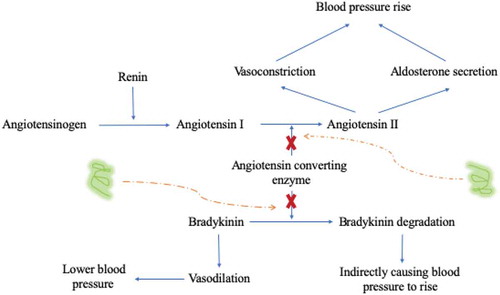
Current researches suggests that the theoretical approach to decrease hypertension via the methods by inhibiting angiotensin converting enzyme (ACE) activity to further relax the arterioles on lower angiotensin levels .[Citation54] ACE plays a role in blood pressure in the human renin-angiotensin system (RAS) and kallikrein-kinin system (KKS) .[Citation55] Importantly, its main mechanism is shown in . Angiotensin I is converted to angiotensin II in the RAS. Angiotensin II is the main hormone regulating electrolyte balance, cardiovascular activity and raising blood pressure. Angiotensinogen is hydrolyzed to angiotensin I by Renin, and then, ACE catalyzes angiotensin I to angiotensin II which could enhance vasoconstriction and stimulate aldosterone secretion to cause an increase in blood pressure. In the kinin release-kinin system (KKS), ACE can inactivate the degradation of Bradykinin degradation with dilated vasculature .[Citation56–58] Therefore, we can quote the exogenous short peptide to inhibit the ACE activity above two systems to achieve the goal of lowering blood pressure. In addition, it is also a common application with angiotensin receptor antagonists to enhance the body’s capability to absorb the Na+ within the systems .[Citation59]
Being focused on the mechanism of hypertension study, several bioactive peptides with significant effects have been decomposed with enzymes assisted from sesame protein. It has been isolated six groups peptides with ACE inhibitory activity extremely short peptide from sesame powder by .[Citation60] All of these components are administered to spontaneously hypertensive mice at a dose of 1–10 mg/kg. It can be allowed to find a significant and transient decrease in their blood pressure. In this model, Leu-Val-Tyr, Leu-Gln-Pro and Leu-Lys-Tyr expressed with strong ACE inhibitory activities, and their IC50 values are 0.92 mol/L, 0.50 mol/L and 0.48 mol/L, respectively. Liang Yu-jie using Alcalase enzyme immobilize on self-made magnetic chitosan microspheres is used to hydrolyze sesame meal protein .[Citation41] Enzymatic hydrolyzate is separated and purified by 5 kDa ultrafiltration membrane, and then separated and desalted by dextran G-15 gel chromatography. ACE inhibitory activity of component II is the highest, with IC50 value of 1.624 mg/mL.
The function-activity relationship of the anti-hypertension peptide is not only related to the amino acid composition, but also expresses a clear association with the molecular weight interval. For instance, Chatterjee et al. obtain ACE inhibitory peptide by the method via ultrafiltration, a small peptide under 3 kDa, which is extracted from sesame skin after treatments with pepsin, papain and alkalize .[Citation9] Meanwhile, Iino et al.[Citation61] has filtered tri-peptides with ACE inhibitory activities in the thermolysin digest of sesame with the array of not only Leu-Ser-Ala, Val-Ile-Tyr, and Leu -Val-Tyr, but also Leeu-Gln-Pro, Leu-Lys-Tyr, Ile-Val-Tyr. All these antihypertensive activity are limited to the amino acids composition, such as Arg, Tyr, Ala, Val, Leu, Ile, Gly, Phe and Pro according to MALDI-TOF sequencing method. Similarly, these peptides are discovered in corn, Antarctic krill and wheat germ, respectively. Besides, three peptide fractions with the antihypertensive activity from sesame contained molecular weight under 3 kDa, 3–10 kDa, and upon10 kDa by the study of, [Citation62] respectively. It is noted that the lower the molecular weight, the better the antihypertensive expressed. After matching with the amino acid sequence of them existed, it is confirmed the ACE inhibitory activity of the peptide, which is consistent with the results of related research on the mechanism of hypertension with the bioactive peptide.
Other biological activities of sesame peptide
Bacteriostatic activities
The bactericidal mechanism of bioactive peptides out of cells is mainly focused on the cell membranes. The lipid-water amphiprotic structure of it can selectively bind to the bacterial cell film to form pores, which destroys its membrane integrity of itself to exert bactericidal action.
Active peptides with bacteriostatic action from sesame has been obtained by the researchers. Ranjana[Citation18] and others find that the small peptide under 1kDa shows stronger antibacterial activity beyond it during 3 kDa to 5 kDa. Moreover, its inhibitory activity against P. aeruginosa is significantly greater than that of B. subtilis. These probably due to that the synthesis of “tetrahydrofolate” is inhibited by the high content of Met in the sesame peptide, which further affects the DNA replication in the bacterial cells to achieve the antibacterial function. Moreover, the variety of sesame would not produce ambiguity on the conditions of the antimicrobial peptide. For instance, peptide approximately 5 kDa can effectively inhibit Klebsiella sp. ability which causing human pneumonia and pathological infections from sesame .[Citation63] In total, the basic mechanism of bactericidal action of most active peptides is abided by principles, destroying the integrity of the bacterial cell membrane, resulting in a large outflow of cell contents and a large influx of extracellular water, changing in intracellular osmotic pressure, and then, the bacteria will be died without membrane protection.
Thrombolysis
The occurrence of thrombotic disorders is mainly related to fibrinogen in the blood. The reports also shows that sesame peptide obtained by trypsin hydrolysis can degrade thrombin-induced fibrin clots, lower blood viscosity, in which effectively removes fibrinogen in the blood in order to dissolve thrombus rapidly .[Citation64] Although a variety of thrombolytic active substances have been obtained from non-foodborne animal and plant proteins, [Citation65–67] most of these anticoagulant peptides are poor in safety which is extracted from the venom of blood-sucking animals. These findings will express the potential to make sesame peptides one of the few food-borne bioactive peptides with hemolysis.
Complementary metal chelate peptide
A polypeptide metal element chelate is a product in which N-terminal amino group, C-terminal carboxyl group, amino acid side chain, and a carbonyl group in the peptide chain are mainly connected with all kinds of metal by a coordinate bond to form a stable chelate compound .[Citation68] Here, it is especially important to synthesize metal chelation peptides and its application. The number of six metal chelate peptides is isolated from the trypsin hydrolyzate of sesame, [Citation69] which are Ser-Met, Leu-Ala-Asn, Ile-Ala-Asn, Arg-Lys-Arg, Arg-Gln-Arg, Asn-Cys-Ser, respectively. Subsequently, three metal chelate peptides of Ser-Met, Leu-Ala-Asn and Asn-Cys-Ser are synthesized, and then the metal chelation ability of the three synthetic peptides is determined. Among them, Asn-Cys-Ser shows the best chelating ability of zinc and iron, even higher than that of L-Glutathione (GSH).
It holds great superiority for sesame metal chelate peptide, mainly due to its small equipment investments, easy industrialization, inexpensive cost, and most importantly can also be added as a nutrition fortifier to food or animal feed. For example, C. Wang et al. Zn2+ express strong bonding ability with carboxyl and amino group in the sesame peptide, the hydroxyl group of Ser and the sulfur group of Cys .[Citation16,Citation69] At the same time, the carbonyl or the amide group in the peptide bond with the oxygen element in the water regularly participating in the coordination with Zn2+ through weak interaction. Since the characteristics of easy absorption in the body of peptides, this study precisely confirms that sesame active peptide can form a chelate with Fe2+ and Zn2+, which can be applied to food of patients with iron deficiency anemia and zinc deficiency. Unfortunately, sesame peptides are not found to react with metallic elements such as calcium, copper, and selenium so far. This gap will be another research guide for sesame peptides in the future.
Conclusion
This review summarizes the research on the method of using sesame oil industrial processing by-products to produce sesame peptide and its biological functional activities, with an emphasis on the overview of the functional value of sesame peptide for human health. Studies have shown that the research on the preparation method and functional value of sesame peptides has made great progress, but it is far inferior to the research on active peptides derived from the seeds of similar plants such as soybean peptides and corn peptides. Most of them have only obtained certain functions mixture. There are relatively fewer studies on further purification and structural identification of sesame peptides, so the separation, purification and structural identification of sesame peptides are still the focus of future research. In addition, no related reports on the functional fields of sesame peptides such as human immunity and nerve activity have been found. Therefore, research and development of sesame active peptides still require a lot of work.
Highlights
1. Sesame oil byproducts are promising sources of bioactive peptides.
2. It has not been reported whether different oil production methods have any effect on the peptides in sesame.
3. Sesame-derived antioxidant and ACE inhibitory peptides have been investigated, but lack of animal experiments and identification.
4. Sesame-derived Bacteriostatic activities, thrombolysis and metal chelate peptides just have been simple research.
Additional information
Funding
References
- Bedigian, D.;. History and Lore of Sesame in Southwest Asia[J]. Econ. Bot. 2004, 58, 329–353. DOI: 10.1663/0013-0001(2004)058.
- Were, B. A.; Onkware, A. O.; Gudu, S.; Welander, M.; Carlsson, A. S. Seed Oil Content and Fatty Acid Composition in East African Sesame (Sesamum Indicum L.) Accessions Evaluated over 3 years[J]. Field Crops Res. 2006, 97, 254–260. DOI: 10.1016/j.fcr.2005.10.009.
- Yin, W.; Washington, M.; Yang, X.; Lu, A.; Ma, X.; Rui, S.; Wang, X.; Zhao, R. Consumer Acceptability and Sensory Profiling of Sesame Oils Obtained from Different processes[J]. Grain Oil Sci. Technol. 2020. DOI: 10.1016/j.gaost.2020.04.001.
- Alpaslan, M.; Hayta, M. Rheological and Sensory Properties of Pekmez (Grape Molasses)/tahin (Sesame Paste) blends[J]. J. Food Eng. 2002, 54, 89–93. DOI: 10.1016/s0260-8774(01)00197-2.
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of Protein Oxidation and Enhancement of Gel Properties of Silver Carp (Hypophthalmichthys Molitrix) Surimi by Addition of Protein Hydrolysates Derived from Surimi Processing by-products[J]. Food Chem. 2020, 316, 126343. DOI: 10.1016/j.foodchem.2020.126343.
- Orruño, E.; Morgan, M. R. A. Purification and Characterisation of the 7S Globulin Storage Protein from Sesame (Sesamum Indicum L.) [J]. Food Chem. 2007, 100, 926–934. DOI: 10.1016/j.foodchem.2005.10.051.
- Shu, Z.; Liu, L.; Geng, P.; Liu, J.; Shen, W.; Tu, M. Sesame Cake Hydrolysates Improved Spatial Learning and Memory of mice[J]. Food Biosci. 2019, 31, 100440. DOI: 10.1016/j.fbio.2019.100440.
- FAO. Food and Agriculture Organization Corporate Statistical Database, 2020 FAO. Food and Agriculture Organization Corporate Statistical Database (2018). http://www.fao.org/faostat/en/#data/QC/ Accessed 14 May 2020.
- Chatterjee, R.; Dey, T. K.; Ghosh, M.; Dhar, P. Enzymatic Modification of Sesame Seed Protein, Sourced from Waste Resource for Nutraceutical application[J]. Food Bioprod. Process. 2015, 94, 70–81. DOI: 10.1016/j.fbp.2015.01.007.
- Boloorforooshan, M.; Markakis, P. Markakis Protein Supplementation of Navy Beans with sesame[J]. J. Food Sci. 1979, 44, 390–392. DOI: 10.1111/j.1365-2621.1979.tb03795.x.
- Abeyrathne, E. D. N. S.; Huang, X.; Ahn, D. U. Antioxidant, Angiotensin-converting Enzyme Inhibitory Activity and Other Functional Properties of Egg White Proteins and Their Derived Peptides – A review[J]. Poultr. Sci. 2018, 97, 1462–1468. DOI: 10.3382/ps/pex399.
- FitzGerald, R. J.; Cermeño, M.; Khalesi, M.; Kleekayai, T.; Amigo-Benavent, M. Application of in Silico Approaches for the Generation of Milk Protein-derived Bioactive peptides[J]. J. Funct. Foods. 2020, 64, 103636. DOI: 10.1016/j.jff.2019.103636.
- Wang, G.; Zhou, G.; Ren, H.; Xu, Y.; Yang, Y.; Guo, L.; Liu, N. Peptide Biomarkers Identified by LC–MS in Processed Meats of Five Animal species[J]. J. Food Compost. Anal. 2018, 73, 47–54. DOI: 10.1016/j.jfca.2018.07.004.
- Korhonen, H.; Pihlanto, A. Food-derived Bioactive Peptides-opportunities for Designing Future foods[J]. Curr. Pharm. Des. 2003, 9, 1297–1308. DOI: 10.2174/1381612033454892.
- Hartmann, R.; Meisel, H. Food-derived Peptides with Biological Activity: From Research to Food applications[J]. Curr. Opin. Biotechnol. 2007, 18, 163–169. DOI: 10.1016/j.copbio.2007.01.013.
- Wang, C.; Wang, C.; Li, B.; Li, H. Zn (II) Chelating with Peptides Found in Sesame Protein Hydrolysates: Identification of the Binding Sites of complexes[J]. Food Chem. 2014, 165, 594–602. DOI: 10.1016/j.foodchem.2014.05.146.
- Yang, Q.; Cai, X.; Huang, M.; Wang, S. A Specific Peptide with Immunomodulatory Activity from Pseudostellaria Heterophylla and the Action mechanism[J]. J. Funct. Foods. 2020, 68, 103887. DOI: 10.1016/j.jff.2020.
- Das, R.; Dutta, A.; Bhattacharjee, C. Preparation of Sesame Peptide and Evaluation of Antibacterial Activity on Typical pathogens[J]. Food Chem. 2012, 131, 1504–1509. DOI: 10.1016/j.foodchem.2011.09.136.
- Wong, F.; Xiao, J.; Wang, S.; Ee, K.; Chai, T. Advances on the Antioxidant Peptides from Edible Plant sources[J]. Trends Food Sci. Technol. 2020, 99, 44–57. DOI: 10.1016/j.tifs.2020.02.012.
- Yang, M.; Cui, G.; Zhao, M.; Wang, C.; Wang, L.; Liu, H.; Peng, S. The Effect of Complexation of Cu (II) with P6A Peptide and Its Analogs on Their Thrombolytic activities[J]. Int. J. Pharmaceutics. 2008, 362, 81–87. DOI: 10.1016/j.ijpharm.2008.06.014.
- Jiang, X.; Pan, D.; Zhang, T.; Liu, C.; Zhang, J.; Su, M.; Wu, Z.; Zeng, X.; Sun, Y.; Guo, Y. Novel Milk Casein–derived Peptides Decrease Cholesterol Micellar Solubility and Cholesterol Intestinal Absorption in Caco-2 cells[J]. J. Dairy Sci. 2020, 103, 3924–3936. DOI: 10.3168/jds.2019-17586.
- Zhao, Y.; Zhang, L.; Tao, J.; Chi, C.; Wang, B. Eight Antihypertensive Peptides from the Protein Hydrolysate of Antarctic Krill (Euphausia Superba): Isolation, Identification, and Activity Evaluation on Human Umbilical Vein Endothelial Cells (Huvecs) [J]. Food Res. Int. 2019, 121, 197–204. DOI: 10.1016/j.foodres.2019.03.035.
- Aguilar-Toalá, J. E.; Hernández-Mendoza, A.; González-Córdova, A. F.; Vallejo-Cordoba, B.; Liceaga, A. M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin products[J]. Peptides. 2019, 122, 170170. DOI: 10.1016/j.peptides.2019.170170.
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and Prospects of Bioinformatics Analysis for Studying Bioactive Peptides from Food-derived Protein: Sequence, Structure, and functions[J]. Trends Anal. Chem. 2018, 105, 7–17. DOI: 10.1016/j.trac.2018.04.005.
- Pang, G.; Chen, Q.; Hu, Z.; Xie, J. Bioactive Peptides: Absorption, Utilization and Functionality[J]. Chin. J. Food Sci. 2013, 9, 375–391.
- Bayliss, W. M.; Starling, E.; Bayliss, W. M.; Starling, E. Preliminary Communication on the Causation of the So-called\”peripheral Reflex Secretion\” of the pancreas[J]. Lancet. 1902, 4099, 813. DOI: 10.1016/S0140-6736(01).
- de Castro, R. J. S.; Sato, H. H. Biologically Active Peptides: Processes for Their Generation, Purification and Identification and Applications as Natural Additives in the Food and Pharmaceutical industries[J]. Food Res. Int. 2015, 74, 185–198. DOI: 10.1016/j.foodres.2015.05.013.
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M. C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant Peptides Released from Gastrointestinal Digestion of “Stracchino” Soft Cheese: Characterization, in Vitro Intestinal Protection and bioavailability[J]. J. Funct. Foods. 2016, 26, 494–505. DOI: 10.1016/j.jff.2016.08.021.
- Benkhelifa, H.; Bengoa, C.; Larre, C.; Guibal, E.; Popineau, Y.; Legrand, J. Casein Hydrolysis by Immobilized Enzymes in a Torus reactor[J]. Process Biochem. 2005, 40, 461–467. DOI: 10.1016/j.procbio.2004.01.022.
- Guo, Y.; Jiang, X.; Xiong, B.; Zhang, T.; Zeng, X.; Wu, Z.; Sun, Y.; Pan, D. Production and Transepithelial Transportation of angiotensin-I-converting Enzyme (Ace)-inhibitory Peptides from Whey Protein Hydrolyzed by Immobilized Lactobacillus Helveticus proteinase[J]. J. Dairy Sci. 2019, 102, 961–975. DOI: 10.3168/jds.2018-14899.
- Li, F.; Chen, N.; Zhang, Z. The Optimum Choice of Enzyme and the Determination of the Hydrolysiscondition of Sesame peptide[J]. Chin. J. Sci. Technol. Cereals, Oils Foods. 2007, 2, 50–51. DOI: 10.16210/j.
- Hou, L.; Liu, Y.; Wang, X.; Yu, X.; Li, Y.; Shen, Z. Optimization of the Preparation of Zinc (Zn2+) Chelating Peptides from Cold-pressed Sesame Protein hydrolysates[J]. Chin. J. Sci. Technol. Cereals Oils Foods. 2014, 6, 83–87. DOI: 10.16210/j.cnki.1007-7561.2014.06.008.
- Zhao, S.; Zhang, Y.; Yang, C.; Wei, M.; Wang, T. Preparation of Peptide from Sesame Meal by Enzymatic hydrolysis[J]. China Oils Fats. 2012, 11, 28–31.
- Xiao, Y.; Duan, Y.; Ding, H. Research on Hydrolyze Condition of Sesame Albumen Enzyme[J]. Chin. J. Acad. Period. Farm Products Process. 2009, 6, 59–63.
- Lu, X.; Jiang, M.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Screening of Protease for Preparing Antioxidant Peptide from Sesame protein[J]. China Oils Fats. 2018, 11, 28–33.
- Latif, S.; Anwar, F. Aqueous Enzymatic Sesame Oil and Protein extraction[J]. Food Chem. 2011, 125, 679–684. DOI: 10.1016/j.foodchem.2010.09.064.
- Tang, Z.; Li, K.; Peng, M.; Li, Z. Study on the Preparation of Sesame Peptides by Enzymatic Hydrolysis of Sesame meal[J]. Chin. J. Grain Oil Technol. Econ. 2013, 2, 48–51.
- Chen, Y.; Wang, W.; Shen, Z.; Yu, D. Study on the Compound Hydrolytic Process if Sesame proteins[J]. Chin. J. Food Res. Dev. 2006, 9, 17–20.
- Wang, S.; Zhang, C.; Qi, B.; Sui, X.; Jiang, L.; Li, Y.; Wang, Z.; Feng, H.; Wang, R.; Zhang, Q. Immobilized Alcalase Alkaline Protease on the Magnetic Chitosan Nanoparticles Used for Soy Protein Isolate hydrolysis[J]. Eur. Food Res. Technol. 2014, 239, 1051–1059. DOI: 10.1007/s00217-014-2301-1.
- Gao, M.; Miao, J.; Cao, Z.; Dong, Y.; Lu, Z. Study on Preparation of Complex Amino Acids by Hydrolyzing Sesame Protein Using Co-immobilized Double enzymes[J]. J. Yangzhou Univ. (Agricultural and Life Science Edition). 2008, 4, 95–98. DOI: 10.16872/j.cnki.1671-4652.2008.04.023.
- Liang, Y.; Lu, X.; Huang, J.; Xie, X. Study on Preparation and Enzymatic Properties of Immobilized Alcalase Alkaline Protease Magnetic Chitosan microspheres[J]. Chin. J. Food Fermentat. Ind. 2014, 8, 66–71. DOI: 10.13995/j.cnki.11-1802/ts.2014.08.013.
- Dong, Y.; Zheng, W. Study on the Extraction Technology of from Sesame Cake fermentation[J]. Chin. J. Food Res. Dev. 2007, 4, 20–23.
- Chen, X.; Qin, W.; Ma, L.; Wang, Y. Study on the Production of Sesame Protein Peptides with Microorganism fermentation[J]. Chin. J. Sci. Technol. Food Ind. 2010, 6, 237–238+241. DOI: 10.13386/j.1002-0306.2010.06.003.
- Qian, S.; Zhao, S.; Wei, M.; Xue, Z.; Wang, J. Optimization of Fermentation Conditions of Producing Polypeptide from Sesame Meal by Response Surface methodology[J]. China Oils Fats. 2013, 1, 20–23.
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant Activity and Sensory Characteristics of Maillard Reaction Products Derived from Different Peptide Fractions of Soybean Meal hydrolysate[J]. Food Chem. 2018, 243, 249–257. DOI: 10.1016/j.foodchem.2017.09.139.
- Makino, N.; Maeda, T.; Oyama, J.; Sasaki, M.; Higuchi, Y.; Mimori, K.; Shimizu, T. Antioxidant Therapy Attenuates Myocardial Telomerase Activity Reduction in Superoxide Dismutase-deficient mice[J]. J. Mol. Cell. Cardiol. 2011, 50, 670–677. DOI: 10.1016/j.yjmcc.2010.12.014.
- Esfandi, R.; Walters, M. E.; Tsopmo, A. Antioxidant Properties and Potential Mechanisms of Hydrolyzed Proteins and Peptides from cereals[J]. Heliyon. 2019, 4, e01538. DOI: 10.1016/j.heliyon.2019.e01538.
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A Specific Antioxidant Peptide: Its Properties in Controlling Oxidation and Possible Action mechanism[J]. Food Chem. 2020, 327, 126984. DOI: 10.1016/j.foodchem.2020.126984.
- Shao, Y.; Dong, Y.; Yang, J. Preparation of Sesame Peptides and Their Antioxidant Activities[J]. Jiangsu J. Agric. Sci. 2009, 4, 900–904.
- Ghribi, A. M.; Sila, A.; Przybylski, R.; Nedjar-Arroume, N.; Makhlouf, I.; Blecker, C.; Attia, H.; Dhulster, P.; Bougatef, A.; Besbes, S. Purification and Identification of Novel Antioxidant Peptides from Enzymatic Hydrolysate of Chickpea (Cicer Arietinum L.) Protein concentrate[J]. J. Funct. Foods. 2015, 12, 516–525. DOI: 10.1016/j.jff.2014.12.011.
- Fazhi, X.; Huihui, P.; Yang, L.; Lumu, L.; Kun, Q.; Xioling, D. Separation and Purification of Small Peptides from Fermented Sesame Meal and Their Antioxidant Activities[J]. Protein Pept. Lett. 2014, 21, 966–974. DOI: 10.2174/0929866521666140411113021.
- Li, G.; Ding, X. Protease Hydrolysis of Sesame Protein and Hydrolysate Antioxidation[J]. J. Chin. Cereal. Oils Assoc. 2006, 1, 104–108.
- Chen, X.; Liu, A.; Chen, X.; Lan, J. Optimization of Enzymatic Preparation of Antioxidant Black Sesame Protein Hydrolysate[J]. Chin. J. Food Sci. 2010, 21, 85–88.
- Zhao, Y.; Xu, C. Structure and Function of Angiotensin Converting Enzyme and Its Inhibitors[J]. Chin. J. Biotechnol. 2008, 24, 171–176. DOI: 10.13345/j.cjb.2008.02.027.
- Campbell, D. J.;. The Renin–angiotensin and the Kallikrein–kinin systems[J]. Int. J. Biochem. Cell Biol. Renin-Angiotensin Syst. 2003, 35, 784–791. DOI: 10.1016/S1357-2725(02).
- Erdös, E. G.;. The ACE and I: How ACE Inhibitors Came to be[J]. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1034–1038.
- Yan, W.; Lin, G.; Zhang, R.; Liang, Z.; Wu, L.; Wu, W. Studies on Molecular Mechanism between ACE and Inhibitory Peptides in Different Bioactivities by 3D-QSAR and MD simulations[J]. J. Mol. Liq. 2020, 304, 112702. DOI: 10.1016/j.molliq.2020.112702.
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a Novel ACE-inhibitory Peptide from Casein and Evaluation of the Inhibitory mechanisms[J]. Food Chem. 2018, 256, 98–104. DOI: 10.1016/j.foodchem.2018.02.107.
- Wohlfart, P.; Wiemer, G. Interactions between the Renin-Angiotensin and the Kallikrein-Kinin System[M]Angiotensin. Vol. II. Springer Berlin Heidelberg. 2004, 359–373. https://doi.org/10.1007/978-3-642-18497-0_16
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive Effect of Angiotensin I-Converting Enzyme Inhibitory Peptides from a Sesame Protein Hydrolysate in Spontaneously Hypertensive Rats[J]. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. DOI: 10.1271/bbb.70.1118.
- Iino, T.; Ogura, K.; Asami, S. (2010). Angiotensin-converting enzyme inhibitory peptides.
- Wang, Z.; Pei, J.; Yan, J.; Ma, H.; Wang, L.; Jiang, M. Effects of Ultrafiltration on ACE-Inhibitory and Antioxidant Activities of Sesame Protein Hydrolysates[J]. J. Chin. Cereal. Oils Assoc. 2015, 8, 58–63.
- Costa, F. T.; Neto, S. M.; Bloch, C.; Franco, O. L. Susceptibility of Human Pathogenic Bacteria to Antimicrobial Peptides from Sesame Kernels[J]. Curr. Microbiol. 2007, 55, 162–166. DOI: 10.1007/s00284-007-0131-0.
- Liu, B.; Chiang, P. Production of Hydrolysate with Antioxidative Activity and Functional Properties by Enzymatic Hydrolysis of Defatted Sesame (Sesamum Indicum L.) [J]. Int. J. Appl. Sci. Eng. 2008, 6, 73–83. DOI: 10.1023/B:JTAN.0000046110.24212.bb.
- Barker, P. L.; Webb, R. R. Antiplatelet and Antithrombotic Agents: From Viper Venom Proteins, to Peptides and Peptidomimetics, to Small Organic molecules[J]. Adv. Med. Chem. 1995, 3, 57–111. DOI: 10.1016/S1067-5698(06)80004-0.
- Harrison, L. M.; Córdova, J. L.; Cappello, M. Ancylostoma Caninum Anticoagulant Peptide-5: Immunolocalization and in Vitro Neutralization of a Major Hookworm anti-thrombotic[J]. Mol. Biochem. Parasitol. 2001, 115, 101–107. DOI: 10.1016/S0166-6851(01)00276-6.
- Lee, J.; Park, J.; Yeom, J.; Han, E. H.; Lim, Y.-H. Inhibitory Effect of Bee Venom on Blood Coagulation via Anti-serine Protease activity[J]. J. Asia-Pacific Entomol. 2017, 20, 599–604. DOI: 10.1016/j.aspen.2017.03.023.
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of Iron-chelating Peptides from Pacific Cod Skin Gelatin and the Possible Binding mode[J]. J. Funct. Foods. 2017, 35, 418–427. DOI: 10.1016/j.jff.2017.06.013.
- Wang, C.; Li, B.; Ao, J. Separation and Identification of Zinc-chelating Peptides from Sesame Protein Hydrolysate Using IMAC-Zn2+ and LC–MS/MS[J]. Food Chem. 2012, 134, 1231–1238. DOI: 10.1016/j.foodchem.2012.02.204.