ABSTRACT
This study was aimed to investigate volatile compounds of white and brown teff grain samples using the hydro-distillation and its extract in dichloromethane and n-hexane by gas chromatography-mass spectrometry. The optimized time for the hydro-distillation of the white and brown teff grain samples was nine hours. The dominant volatile compounds identified in the white and brown teff grain samples of the dichloromethane extract corresponding to peak area proportion were furfural (29.9%, 38.1%) and 5-methyl-2-furancarboxaldehyde (15.1%, 23.8%), respectively. The relative amount of dominant volatile compounds determined in the white teff grain sample of the n-hexane extract were 10.2% and 7.27% for the 5-methyl-2-furancarboxaldehyde and 2-[(methylthio)methyl]furan, respectively, and 18.4%, 16.0%, 12.5% and 9.73%, for the furfural, 5-methyl-2-furancarboxaldehyde, 2-[(methylthio)methyl]furan and benzeneacetaldehyde, respectively, for the brown teff grain sample. This study revealed that dichloromethane was efficient to extract more volatile compounds in the white teff grain sample than in the brown teff grain sample, whereas n-hexane was efficient to extract almost equal number of volatiles in both white and brown teff samples. Besides, aldehydes were the major constituents of the white and brown teff grain samples in both dichloromethane and n-hexane extracts.
Introduction
Cereals are the major staple foods which supply large quantity of energy, carbohydrates, protein, micronutrients and fiber to the human diet.[Citation1] Nutritional quality of food is the most important parameter for maintaining human health and complete physical well-being since nutritional well-being is the driving force for development and maximization of human genetic potential.[Citation2] In recent times, the term “super grain” has become popular and many researchers appreciated whole grain foods as the source of nutrition. Several medical and epidemiological studies steadily reported that higher consumptions of whole grain are firmly connected with reduced risk of acute and chronic diseases, including type 2 diabetes, cardiovascular disease and certain cancers, namely colorectal cancer.[Citation3]
Teff [Eragrostis tef (Zuccagni) Trotter] is a self-pollinated, annual and tropical principal cereal belonging to the family of Poaceae, subfamily Eragrostoidae, tribe Eragrosteae, and genus Eragrostis, where believed to have grown and originated in Ethiopia.[Citation4,Citation5] Teff grain is one of the smallest cereals being oval-shaped and comprised of germ, pericarp and endosperm layers.[Citation6] The color of teff can vary from white (ivory) to dark brown (black) depending on the variety.[Citation2] In Ethiopia, teff is cultivated on more than 2.7 million hectares, accounting for 31% of total cereals and 21% of gross cereal production. Teff grain is a staple for more than 50 million Ethiopian and used to make injera (fermented flatbread), porridge, and kitta/chapatti, as well as white bread, brown bread, gluten-free “sprits” (Dutch shortcake cookie), gluten-free (sponge) cake, gluten-free kanos (Dutch almond fingers), and Dutch almond tartlets, pancakes, and gluten-free portugeesjes (Dutch frangipane cakes).[Citation7]
Teff is an endemic cereal but recently appreciated and used by many people throughout the world due to its excellent nutritional value as grain for human consumption without fortification and as forage for livestock. Teff tolerates anoxic situations better than maize, wheat and sorghum and is resistant to many pests and diseases,[Citation2,Citation8,Citation9] and has the potential of growing in every part of the world.[Citation2] Teff is leading all cereal grains by a wide margin having a high concentration of a variety of nutrients like minerals, amino acids, dietary fibers, proteins, dietary polyphenols, lipids, starch, carbohydrates and vitamins.[Citation5,Citation10,Citation11] It contains all essential amino acids that make it comparable to egg.[Citation2] Food with a balanced nutritional composition is easily absorbed by human body and all cells require these substances in appropriate amounts to maintain their homeostasis foods containing the so called chemo-preventive agents which do have the potential to increase life quality and expectancy.[Citation12]
Nowadays, there has been increasing interest and global demand of teff throughout the world due to its perceived better nutritional quality which plays an important role in the food security compared to other grains.[Citation3,Citation13,Citation14] The flour of teff is whole grain because of the very small grain size, a source of bioactive compounds such as polyphenols and is rich in fiber due to the incorporate of the bran components.[Citation7] The teff grain is gluten-free and has great potential to be formulated into a range of food/beverage products to aid people with celiac disease.[Citation4] Celiac disease (CD) is a genetically based autoimmune condition that results in permanent sensitivity to the protein gluten.[Citation15] It is characterized by chronic inflammation of the intestinal mucosa, atrophy of intestinal villi and several clinical manifestations. Its prevalence has increased in recent years.
Due to the unique chemical composition, higher amount of fiber content and gluten-free property, teff is getting acceptance as medicinal ingredient and a range of health benefits have been associated with it.[Citation2,Citation4,Citation16] For instance, teff showed in vitro anti-oxidative activities, and can improve the hemoglobin level in human body because it has high levels of iron and help to prevent malaria and incidence of anemia; it has a lower glycemic index (GI) and can avoid diabetes.[Citation4,Citation16] Not only this but also, teff does have longer shelf life and slow staling of its bread products compared to wheat, sorghum, rice, barley and maize.[Citation14] Because of such health effects and attractive nutrients, many efforts have been made in the laboratories to produce teff-based food/beverage products in recent years.[Citation17,Citation18]
Teff accounts for about two-third of the daily protein intake in the diet of Ethiopian population and has recently got a global attention particularly as a “healthy food” due its risk lowering of various diseases and exerting health-promoting effects.[Citation3] In comparison to widely consumed cereals like rice, wheat, corn and sorghum, the dietary composition and health benefits of teff are less studied. However, in connection to its medicinal values, interests are growing in many countries to utilize teff for the production of gluten-free foods.[Citation19]
The volatile profiles of most widely consumed cereals have been studied.[Citation1,Citation20–26] However, to the best of the researchers’ knowledge, no information is available regarding volatile compounds of teff grain [Eragrostis tef (Zuccagni) Trotter] varieties. Hence, the objectives of this study were (i) to optimize the appropriate hydro-distillation time of the white and brown teff grain samples using Clevenger apparatus, (ii) to identify and quantify the volatile compounds present in the white and brown teff grain samples using GC-MS method, and (iii) to investigate the extraction efficiency of dichloromethane and n-hexane solvents toward the volatile compounds present in the white and brown teff grain samples.
Materials and methods
Apparatus and instrument
An electronic balance (ARA520, China), Clevenger apparatus, hot plate ceramic magnetic stirrer (JIJE, Private Limited Company), Agilent gas chromatograph equipped with a mass spectrometer detector and Agilent automatic injector spectrometer (Agilent Technologies, 7890A GC-MS, USA) were used.
Chemicals
Dichloromethane (>99%, Fisher Scientific, UK) and n-hexane (>99%, Fisher Scientific, UK) GC/MS grade were used for the extraction of volatile compounds after hydro-distillation of white and brown teff grains. Anhydrous sodium sulfate (Techno, Pharmachem, Bahadurgarh, India) was used for drying the dichloromethane and n-hexane extract.
Sample collection
White and brown varieties of teff samples were collected from Amhara Region, North Shewa Zone, Minjar Shenkora District, Ethiopia, because of its higher production, during December 2020 from the local market. About 3 kg of each teff sample was collected from the local market and stored in paper bags under room temperature conditions.
Extraction of volatile compounds by hydro-distillation
The volatile compounds were extracted from the teff grain by hydro-distillation according to the procedure of Chughtai et al.[Citation1] with some modifications. Sample of teff grain (200 g) was thoroughly washed three times by tap water followed by distilled water and transferred into 2 L round bottom flask with 1 L of distilled water. The mixtures were distilled for 5, 7 and 9 hours by applying magnetic stirrer throughout, at atmospheric pressure to optimize the time. The distillate was transferred to 100 mL of separatory funnel, 4 mL of dichloromethane was added, shaken properly for 5 min and the organic phase was separated. The same procedure was also used for the extraction in n-hexane. Finally, the extract was dried over anhydrous Na2SO4 to remove residual water content, and the dried extract was transferred into a 1.5 mL glass vial for gas chromatography-mass spectrometry (GC-MS) analysis.
It should be noted that the hydro-distillation using the teff flour was attempted. However, it resulted in too much bubbling and went up to receiver and condenser. Therefore, the whole grain was used for hydro-distillation. The grain has very small size and has no bran which resulted in successful hydro-distillation.
Analysis of volatile compounds
The GC-MS analysis was carried out using Agilent technologies 7820A GC and 5977E MSD system equipped with auto-sampler. Chromatographic separations were conducted using DB1701 capillary column with 30 m length, 0.25 mm internal diameter and 0.25 μm column phase thickness. Injection mode was split-less with helium as carrier gas and 1 μL of the sample was injected at a constant flow rate of 1 mL/min to the inlet heated to 275°C. Initial oven temperature was 60°C with 2 min hold time then heated to 200°C with ramp of 10°C/min and 3°C/min to 240°C. Conditions used for the mass spectrometer were a temperature of 230°C for the ion source and 150°C for quadruple, scan range 40–650 m/z, operating in positive electron impact mode with ionization energy of 70 eV. Chromatographic and mass spectral data were processed by the instrument built in software (MS Mass Hunter; Agilent Technologies, USA). The volatile components were identified from the chromatograph library (NIST-14) and by the MS fragmentation pattern of the authentic chemicals. The GC-MS analysis was run in triplicate.
Results and discussion
Tentative identification and quantification of volatile compounds of teff grain
The volatile compounds from the white and brown teff varieties were, firstly, extracted using the Clevenger apparatus (hydro-distillation) followed by further extraction using dichloromethane and n-hexane, separately, and subjected to GC-MS analysis. The distillation time was conducted at 5, 7, and 9 hours and the optimum time for distillation was 9 hours since the number of volatile compounds were increased as the distillation time increased. Longer distillation time was not studied since it was not convenient. Tentative identification of individual volatile constituents was based on comparison of mass spectra of the sample with those present in the National Institute of Standards and Technology (NIST-14) mass spectral data library found in the GC-MS database and the individual volatile compound concentration expressed as percent peak was relative to the total peak area from GC-MS analysis of the sample. Accordingly, a total of 16 volatile compounds in the white teff sample and 3 volatile compounds in the brown teff sample were identified in the dichloromethane extract. While a total of 10 volatile compounds in the white teff sample and 11 volatile compounds in the brown teff sample were identified in the n-hexane extract. The chromatograms are presented in and the data are summarized in for the white and brown teff samples in the dichloromethane extract, respectively. The chromatograms of the n-hexane extract are presented in and the data are summarized in for the white and brown teff samples, respectively.
Table 1. The peak number, name of volatile compounds, their chemical class, retention time (RT) and percentage peak area (mean±SD, n = 3) of the identified compounds in the white teff grain sample by GC-MS method from the dichloromethane extract
Table 2. The peak number, name of volatile compounds, their chemical class, retention time (RT) and percentage peak area (mean±SD, n = 3) of the identified compounds in the brown teff sample by GC-MS method from the dichloromethane extract
Table 3. The peak number, name of volatile compounds, their chemical class, retention time (RT), percentage peak area (mean±SD, n = 3) and matching of the identified compounds in the white teff grain samples by GC-MS method from the n-hexane extract
Table 4. The peak number, name of volatile compounds, their chemical class, retention time (RT), percentage peak area (mean±SD, n = 3) and matching of the identified compounds in the brown teff grain sample by GC-MS method from the n-hexane extract
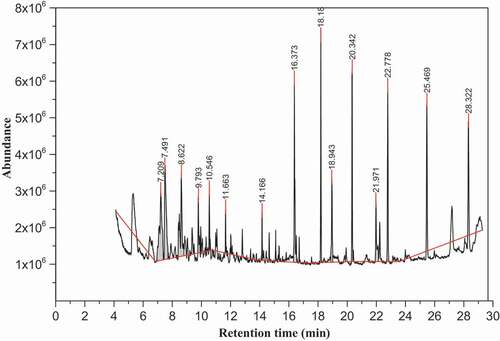
Figure 1. The chromatogram of volatile compounds of white teff grain sample in dichloromethane extract
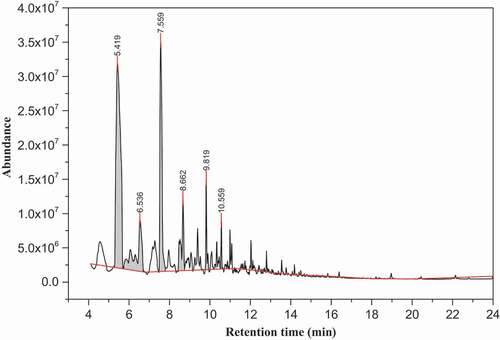
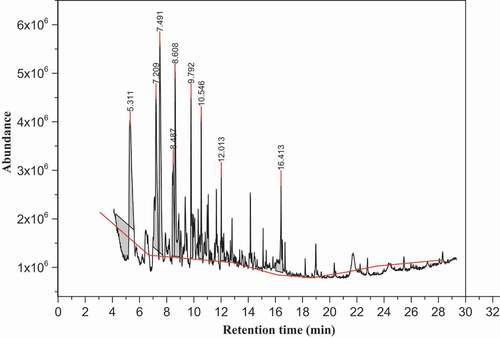
Figure 2. The chromatogram of volatile compounds of brown teff grain sample in dichloromethane extract
The chromatogram in shows the peak profile of volatile compounds in the white teff grain sample from the dichloromethane extract determined by GC-MS method. As can be seen in , sixteen volatiles having ≥80% matching with the NIST-14 database were identified and the compounds were eluted in the range of 5.45 min up to 14.5 min in the white teff sample. The most intense compounds were furfural and 5-methyl-2-furancarboxaldehyde. revealed the peak number, name of volatile compounds, their chemical class, retention time (RT), percentage peak area and matching of the identified compounds in the white teff sample by GC-MS method in the dichloromethane extract. The volatile compounds presented in were selected based on the NIST-14 matching quality showing ≥80%.
indicated that the identified volatile compounds in the white teff grain sample of the dichloromethane extract includes a class of aldehydes, ketones, alcohols, hydrocarbons, pyrroles, indoles, esters and terpene with high quality of NIST-14 matching ≥80% in the GC-MS database. Among these, the predominant compounds corresponding to peak area proportion were furfural (29.9%) and 5-methyl-2-furancarboxaldehyde (15.1%) at retention time of 5.42 min and 7.56 min, respectively, followed by 1-(2-furanyl)ethanone (5.11%) and benzeneacetaldehyde (3.16%) at retention time of 6.54 min and 8.66 min, respectively. From the data in , it was possible to conclude that aldehydes were the major constituents of the white teff grain sample volatiles in the dichloromethane extract followed by ketones and alcohols and lower content of furans, pyrrole, indoles and ether, respectively.
Cramer et al.[Citation20] stated that aldehydes, alcohols and ketones are the common lipid oxidation products of cereal grains. According to Liu et al.,[Citation21] aldehydes are typically obtained from the autoxidation and enzymolysis oxidation of the carbon-carbon double bond of unsaturated fatty acids found in cereals and have a significant effect on the fragrance of a cereal product due to their low odor threshold values. It has been suggested that ketones are formed by the autoxidation of fatty acids, especially unsaturated fatty acids. Ketones are responsible for the soapy and fruity flavors used in food. Furthermore, alcohols are commonly produced by the decomposition of fatty acid secondary hydroperoxides. They usually contribute the fruity, floral, and grassy flavors to cereal. In addition, hydrocarbons could be formed as a consequence of the decarboxylation and breaking of higher fatty acid carbon-carbon chains.
Chai et al.[Citation22] have identified a total of 92 volatile compounds in wheat flour samples, of which benzeneacetaldehyde, furfural, 2-furanmethanol, 1-(2-furanyl)ethanone were among the reported compounds which were also identified in this study. Besides, Gao et al.[Citation23] have identified about 65 volatiles in rice bran and 2-furanmethanol, furfural, 2-methoxy-4-vinylphenol and 5-methyl-2-furancarboxaldehyde were among the compounds. The main characteristic odor compound of rice bran was 2-methoxy-4-vinylphenol and was detected in this study. On top of this, the study conducted by Cramer et al.[Citation20] indicated that barley flour exhibits a higher content of aldehydes, alcohols, and furans and a lower content of ketones. The present study has revealed higher content of aldehydes which is in agreement to the Cramer et al.[Citation20] but lower amount of furans. Liu et al.,[Citation21] has reported volatile compounds in millet bran with their relative concentrations like furfural (0.05%), 2-furanmethanol (0.13%), 1-(2-furanyl)ethanone (0.27%) and benzeneacetaldehyde (2.03%) which have been identified as odor active compounds in some cereals and derived products and are lower in content compared with the present study. Besides, Hinge et al.[Citation24] has identified 1 H-indole and 2-methoxy-4-vinylphenol volatiles in rice.
revealed the chromatogram peak profile of volatile compounds in the brown teff sample from the dichloromethane extract determined by GC-MS method. As can be seen in , only three compounds mainly furfural, 5-methyl-2-furancarboxaldehyde and benzeneacetaldehyde at the retention time of 5.41 min, 7.55 min and 8.65 min were identified by GC-MS method and the most intense compounds were furfural and 5-methyl-2-furancarboxaldehyde. As shown in , only aldehydes were identified in the brown teff sample and the relative contents of dominant volatile compounds determined were 38.1% and 23.8% for the furfural and 5-methyl-2-furancarboxaldehyde, respectively. Compared with the white teff sample of the dichloromethane extract, the numbers of volatile compounds identified in the brown teff sample were very few. This fact could be used to differentiate the two varieties of the teff samples. This might confirm that the dichloromethane was not suitable and efficient to extract all the volatile compounds present in the brown teff sample. Furfural and 5-methyl-2-furancarboxaldehyde which were the main volatiles of the brown teff sample from the dichloromethane extract were identified from the rice bran as reported by Gao et al.[Citation23] and benzeneacetaldehyde from the wheat flour as conducted by Chai et al.[Citation22]
showed the chromatogram peak profile of volatile compounds of white teff sample from the n-hexane extract determined by GC-MS method. The volatile compounds which have revealed a NIST-14 matching ≥80% were eluted in the range of 7.22 min up to 27.2 min in the white teff sample and the most intense compounds were 5-methyl-2-furancarboxaldehyde (7.49 min), methylhexadecanoate (18.9 min), benzeneacetaldehyde (8.62 min), 1-(2-furanylmethyl)-1 H-pyrrole (10.55 min), 2-[(methylthio)methyl]furan (7.21 min) and (E)-5-octadecene (14.17 min).
As can be seen in , the relative amount of dominant volatile compounds determined in the white teff sample in the n-hexane extract were 10.2% and 7.27% for the 5-methyl-2-furancarboxaldehyde and 2-[(methylthio)methyl]furan, respectively. The aldehydes were the main volatile components of the white teff in the n-hexane extract followed by furans, terpene and esters and lower amount of hydrocarbons and pyrrole class of compounds. Ashokkumar et al.[Citation25] have identified 31 volatile compounds in the popular South Indian traditional and modern rice varieties among which were squalene and methyl stearate with relative content of 2.5–6.2% and 0.6–1.8%, respectively, and is comparable with the present study.
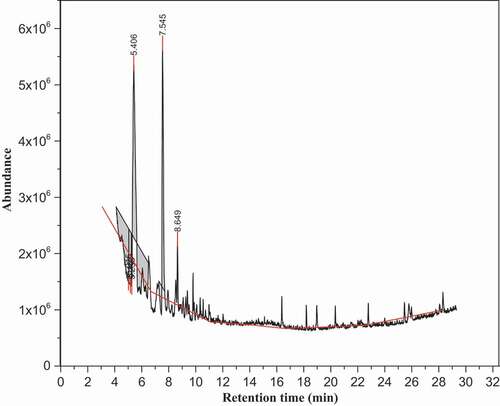
depicted the chromatogram peak profile of volatile compounds in the brown teff sample from the n-hexane extract determined by GC-MS method. The volatile compounds which have revealed a NIST-14 matching ≥80% were eluted in the range of 5.31 min up to 16.4 min in the brown teff sample and the most intense compounds were 5-methyl-2-furancarboxaldehyde (7.49 min), benzeneacetaldehyde (8.61 min), 2-(2-furanylmethyl)-5-methylfuran (9.79 min), 2-[(methylthio)methyl]furan (7.21 min), furfural (5.31 min), 2,2ʹ-methylenebis furan (8.48 min), 4-ethyl-2-methoxyphenol (12.0 min) and cetene (16.4 min).
As can be seen in , the relative contents of main volatile compounds determined in the brown teff sample were 18.4%, 16.0%, 12.5% and 9.73%, for the furfural and 5-methyl-2-furancarboxaldehyde, 2-[(methylthio)methyl]furan, benzeneacetaldehyde, respectively. The numbers of volatile compounds identified from the brown teff sample in the n-hexane extract were comparable with the white teff sample of the n-hexane extract. Aldehydes were the main quantitative constituents of brown teff sample in the n-hexane extract followed by furans and lower contents of pyrrole, alcohol and hydrocarbons, respectively. This might indicate that the n-hexane solvent was suitable and efficient to extract the volatile compounds present in the brown teff sample than dichloromethane.
Some volatile compounds identified in this study were reported by other authors in different cereals like furfural in sorghum and millet,[Citation1] benzeneacetaldehyde in rice,[Citation26] 5-methyl-2-furancarboxaldehyde in rice bran[Citation23] and 1-tetradecene in rice.[Citation24] There is no literature about the volatile compounds in the teff [Eragrostis tef (Zuccagni) Trotter] varieties. To the best of our knowledge, this is the first study about the volatiles of white and brown teff in the extract of dichloromethane and n-hexane solvents.
Conclusion
This is the first study on the volatile compounds in the white and brown teff samples. Nine hours was the appropriate optimized time for the hydro-distillation of the white and brown teff samples. A total of 16 volatile compounds in the white teff sample and 3 volatile compounds in the brown teff sample were identified from the dichloromethane extract. Compared with the white teff sample of the dichloromethane extract, the numbers of volatile compounds identified in the brown teff sample were very few. This fact could be used to differentiate the two varieties of the teff samples. While a total of 10 volatile compounds in the white teff sample and 11 volatile compounds in the brown teff sample were identified from the n-hexane extract. The study revealed that aldehydes were the major constituents of white and brown teff in both dichloromethane and n-hexane extracts which have a significant effect on the fragrance of a cereal product due to their low odor threshold values. Some volatile compounds identified in this study were also reported in different cereals like sorghum, millet and rice. In addition, dichloromethane was efficient to extract more volatile compounds in the white teff sample than in the brown teff sample, whereas n-hexane was efficient to extract almost equal number of volatiles in both white and brown teff samples.
Acknowledgments
The authors express their gratitude to the Department of Chemistry for providing laboratory facilities. Hagos Yisak is thankful to Ethiopian Police University, Ethiopia, for sponsoring his study.
Additional information
Funding
References
- Chughtai, M. F. J.; Pasha, I.; Anjum, F. M.; Nasir, M. A. Characterization of Sorghum and Millet with Special Reference to Fatty Acid and Volatile Profile. Turk. J. Agric. Food Sci. Technol. 2015, 3(7), 515–521.
- Sharma, K.; Chauhan, E. S. Nutritional Composition, Physical Characteristics and Health Benefits of Teff Grain for Human Consumption: A Review. Pharma Innov. J. 2018, 7(10), 3–7.
- Satheesh, N.; Fanta, S. W.; Yildiz, F. Review on Structural, Nutritional and Anti-nutritional Composition of Teff (Eragrostis tef) in Comparison with Quinoa (Chenopodium quinoa Willd.). Cogent Food Agric. 2018, 4(1), 1546942. DOI: https://doi.org/10.1080/23311932.2018.1546942.
- Gebremariam, M. M.; Zarnkow, M.; Teff, B. T. (Eragrostis tef) as A Raw Material for Malting, Brewing and Manufacturing of Gluten-Free Foods and Beverages: A Review. J. Food Sci. Technol. 2014, 51(11), 2881–2895. DOI: https://doi.org/10.1007/s13197-012-0745-5.
- Yilmaz, H. O.; Arslan, M. Teff: Nutritional Compounds and Effects on Human Health. Acta Sci. Med. Sci. 2018, 2(9), 15–18.
- Serna-Saldivar, S. O.; Espinosa-Ramírez, J. Grain Structure and Grain Chemical Composition. Sorghum and Millets 2nd Ed. Am. Assoc. Cereal Chem. 2019, 85–129. DOI: https://doi.org/10.1016/B978-0-12-811527-5.00005-8.
- Shumoy, H.; Raes, K. Antioxidant Potentials and Phenolic Composition of Tef Varieties: An Indigenous Ethiopian Cereal. Cereal Chem. 2016, 93(5), 465–470. DOI: https://doi.org/10.1094/CCHEM-10-15-0210-R.
- Kibatu, G.; Chacha, R.; Kiende, R. Determination of Major, Minor and Trace Elements in Tef Using Portable Total X-Ray Fluorescence (TXRF) Spectrometer. EC Nutr. 2017, 9(1), 51–59.
- Gebregewergis, A.; Chandravanshi, B. S.; Redi-Abshiro, M. Levels of Selected Metals in Teff Grain Samples Collected from Three Different Areas of Ethiopia by Microwave Plasma-Atomic Emission Spectroscopy. Bull. Chem. Soc. Ethiop. 2020, 34(3), 449–462. DOI: https://doi.org/10.4314/bcse.v34i3.2.
- Fekadu, D.; Abate, S.; Kore, T.; Agza, B.; Asaminew, G.; Shiferaw, L. Nutrition of Tef (Eragrostis tef) Recipes. Food Sci. Quality Manage.. 2015, 45, 18–22.
- Gangopadhyay, N.; Rai, D. K.; Brunton, N. P.; Gallagher, E.; Hossain, M. B. Antioxidant-Guided Isolation and Mass Spectrometric Identification of the Major Polyphenols in Barley (Hordeum vulgare) Grains. Food Chem. 2016, 210, 212–220. DOI: https://doi.org/10.1016/j.foodchem.2016.04.098.
- Goersch, M. C. S.; Schafer, L.; Tonial, M.; Oliveira, V. R.; Ferraze, A. B. F.; Fachini, J.; Silva, J. B.; Niekraszewicz, L. A. B.; Rodrigues, C. E.; Pasquali, G.; et al. Nutritional Composition of Eragrostis teff and Its Association with the Observed Antimutagenic Effects. Royal Soc. Chem. Adv. 2019, 9, 3764–3776.
- Dame, Z. T.;. Analysis of Major and Trace Elements in Teff (Eragrostis tef). J. King Saud Uni.-Sci. 2020, 32(1), 145–148. DOI: https://doi.org/10.1016/j.jksus.2018.03.020.
- Gebru, Y. A.; Hyun-II, J.; Young-Soo, K.; Myung-Kon, K.; Kwang-Pyo, K. Variations in Amino Acid and Protein Profiles in White versus Brown Teff (Eragrostis tef) Seeds, and Effect of Extraction Methods on Protein Yields. Foods. 2019, 8(6), 202. DOI: https://doi.org/10.3390/foods8060202.
- Alaunyte, I.; Development of Nutrient-Rich Teff Bread and Its Effects on Iron Status and Exercise Performance in Female Runners. PhD Dissertation, Manchester Metropolitan University. (2013). Available at: http://www.espace.mmu.ac.uk/espace/bitstream/2173/313142/1/HESIS%20definitive%20copy.pdf
- Salawu, S. O.; Bester, M. J.; Duodu, K. G. Phenolic Composition and Bioactive Properties of Cell Wall Preparations and Whole Grains of Selected Cereals and Legumes. J. Food Biochem. 2014, 38(1), 62–72. DOI: https://doi.org/10.1111/jfbc.12026.
- Gebremariam, M. M.; Abegaz, K.; Zarnkow, M.; Becker, T. Studies on the Mashing Conditions of Teff (Eragrostis tef) Malt as a Raw Material for Lactic Acid-fermented Gluten-free Beverage. Int. J. Food Sci. Technol. 2015, 50(9), 2032–2037. DOI: https://doi.org/10.1111/ijfs.12854.
- Giuberti, G.; Gallo, A.; Fiorentini, L.; Fortunati, P.; Masoero, F. In Vitro Starch Digestibility and Quality Attributes of Gluten Free ‘Tagliatelle’ Prepared with Teff Flour and Increasing Levels of a New Developed Bean Cultivar. Starch-Stärke. 2016, 68(3–4), 374–378. DOI: https://doi.org/10.1002/star.201500007.
- Barretto, R.; Buenavista, R. M.; Rivera, J. L.; Wang, S.; Prasad, P. V.; Siliveru, K. Teff (Eragrostis tef) Processing, Utilization and Future Opportunities: A Review. Int. J. Food Sci. Technol. 2020. DOI: https://doi.org/10.1111/ijfs.14872.
- Cramer, A.-C. J.; Mattinson, D. S.; Fellman, J. K.; Baik, B.-K. Analysis of Volatile Compounds from Various Types of Barley Cultivars. J. Agric. Food Chem. 2005, 53(19), 7526–7531. DOI: https://doi.org/10.1021/jf0506939.
- Liu, J.; Tang, X.; Zhang, Y.; Zhao, W. Determination of the Volatile Composition in Brown Millet, Milled Millet and Millet Bran by Gas Chromatography/Mass Spectrometry. Molecules. 2012, 17(3), 2271–2282. DOI: https://doi.org/10.3390/molecules17032271.
- Chai, D.; Li, C.; Zhang, X.; Yang, J.; Liu, L.; Xu, X.; Du, M.; Wang, Y.; Chen, Y.; Dong, L. Analysis of Volatile Compounds from Wheat Flour in the Heating Process. Int. J. Food Eng. 2019, 15(10), 1–13. DOI: https://doi.org/10.1515/ijfe-2019-0252.
- Gao, C.; Li, Y.; Pan, Q.; Fan, M.; Wang, L.; Qian, H. Analysis of the Key Aroma Volatile Compounds in Rice Bran during Storage and Processing via HS-SPME GC/MS. J. Cereal Sci. 2021, 99, 103178. DOI: https://doi.org/10.1016/j.jcs.2021.103178.
- Hinge, V. R.; Patil, H. B.; Nadaf, A. B. Aroma Volatile Analyses and 2AP Characterization at Various Developmental Stages in Basmati and Non-Basmati Scented Rice (Oryza sativa L.) Cultivars. Rice. 2016, 9(1), 1–22. DOI: https://doi.org/10.1186/s12284-016-0113-6.
- Ashokkumar, K.; Govindaraj, M.; Vellaikumar, S.; Shobhana, V. G.; Karthikeyan, A.; Akilan, M.; Sathishkumar, J. Comparative Profiling of Volatile Compounds in Popular South Indian Traditional and Modern Rice Varieties by Gas Chromatography-Mass Spectrometry Analysis. Front. Nutr. 2020, 7, 1–13. DOI: https://doi.org/10.3389/fnut.2020.599119.
- Dias, L. G.; Hacke, A.; Bergara, S. F.; Villela, O. V.; Mariutti, L. R. B.; Bragagnolo, N. Identification of Volatiles and Odor-Active Compounds of Aromatic Rice by OSME Analysis and SPME/GC-MS. Food Res. Int. 2021, 142, 110206. DOI: https://doi.org/10.1016/j.foodres.2021.110206.