 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this study was to assess the impact of innovative pre-drying treatments (ultrasound and microwave) on the physical, chemical, and functional properties pumpkin flour in comparison to untreated and freeze-dried (optimal control) flours. The impact was primarily related to the improvement of the flour’s nutritional, phytochemical, and functional properties. The pretreated flours were superior to untreated flour and were nearer in quality to freeze-dried flour. The moisture, ash, crude fat, crude protein, crude fiber, and carbohydrate contents of flours ranged from 7.57 to 8.23%, 5.73 to 6.57%, 1.17 to 1.85%, 8.72 to 11.32%, 10.92 to 13.11%, and 61.47 to 64.23%, respectively. Among the pre-drying treatment, 20UM depicts the highest color change and retention of the bioactive component in pumpkin flour. The results also showed that the 30UM pry-dried flour had higher water solubilities index (11.83%), water absorption (9.75 g/g), and oil absorption capacities (2.28 mL/g).
Introduction
Fruits and vegetables are consumed worldwide in both fresh and processed forms. Pumpkin is a traditional crop considered an excellent source of provitamin A carotenoids, which are very helpful in preventing vitamin A deficiency .[Citation1] Carotenoids are phytochemicals thought to reduce the risk of certain degenerative diseases and are responsible for the attractive color of many fruits and vegetables .[Citation2] Nevertheless, very little has been done on a pumpkin to overcome undernourishment, food poverty and income generation even in a favorable ecological conditions throughout East Africa .[Citation3] As such, it remains underutilized and less considered by many households. Due to the high moisture content (90–92%), pumpkins are bulky and difficult to handle and transport . Hence, most pumpkins produced are consumed in the area of production. Processing can transform pumpkins from perishable produce to stable foods with a long shelf-life, facilitating global transportation and distribution .[Citation4] For these reasons, freezing and drying may be suitable techniques for processing and extending pumpkins shelf life. Currently, powders are the core processed products of pumpkins.
Air drying is generally favored over freeze drying due to its low operating costs and shorter drying times. In conventional air drying, high temperatures are employed that adversely affect the texture, color, and nutritional value of the products in question .[Citation5] One way to produce high-quality dried products is to use pre-drying treatments, improving product quality .[Citation6] Hence, possible pre-dryig treatment methods that reduce drying time while maintaining quality are imperative. Pre-drying treatment helps to reduce undesired changes such as antioxidant activity reduction, color, and textural changes. Additionally, it reduces drying time by relaxing tissue structure and producing a superior dry product .[Citation7] In this sense, recent advances in the use of emerging food processing technologies have received considerable attention in reducing various adverse changes in final dried product properties. Among the so-called emerging technologies, ultrasound is a promising non-thermal pre-drying treatment commonly used before drying various agricultural products to improve mass transfer, drying time, reduce processing cost, and maintain quality properties .[Citation8] The application of ultrasound produces a sponge-like effect on the surface of the solid food samples, resulting in the formation of microspores in the food material .[Citation9] Also, ultrasonic pretreatment is a favorite because the process can be performed at low temperatures, which shrinks the probability of food degradation[Citation10] and permits the removal of moisture content from solids without producing a liquid phase change. The utilization of microwave energy has attracted much attention as it is said to improve the drying process by reducing processing time and operating costs .[Citation7] Applying microwave heating as a pre-drying treatment to moist material for drying operations results in internal moisture being heated up and migrating to the surface due to pressure differences for subsequent drying .[Citation11] Therefore, this study aimed to investigate the effect of combined ultrasound and microwave technologies as a pre-drying treatment on the physical, chemical and functional properties of pumpkin flour qualities.
Materials and methods
Chemicals
Standards and reagents used were gallic acids (97.5–102.5% sigma Aldrich, China), phytic acid sodium salt hydrate (Sigma Aldrich, Switzerland), (+-) Catechin hydrate (≥96.0%, Sigma Aldrich, China), Quercetin (≥98%, sigma Aldrich, Germany), Folin-Ciocalteu’s (2 N, Sigma Aldrich, USA), Vanillin (≥ 99.5%, UNI-CHEM, Roth, France), 2,2-Diphenyl-1-picrylhydrazyl (Sigma Aldrich, Germany), Aluminum Chloride (99% Loba chemic, India), Methanol (M.wt. = 34.02 g/mol, Biochem chemopharma, France), Nitric acid (69% Loba chemic, India) and Sodium carbonate(≥99.5% Carl Roth GmbH, Karlsruhe). All chemicals were of analytical grade.
Material collection and preparation
The fresh, healthy, and ripped pumpkin bought from a local market was washed properly in distilled water, peeled, and cleaned by discarding the seeds. Only the fleshy part was taken and sliced into (15 × 15 × 4) mm3 pieces. Slices were washed with distilled water, drained, and mopped with a paper towels. Untreated and freeze-dried (Lablyo plus, Germany) samples were used as a control sample to compare the effect of pre-drying methods; the remaining samples were treated with the following pre-drying methods before drying at 60°C in fluidized bed drier (TG 200, Germany). Dried slices were milled (Model BH24 1DY, Armfield, England) and then sieved through a standard 500-micron mesh sieve. The obtained flours were wrapped in aluminum foil bags and were kept at 4°C in a desiccator before use.
Microwave pretreatment
FA programmable domestic microwave oven (Model-CE107BT, Samsung, Thailand) with a maximum output of 900 W at 2450 MHz was used. For processing, approximately 100 g of sample in slice form was spread evenly on the tissue paper and placed on the rotatable turntable to confirm the uniformity of microwave energy absorbed by each sample. The microwave oven was allowed to operate at a 300 W power level for 6 min,[Citation12] followed by cold water dipping to avoid the residual heating effect.
Ultrasonic pretreatment
A set of experimental pumpkin slice samples were immersed in distilled water and subjected to ultrasonic waves for 10, 20, and 30 min .[Citation10,Citation13] Experiments were performed in an ultrasonic bath (Model-EU-28, Akin Electronic, Turkey) at ambient water temperature (30°C) without mechanical agitation. The temperature raised during the experiments was lower than ±2°C at each treatment. Keep the water to fruit ratio at 4:1 by weight basis. Experiments were performed in isolated 250 mL Erlenmeyer flasks to keep away from interference among the samples and runs. For the combined treatment, other parts were further microwave blanched (Samsung, Model-CE107BT, Thailand) for six (6) min at 300 W power level as above after draining and wiping .
Drying methods
Freeze-drying
A pumpkin slices were dried using a laboratory-scale freeze dryer (Lablyo plus, Germany) as described by Marques, Prado, & Freire .[Citation14] One hundred grams of each sliced sample was put in the tray, frozen at −18C for 3 hours, and then placed inside the freeze dryer chamber at 52 pa and −52°C. Thermocouple probes were used to regulate and adjust the product temperature on each tray during drying. The dried slices were milled (Model BH24 1DY, Armfield, England), sealed in aluminum foil bags and stored at 4°C until further tests were carried out.
Fluidized bed drying
The slices were drained well and spread for drying using a fluidized bed (TG 200, Germany) drier at 60C with constant fluidizing drying air velocity set to 1.5 m/s to achieve fluidization .[Citation15] After being subjected to drying, weighing every interval of time until constant weight is achieved. The dried pumpkin slices were then grounded using a laboratory grinder (Model BH24 1DY, Armfield, England) and sieved through a 500-micron mesh sieve to remove coarse fiber. Pumpkin flours were sealed in aluminum foil bags and stored at 4°C for further analysis .[Citation15]
Physical property analysis
Color
Color analysis was carried out using Hunter Lab Colorimeter, Minolta. The color readings were presented as L*, a*, b* format where a* value ranges from −100 (greenness) to +100 (redness), the b* value varies from −100 (blueness) to +100 (yellowness), whereas the L* value, elucidate the degree of lightness, extend from 0 (black) to 100 (white). Siddiq et al.[Citation16] The black and white tile was used for instrument calibration before color measurement. The chroma factor (C*) was calculated by converting the Cartesian coordinates (b*, a*) based on the following formula[Citation17]:
Hue angle shows a relation between a* and b* and was calculated according to the formula[Citation17]:
Color changes in comparison to the negative control sample are obtained from the following formula:
Water activity, total soluble solids, and pH
The water activity of the pumpkin flours were measured by a water activity meter (HD-3A, NanBei, China). Total soluble solids (TSS) were determined for each sample according to the AOAC[Citation18] method using a digital refractometer (Model-A670, Hanon, China) at 25°C and expressed as °Brix. A 10 g of sample was balanced and dissolved in a beaker containing 25 mL of distilled water to form a slurry. It was placed for 10 min with constant stirring. The pH was then determined according to official methods AOAC[Citation19] with a pH meter (BANTE Multiparameter/China).
Chemical properties
Proximate values of the pumpkin flour samples were determined following the official methods AOAC [Citation18] Moisture content (MC) was determined by laboratory oven (Model 10-D1391/AD, SCA) set at 105°C. Total crude protein content (N × 6.25) was analyzed using the Kjeldahl method (K1160, Hanon, China) according to AOAC method No. 978.04. The total fat content was evaluated by using a soxhlet extractor following AOAC method No. 930.09. The total ash content was examined by using muffle furnace (MKF-07,natek, Turkey), following AOAC method No. 930.05. Crude fiber analysis was performed by neutralization method (BXB-06, Guangzhou, China), according to AOAC method No. 930.10. Carbohydrate was calculated by difference.
Phytochemical analysis
Phytate content
The phytate content of the samples was find out according to the method described in Deme et al. .[Citation20] One hundred milligrams of the sample were extracted with 10 mL of 0.2 N HCl in a mechanical shaker for one hour at room temperature. The extract was centrifuged at 3000 rpm for 30 min. Pure supernatant was used for phytate assessment. One milliliter of Wade reagent (containing 0.03% solution of FeCl3 · 6H2O and 0.3% of sulfosalicylic acid in water) was added to 3 mL of the sample solution (supernatant), and the mixture was vortexed for 5 s, and then read the absorption at 500 nm (Perkin Elmer Lamda 950 UV/Vis/NIR, UK) against a blank solution (3 mL extract solution mixed with 2 mL of 2.4% HCl). The sodium salt of phytic acid (5–36 mg/mL) was used as the standard to draw the calibration curve. Phytate concentration was estimated from a standard curve and the results were expressed as mg phytate per 100 g of dry matter.
Tannin content
tannin content was determined by the method of Gemed,[Citation21], modified from Burns (1971), using catechin as the tannin standard. About 1.0g of each sample was weighed in triplicates in a screw-cap test tube and extracted with 10 ml of 1% HCl in methanol for 24 hours at room temperature with mechanical shaking. After shaking for 24 hours, the solution was centrifuged at 1000 rpm for 5 minutes. Mix 1 mL of supernatant with 5 mL of vanillin-HCl reagent (mix equalt volume of 8% concentrated HCl in methanol and 4% Vanillin in methanol). D-catechin was used as a standard for tannin determination. A 0.01 g of D- catechin was measured and dissolved in 50 mL of 1% HCl in methanol, serving as a stock solution. A 0, 0.2, 0.4, 3, 0.6, 0.8, and 1 mL of stock solution were measured in a test tube and the volume of each test tube was regulated to 1 mL with 1% HCl in methanol. To each test tube, added 5 ml of vanillin-HCl reagent. Zero the spectrophotometer by using distilled water before evaluating the absorbance of sample solutions and the standard solution after 20 minutes at 500 nm (Perkin Elmer Lamda 950 UV/Vis/NIR, UK). The calibration curve was plotted from the sequence of standard solutions as absorbance versus concentration and the slope and intercept were used for calculation.
Oxalate
It was assessed by the AOAC[Citation18] method. One gram of the sample was taken in a 100-mL conical flask. Seventy-five milliliters of 3 mol/L H2SO4 were poured and the solution was stirred occasionally with a magnetic stirrer for about 1 h and then clarified using Whatman No. 1 filter paper. The clarified samples (extract) (25 mL) were gathered and titrated against hot (80–90°C) 0.1 N KMnO4 solution to the point when a faint pink color was observed that persisted for at least 30 sec. Results were outlined as oxalate in mg per g of sample, and the concentration of oxalate in every sample was recorded from the calculation:
Analysis of antioxidant activities
Sample extraction
Samples were extracted according to previously defined procedures Ferreira et al. .[Citation22] Extract 10 g of pumpkin flour with 100 mL of methanol for 24 h using a temperature shaker incubator (ZHWY-103B) at 25°C and 150 rpm, then clarified through Whatman No. 1 paper. Again extract the residue with above two additional 100 mL portions of methanol. The combined methanolic extracts were then evaporated to dry using a Rota evaporator (R-300, Buchi, Switzerland) at 40°C, redissolved in methanol at a 50 mg/mL concentration, and stored at 4°C for further use.
Total phenolic content
Total phenolic content (TPC) was assessed using the Folin Ciocalteu assay and gallic acid (GA) as the standard, according to Xu & Chang.[Citation23] The mixture of the sample solution (50 µL), distilled water (3 mL), 250 µL of Folin-Ciocalteu’s reagents solution, and 7% NaCO3 (750 µL) was vortexed and incubated for 8 min at room temperature. Then, a dose of 950 µL of distilled water was added. The mixture was left at room temperature for two hours. Read the absorbance at 765 nm (Perkin Elmer Lamda 950 UV/Vis/NIR, UK) against distilled water as a blank. Gallic acid was used to construct the standard curve of 20–100 µg/mL (r = 0.99). The results were mean ± standard deviation and indicated as mg of gallic acid equivalents/g of extract (GAEs). The total phenolic content of gallic acid equivalent (GAE) in flour extracts was determined by the following formula:
where C is the total content of phenolic compounds, mg/g fresh material, in GAE; c is the concentration of gallic acid determined from the calibration curve; V is the volume of extract, L; m is the weight of extract, g.
Total flavonoid content
Total flavonoid content was estimated using the colorimetric method previously described by Xu & Chang.[Citation23] Briefly, mix 0.25 mL of the flour extract or quercetin standard solution with 1.25 mL of distilled water in a test tube, then add by 75 µL of 5% NaNO2 solution. After 6 min, add 150 µL of 10% AlCl3.6H2O solution, let stand for an additional 5 min, and then add 0.5 mL of 1 M NaOH. The mixture was accustomed to 2.5 mL with distilled water and stirred well. Absorbance was immediately assessed against the blank (the same mixture without the sample) at 510 nm using a UV-Visible Spectrophotometer (Lamda 950 UV/Vis/NIR, Perkin Elmer, UK). Results were evaluated and expressed as quercetin equivalents (mgQE/g sample) using the calibration curve of (+)-catechin. The Linear range of the calibration curve was 25 to 200 µg/mL (r = 0.99). The extraction was conducted in triplicate.
Free radical scavenging activity
The influence of methanolic extracts on the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was assessed according to Woldegiorgis et.at, [Citation24] A 0.004% DPPH radical solution in methanol was prepared, and then 4 mL of this solution was mixed with 1 mL of numerous concentrations (2–14 mg/mL) of the extracts in methanol. Finally, the samples were incubated for 30 min at room temperature in the dark. Scavenging ability was read spectrophotometrically (UV/Vis/NIR, Lamda 950 Perkin Elmer, UK) by checking the decline in absorbance at 517 nm. The absorption maxima were first settled by scanning freshly prepared DPPH from 200 to 800 nm by the scan way of the spectrophotometer. Ascorbic acid was used as a standard without extract and as the control. Inhibition of free radical DPPH in percent (I%) was then calculated:
where A0 is the absorbance of the control and A1 is the absorbance of the sample.
Total carotene content
It was estimated according to the method by de Carvalho et al .[Citation25] Approximately 1 g of the sample was taken in a mortar and crushed by adding 25 mL of acetone. This extract was filtered and collected in a volumetric flask. This extraction procedure was repeated until the sample became colorless. The extract was pooled together and transferred to a 500 mL separating funnel containing 40 mL of petroleum ether.Acetone was removed from the extract by the slow addition of distilled water.The addition of distilled water resulted in the separation of two-phases . Repeat the process by discarding the aqueous phase until no residual solvent remained. Then extract was transferred to a funnel containing 15 g of anhydrous sodium sulfate to a 50 mL volumetric flask. Read the sample absorbance at 450 nm after making up the volume to 50 mL by petroleum ether. The total carotenoid content was determined using the following formula:
where A = absorbance; V = total extract volume (mL); P = Sample weight (g); A11cm % = 2592 (Beta carotene extinction coefficient in petroleum ether).
Functional properties
Bulk density
Bulk density (g/mL) was determined in a graduated cylinder by lightly adding 2 g of pumpkin flour into an empty 10 mL graduated cylinder and holding the cylinder on a vortex vibrator (XH-B, Hinotek, China) for 1 min. The volume was read and recorded. The measurements were made in triplicate. The ratio of the mass of the powder to the volume occupied in the cylinder decides the bulk density value in g/mL using Eq. (3) .[Citation26]
where,W = grams of pumpkin powder; V = measuring volume
Water absorption capacity and water solubility index
It was assessed according to Que et al. .[Citation15] Pumpkin flour (1 g) and water (10 mL) were vigorously mixed in a weighted 15 mL centrifuge tube, incubated in a 37°C water bath for 30 min, and then centrifuged (3000 * g, 10 min) (TGL-16, Sichuan Shoke, China). The supernatant was collected in pre-weighed aluminum cans, and the residue was weighed after the water was evaporated at 105C overnight.
Oil absorption capacity
It was determined using the method of Que et al.[Citation15] with slight modifications. Specifically, it was done by mixing pumpkin powder (1 g) and 6 mL of corn oil in a centrifuge tube and stirring it for 30s using a vortex mixer (XH-B, Hinotek, China), followed by centrifugation in a benchtop centrifuge (TGL-16, Sichuan Shoke, China) at 8000 rpm for 10 min. The volume of supernatant was recorded and an average was calculated from triplicate determinations.
Statistical analysis
Statistical analysis was carried out using the software package SAS version 9.0 (SAS Institute, Inc., Cary, North Carolina, USA) using analysis of variance (ANOVA). Tukey’s HSD test at the significance level of 5% (P < .05) was used to determine significant differences among samples.
Result and discussion
The pumpkin flour quality responds differently to the application of ultrasound, microwave, and combined ultrasound followed by microwave pre-drying treatments due to a reduction in drying time through enhanced mass transfer.
Physical properties
The physical properties of pumpkin flour produced under different types of pre-drying treatment show a significant difference (p < .05) in all properties except pH and water activities values (). The data also depicts that water activity increased with ultrasound exposure time and microwave treatment, but it is not significantly (p < .05) different except for freeze-dried flour. In line with this finding, Szadzińska et al.[Citation27] reported that ultrasound application did not influence the dried potatoes’ water activity (aw).
Table 1. Effects of pretreatment methods on drying time, water activity, pH, and TSS content of Pumpkin flour.
Table 2. Effects of pretreatment methods on color properties of Pumpkin flour.
Mothibe et al.[Citation28] also reported that microwave pre-drying treatment can reduce the water activity of dried apples more than ultrasound pretreatment. The results show that a water activity below 0.6, indicates it is microbiologically safe .[Citation29] The pH value of the untreated pumpkin flour (6.46 ± 0.16) was higher than that of pretreated and freeze-dried flours, but there was no significant difference among pretreated flours. This finding also showed that the total soluble solids (TSS) of untreated pumpkin flour (3.97 ± 0.06) were lower than that of freeze-dried (4.53 ± 0.21) and microwave pretreated (4.03 ± 0.15) flours, but higher than that of ultrasound pretreated flours. As reported in previous study, the TSS of untreated pumpkin powder and salt pretreated were 7.5 and 8.3 ○Brix, respectively,[Citation30] which is higher than this finding. The reduction in TSS is reasonable because the longer samples are sonicated, the more soluble solutes are lost. The drying time for 20UM and 30UM pre-dried flour samples were lower than for the other flour samples. The possible reasons could be the faster the liquid flow rates through the food to the boundary where micro-channels are created during the ultrasonic application. A similar reduction in drying time was observed for pineapple,[Citation31] melon[Citation32] and mulberry[Citation33] due to ultrasonic pre-drying treatment.
All color parameters for pretreated and untreated flours are shown in . Color is the first parameter customers use to judge the quality of dried products. The study revealed that the color parameters of pumpkin flours were affected by ultrasound, microwave, and combined pre-drying treatments, as shown in . Pumpkin is a good source of carotene, especially β-carotene, used as a coloring agent .[Citation34] This study indicated that freeze-drying reduced discoloration and preserved the yellowness of pumpkin powders compared to other flours. L* values representing brightness were found to be lower in ultrasound-pretreated flours compared to those of microwave-pretreated flours. Microwaved and ultrasonic pretreatment had a lower L* value than untreated flour (79.80 ± 1.59) but higher than freeze-dried (71.35 ± 3.05) flour. For the ultrasound pretreated flour, the highest L* value was noted for 10U (78.62 ± 1.78), followed by 20U (75.82 ± 2.40) and 30U (74.33 ± 2.89), respectively. Samples pretreated with ultrasound for 20 min followed by 6 min microwave blanching had the highest b* value (44.58 ± 0.71), while samples pretreated at 30 min followed by 6 min microwave blanching had the highest a* value (8.18 ± 0.42). The chroma index for the untreated control flour is significantly different (p < .05) than other flour samples, with the FD (54.07 ± 1.22) recording the highest and FC (40.10 ± 0.54) the lowest. The data reveals that while 20U and 30U show the highest hue angle, but FD shows the lowest, illustrating color purity on how an average person will recognize that color .[Citation17] The data reveals a significant difference between the color change of pretreated and untreated flours. Among the pretreated flours, while the 20UM flour sample noticed the highest color change, the 10U (1.46 ± 0.23 was) samples showed the lowest as compared to the negative control flour, indicating that colors are not close to one another. The highest ∆E was likely due to high differences in the L* (lightness) and b* (yellowness) values. The possible reasons for these difference are the exposure of cellular-bound yellow pigmentation of the pumpkin slice to the cavitation effect of ultrasound .[Citation35] A similar finding was reported by Wang et al.[Citation6] on ultrasound pretreated carrot samples. Generally, untreated flour had significantly (p < .05) lower b* values than the pretreated ones, which means that the latter were less yellow (more orange) since the pretreating helped them to retain their color .[Citation36] This implies that the samples lost their pigment to become lighter. The results conform with previous investigations of longer drying times causing a change in food surface characteristics or producing more significant pigments losses (carotenoids and others), which lead to the color change .[Citation37] Microwave drying has been reported to prevent color damage during drying .[Citation28]
Chemical properties
Proximate
The significant (p < .05) differences observed between the proximate parameters (moisture, protein, fat, fiber, ash, carbohydrate) of the pretreated and untreated flours were presented in . The low moisture content of the freeze-dried flours implied that it would have good storage qualities.
Table 3. Proximate composition of pumpkin flour as affected by pretreatment methods % (g/100 g dry weight basis) .
Why protein change No significant (p < .05) differences were observed between the crude fat and crude fiber values of the pretreated flours. Excluding moisture content, combined pretreatments improve the nutritional content of flour more than ultrasound and microwave pretreatment independently. Freeze-dried pumpkin flour depicted the highest value in all studied parameters except for moisture and carbohydrate content. While 20U and 30U flour samples were higher in nutritional content than FM, 10U flour was lower since it doesn’t have much difference from FC (untreated) flour. So longer (20, 30 min) ultrasound pretreatment time prevents more nutrient loss than microwave (for 6 min, at 300 W) pretreatment, which may be due to thermal effect. The result obtained indicates that 30 min ultrasound pretreated followed by 6 min microwaved blanched flour (30UM) had the highest protein (p < .05) (11.12 ± 0.62%) and crude fiber (12.98 ± 0.38%) (not significant) than others, while lower than freeze-dried flour. The highest two flours in carbohydrates content were 10UM (64.23 ± 0.23%) and 20UM (63.03 ± 1.860%), while the lowest two were FD (61.47 ± 1.03%) and FC (62.63 ± 0.95%). According to Toan et al.,[Citation38] in ash, fat, protein, and crude fiber the content of pumpkin flour was 4.51, 0.85, 7.63, and 4.78, respectively lower than this finding for untreated flour. However, the carbohydrate content was higher, which could be attributed to the high fiber content of these flours. El-Deremy,[Citation39] also reported a similar finding. Khandpur & Gogate,[Citation40] reported that ultrasound pretreatment enhanced the nutritional quality of carrot, spinach, sweet lime, orange, and juices . The high nutrient retention in freeze-dried samples is not surprising as it has been reported to be one of the choosen drying methods to minimize the loss of nutrients and bioactive compounds .[Citation41,Citation42] Generally, the higher nutritional values observed in pumpkin flour in the present study were due to pretreatment methods which increased the loss of moisture and level of nutrients at any given weight. This was also in line with a study conducted by Morris et al.,[Citation14] who concluded that moisture removal might increase the nutrient content, which was the case in all the dried samples. Products with low moisture were generally stored longer[Citation43] due to reduced microbial and chemical activity. In addition, the reason for an increase in protein content during the ultrasound treatment may be due to its mechanical effect that breaks up the food matrix into smaller particles that forms microspores within the food material, thus increasing the surface area, facilitating protein release, and increasing protein yield .[Citation44]
Phytochemical properties
As shown in phytate, tannins, oxylate, total phenols, total flavonoids, and total carotenoids were significantly influenced by pretreatment methods (P < .05). Anti-nutrients (phytate, tannin, and oxalate) in food materials have been reported to form complexes with several mineral elements, thereby making them biologically unavailable for human absorption and utilization. Significant differences (p < .05) were observed in the pretreated flours and controls phytate, tannin, and oxalate content. . The lowest two in phytate content with no significant difference among them were 20UM (110.80 ± 8.12 mg/100 g) and 30UM (110.26 ± 8.66 mg/100 g), while freeze-dried and untreated flour samples had the highest (189.55 ± 0.00 and 142.36 ± 14.31 mg/100 g), respectively.
Table 4. Phytochemical composition of pumpkin flour as affected by pre-drying treatment methods.
The finding indicated that microwave and ultrasound pretreatment reduces phytate, tannin, and oxylate content while combined pretreatment was more effective. With the increase of ultrasonic pre-drying treatment exposure time, phytate, tannins, oxylate content reduction in flour increased, but microwave pre-drying treatment was more effective in reducing phytate content (134.33 ± 11.12 mg/100 g). So probably due to the chemical degradation of phytate to lower inositol phosphates and inositol or cleavage of the phytate ring itself .[Citation45] As shown in the study results, microwave and ultrasonic pretreatment reduced phytate, tannin, and oxalate content, while combined pretreatment was more effective. The high tannin (6.48 ± 0.40 mg/100 g) and phytate (189.55 ± 0.00 mg/100 g) content in the freeze-dried flour may indicate both susceptibility of both to hot temperatures. The content of total phenol (4.36 ± 155.36 mgGAE/g), total flavonoid (1.31 ± 160.61 mgQE/g), and total carotenoid (87.70 ± 4.56 µg/g) in microwave pretreatmented flour was lower than those of flour pretreated by ultrasonic for 20 min and 30 min, but higher than 10 min sonicated and untreated (FC) flour. A similar case was also reported by Kaseke, Opara, & Fawole,[Citation46] who said microwave pretreatment enhanced total carotenoids, total phenolic content, and DPPH radical scavenging capacity of pomegranate seeds than the untreated one. Hayat et al.,[Citation47] also reported that microwave energy could increase the bioavailability of some phenolic compounds by liberating them from the food matrix. Among the pretreated flours, the 20UM flour samples had the highest total phenol, total flavonoid, and total carotenoid, which were 6.31 ± 229.99 mgGAE/g, 1.97 ± 135.22 mgQE/g, and 131.50 ± 9.84 µg/g, respectively. In contrast, the lowest 4.36 ± 155.36 mgGAE/g, 1.28 ± 155.23 mgQE/g, and 81.29 ± 4.56 µg/g were obtained for FM and 10 U flour samples, respectively. It implies that combined pretreatment resulted in higher retention of bioactive compounds than independent ultrasound and microwave pre-drying treatment alone and untreated flour. According to April et al.[Citation48] and Hussain et al.,[Citation2] the total phenol and flavonoid content in pumpkin flour were 2.77 mgGAE/g and 2.46 mgQE/g, 1.35 + 1.24 mgGAE/g, and 0.77 + 0.63 mgQE/g, respectively, all lower than this finding. In line with these (Roongruangsri & Bronlund[Citation49] also stated that pumpkin powder produced from oven dried at 50°C, 60°C, and 70°C contains about 25.99, 16.42, and 17.66 µg/g of total carotenoid content, respectively, lower than this finding. As depicted in , 30UM flour had lower TPC, TFC, and TCC than 20UM flour, it may be due to the extraction of phenolic compounds by water (phenolics losses) during extended ultrasound treatment time .[Citation50] Opalic et al.[Citation51] reported that prolonged sonication reduces dried apples’ the total phenolic, flavonoids content, and antioxidant capacity. As presented in , the content of total phenol, flavonoid, and carotenoids in freeze-dried flour was the highest, followed by 20UM and 30UM pretreated flours. Freeze-dried flour and 20UM flour had the highest total phenolic content, which was 47.46% and 45.72% higher than untreated flour, respectively, while microwave pretreated flour had the lowest total phenolic content (21.31%). The lowest two in total carotenoid retention were observed for 10 U (30.25%) and FM (35.35%) flour as compared to the best-case control sample (freeze-dried). Dini, Tenore, & Dini[Citation52] reported that microwave treatment increased the bioactive compounds and antioxidants activity of pumpkin pulp and also Carpenter & Toftness[Citation53] showed sonication pretreatment preserved it in dried onion slices. High carotenoid contents in flour show a more yellow color (20UM). Carotenoids are considered a significant source of vitamin A, which plays a crucial role in the human body by promoting eyesight, immune system, reproductive system, growth, and development, whereas deficiency of this vitamin is a common cause of infant mortality and blindness .[Citation2]
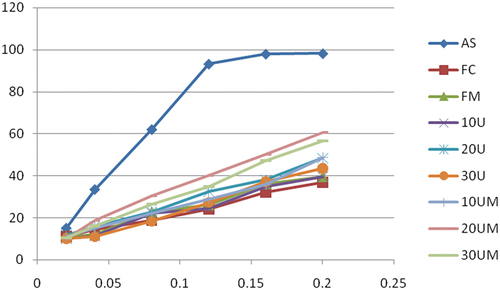
illustrates the antioxidant scavenging activities of extract of pretreated and untreated pumpkin flour relative to ascorbic acid (control). The combined pre-drying treatment showed a stronger DPPH scavenging activity than single pretreatment alone. DPPH scavenging ability increases with the content of total phenol, total flavonoid, and carotenoid in flour, among which FD and 20UM flour were the highest, and 10 U and FC were the lowest. Because of their conjugated double bonds, carotenoids have a strong antioxidant capacity to scavenge free radicals .[Citation54] Although the total flavonoid contents in pumpkin are lower than total phenolic s, even small concentrations of total flavonoid contents possess strong antioxidation potential .[Citation55] This result is consistent with the findings of,[Citation56] who reported that the ultrasonic pretreatment retained higher proportions of antioxidant properties, total phenolic and total flavonoid content compared to apple slices dried without ultrasound pretreatment. This study revealed that 20 minutes of ultrasound followed by 6 minutes of microwave (300 W) pre-drying treatment could be recommended as pre-drying conditions for obtaining the high-phytochemical composition of pumpkin flour. Titikan & Rungarun,[Citation57] also reported that the composition of phenolics and carotenoids may also be affected by fruit and vegetables processing methods.
Figure 1. Free radical scavenging of methanolic extract of pretreated pumpkin flour and controls. AS, ascorbic acid; FC, untreated pumpkin flour; FM, Microwaved pretreated; 10 U, 10 min ultrasound pretreated; 20 U,20 min ultrasound pretreated;30 U,30 min ultrasound pretreated; 10 UM, 10 min ultrasound and 6 min microwaved pretreated; 20 UM, 20 min ultrasound and 6 min microwaved pretreated;30 UM,30 min ultrasound and 6 min microwaved pretreated flours.
Functional properties
exhibited that bulk density, water absorption capacity (WAC), water solubility index (WSI), and oil adsorption capacity (OAC) of pumpkin flour were affected by ultrasonic, microwave, and combined pre-drying treatment methods before drying. Bulk density was reduced with ultrasound and microwave pretreatment, and the effect was more pronounced with an increase in the exposure time of ultrasound treatment. The impact of microwave pre-drying treatment on a bulk density was lower than that of ultrasonic and combined pre-drying treatment. Untreated flour had a higher bulk density (0.52 ± 0.01 g/cc), while freeze-dried flour had a lower bulk density (0.33 ± 0.01 g/cc). Lim et al.[Citation58] indicated that the bulk density of pumpkin pretreated with Ca(OH)2 and blanching then air-fried were 0.400 g/cc and 0.358 g/cc, respectively, comparable to this finding. According to Mirhosseini & Amid,[Citation59] the low bulk density of freeze-dried flour might be due to increased volume rather than mass, although the solubility of freeze-dried flour may be significantly affected by the low bulk density.
As displayed in , ultrasound, microwave, and combined pretreatment of samples generally resulted in greater WAC, WSI, and OAC. In comparison, 30 UM flour had significantly (p < .05) the highest WAC (9.75 ± 0.24 g/g), WSI (11.83 ± 1.0%) and OAC (2.28 ± 0.11 mL/g), but the lowest content was observed for freeze-dried flour (6.13 ± 0.08 g/g, 5.95 ± 0.75%) expect for OAC on untreated flour (1.53 ± 0.06 mL/g), respectively. In addition, M. Asif-Ul-Alam et al.[Citation60] pointed out that the water holding capacity of freeze-dried flour was lower than that of hot air-dried flour. High water absorption capacity improves yield and consistency, gives the body to food,[Citation61] and is an alternative emulsifier for food formulations .[Citation34] Higher water solubility was observed in 30 UM, indicating that more starch had been decomposed. The analysis showed that fluidized bed-dried pumpkin flour had higher water solubility and bulk density than freeze-dried flour, which is consistence with the findings of Que et al. .[Citation15] According to Traynham et al.,[Citation62] fruit and vegetable flours, which have high water absorption and oil adsorption capacities, can impart water-retention and fat-binding properties essential in bakery products and other select food applications. This study showed that with increase of ultrasound exposure time, the water solubility, water absorption capacity, and oil adsorption capacity of pumpkin flour also increased
Table 5. Functional properties of pumpkin flour as affected by pretreatment methods.
Conclusion
Ultrasound combined with microwave pre-drying treatment reduced the drying time than independent pretreatments alone, and the finding indicates that 20 UM treatments were more effective (32.78%) in reducing drying time. The present work describes the possibility of producing high-quality pumpkin flour in terms of the physical, chemical, and functional qualities by ultrasound, microwave, and combined pre-drying treatment. Pre-drying treatment decrease lightness due to the increase in the redness and yellowness of all pretreated samples. The high levels of crude protein and fiber found in pumpkin flour suggest that these raw materials are potential ingredients for developing nutritious foods. The analysis indicates that the flour moisture content and water activity values were within acceptable limits for safe storage. Untreated pumpkin flour showed greater bulk density, WAC, and WSI than freeze-dried flour, but lower than pretreated. Ultrasonication Pre-drying treatment for 20 min followed by microwave (300 W) blanching for 6 min before drying was the best process for maintaining color, total phenolic content, total carotenoids content, and DPPH activities during processing. This information is crucial for preparing pumpkin flour, which can be used as an ingredient for coloring and improving food’s nutritional and medicinal values.
Abbrevaition
Acknowledgments
Author Derese Wodajo would like to thank Wolkite University for providing laboratory facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kim, M. Y.; Kim, E. J.; Kim, Y. N.; Choi, C.; Lee, B. H. Comparison of the Chemical Compositions and Nutritive Values of Various Pumpkin (Cucurbitaceae) Species and Parts. Nutr. Res. Pract. 2012, 6(1), 21–27. DOI: 10.4162/nrp.2012.6.1.21.
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M. A.; Jamil, M. A.; Noreen, S.; Rehman, H. U.; Shabbir, H.; Ramzan, M. A., et al. Determination of Total Phenolic, Flavonoid, Carotenoid, and Mineral Contents in Peel, Flesh, and Seeds of Pumpkin (Cucurbita Maxima). J. Food Process. Preserv. 2021, 45(6), 1–8.
- Kiharason, J. W.; Isutsa, D. K.; Ngoda, P. N. Effect of Drying Method on Nutrient Integrity of Selected Components of Pumpkin Fruit Flour. J. Agric. Biol. Sci 2017, 12(3), 110–116.
- Habwe, F. O.; Walingo, K. M. Food Processing and Preparation Technologies for Sustainable Utilization of African Indigenous Vegetables for Nutrition Security and Wealth Creation in Kenya. Int. Union Food Sci. Technol. no. May 2014 2–9. 2008.
- Rastogi, N. K. Recent Trends and Developments in Infrared Heating in Food Processing. Critical Reviews in Food Science and Nutrition. 2012, 52(9), 737–760. DOI: 10.1080/10408398.2010.508138.
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low Frequency Ultrasound Pretreatment of Carrot Slices: Effect on the Moisture Migration and Quality Attributes by intermediate-wave Infrared Radiation Drying. Ultrason. Sonochem. 2018, 40, 619–628. DOI: 10.1016/j.ultsonch.2017.08.005.
- Önal, B.; Adiletta, G.; Di Matteo, M.; Russo, P.; Ramos, N.; Silva, C. L. M. Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods. 2021, 10(4), 1–18. DOI: 10.3390/foods10040853.
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of Ultrasound Treatment on the Water State in Kiwifruit during Osmotic Dehydration. Food Chemistry. 2014, 144, 18–25. DOI: 10.1016/j.foodchem.2013.05.129.
- Mieszczakowska-Frąc, M.; Dyki, B.; Konopacka, D. Effects of Ultrasound on Polyphenol Retention in Apples after the Application of Predrying Treatments in Liquid Medium. Food and Bioprocess Technology. 2016, 9(3), 543–552. DOI: 10.1007/s11947-015-1648-z.
- Azoubel, P.; Baima, A.; Amorim, R.; Oliveira, S. Effect of Ultrasound on Banana Cv Pacovan Drying Kinetics. Journal of Food Engineering. 2010, 97(2), 194–198. DOI: 10.1016/j.jfoodeng.2009.10.009.
- Adedeji, A. A.; Gachovska, T. K.; Ngadi, M. O.; Raghavan, G. S. V. Effect of Pretreatments on Drying Characteristics of Okra. Drying Technology. 2008, 26(10), 1251–1256. DOI: 10.1080/07373930802307209.
- Coklar, H.; Akbulut, M.; Kilinc, S.; Yildirim, A.; Alhassan, I. Effect of Freeze, Oven and Microwave Pretreated Oven Drying on Color, Browning Index, Phenolic Compounds and Antioxidant Activity of Hawthorn (Crataegus Orientalis) Fruit. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2018, 46(2), 449–456. DOI: 10.15835/nbha46211027.
- Bozk, H.; Ergün, A. R.; Baysal, T. Effects of Electrical and Sonication Pretreatments on the Drying Rate and Quality of Mushrooms. LWT - Food Science and Technology. 2016, 69, 197–202. doi:10.1016/j.lwt.2016.01.032.
- Marques, L. G.; M, M.; Prado; Freire, J. T. Vitamin C Content of freeze-dried Tropical Fruits. Int. Congr. Eng. Food 2011, 22–26.
- Que, F.; Mao, L.; Fang, X.; Wu, T. Original Article Comparison of Hot air-drying and freeze-drying on the Physicochemical Properties and Antioxidant Activities of Pumpkin (Cucurbita Moschata Duch) Flours. International Journal of Food Science and Technology . 2008, 43,1195–1201. doi:10.1111/j.1365-2621.2007.01590.x.
- Siddiq, M.; Ravi, R.; Harte, J. B.; Dolan, K. D. Physical and Functional Characteristics of Selected Dry Bean (Phaseolus Vulgaris L.) Flours. LWT - Food Science and Technology. 2010, 43(2), 232–237. DOI: 10.1016/j.lwt.2009.07.009.
- Mirani, A.; Goli, M. “Production of the Eggplant- - Fiber Incorporated Cupcake and Evaluating Its Chemical, Textural and Colorimetric Properties over a Ten- - Day Storage Time,”. Journal of food processing and preservation. 2021, 45(4), e15311. DOI: 10.1111/jfpp.15311.
- AOAC. Association of Official Analytical Chemists, 17th edn ed.; Washington DC, 2005.
- AOAC. Official Methods of Analysis., Association of Official Analytical Chemist, 15th Editi ed.; Washington D.C, 1990.
- Deme, T.; Haki, G. D.; Retta, N.; Woldegiorgis, A.; Geleta, M. Mineral and anti-nutritional Contents of Niger Seed (Guizotia Abyssinica (L.f.) Cass., Linseed (Linumusitatissimum L.) and Sesame (Sesamumindicum L.) Varieties Grown in Ethiopia. Foods. 2017, 6(4), 1–10. DOI: 10.3390/foods6040027.
- Gemed, H. F. Effects of Boiling Methods on Anti-nutritional Factors of Anchote (Coccinia Abyssinica (Lam.) Cogn) Tubers Grown in Western Ethiopia. Food Sci. Qual. Manag. 2014, 27, 39–40.
- Arca, M.; Montali, A.; Valiante, S.; Campagna, F.; Pigna, G.; Paoletti, V.; Antonini, R.; Barillà, F.; Tanzilli, G.; Vestri, A. Usefulness of Atherogenic Dyslipidemia for Predicting Cardiovascular Risk in Patients with Angiographically Defined Coronary Artery Disease. The American Journal of Cardiology. 2007, 100(10), 1511–1516. DOI: 10.1016/j.amjcard.2007.06.049.
- Xu, B. J.; Chang, S. K. C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. Journal of Food Science. 2007, 72(2), S159–S166. DOI: 10.1111/j.1750-3841.2006.00260.x.
- Woldegiorgis, A. Z.; Abate, D.; Haki, G. D.; Ziegler, G. R. Antioxidant Property of Edible Mushrooms Collected from Ethiopia. Food Chemistry. 2014, 157, 30–36. DOI: 10.1016/j.foodchem.2014.02.014.
- de Carvalho, L. M. J.; Gomes, P. B.; Godoy, R. L. D. O.; Pacheco, S.; Do Monte, P. H. F.; de Carvalho, J. L. V.; Nutti, M. R.; Neves, A. C. L.; Vieira, A. C. R. A.; Ramos, S. R. R., et al. Total Carotenoid Content, α-carotene and β-carotene, of Landrace Pumpkins (Cucurbita Moschata Duch): A Preliminary Study. Food Research International. 2012, 47(2), 337–340.
- Goula, A. M.; Adamopoulos, K. G.; Kazakis, N. A. Influence of Spray Drying Conditions on Tomato Powder Properties. Drying Technology. 2004, 22(5), 1129–1151. DOI: 10.1081/DRT-120038584.
- Szadzińska, J.; Łechtańska, J.; Kowalski, S. J.; Stasiak, M. The Effect of High Power Airborne Ultrasound and Microwaves on Convective Drying Effectiveness and Quality of Green Pepper. Ultrasonics Sonochemistry. 2017, 34, 531–539. DOI: 10.1016/j.ultsonch.2016.06.030.
- Mothibe, K. J.; Zhang, M.; Mujumdar, A. S.; Wang, Y. C.; Cheng, X. Effects of Ultrasound and Microwave Pretreatments of Apple before Spouted Bed Drying on Rate of Dehydration and Physical Properties. Dry. Technol. 2014, 32(15), 1848–1856.
- Jackson Lim Hwa Keen, M. R. I. S. U.; Saleena Taip, F.; Mohd, N. I.; Abdul Aziz, N. Effect of Pre-Treatment on the Physical Properties of Pumpkin Powder. Aust. J. Basic Appl. Sci. 2016, 10(7), 146–151.
- Teferi, Z.; Workneh, T. S.; Woldetsadik, K. Thin-layer Drying of Pumpkin Fruit Slices. Adv. Mater. Res. 2013, 824, 283–292.
- Fernandes, F. A. N.; Linhares, F. E.; Rodrigues, S. Ultrasound as pre-treatment for Drying of Pineapple. Ultrason. Sonochem. 2008, 15(6), 1049–1054.
- Dias da Silva, G.; Barros, Z. M. P.; de Medeiros, R. A. B.; de Carvalho, C. B. O.; Rupert Brandão, S. C.; Azoubel, P. M. Pretreatments for Melon Drying Implementing Ultrasound and Vacuum. Lwt. 2016, 74, 114–119.
- Tao, Y.; Wang , P.; Wang, Y.; Kadam, S. U.; Han Yongbin, Y.; Wang , J.; Zhou, J. Power Ultrasound as a Pretreatment to Convective Drying of Mulberry (Morus Alba L.) Leaves: Impact on Drying Kinetics and Selected Quality Properties. Ultrason. Sonochem. 2016, 31, 310–318. DOI: 10.1016/j.ultsonch.2016.01.012.
- Aziah, A. A. N.; Komathi, C. A. Physicochemical and Functional Properties of Peeled and Unpeeled Pumpkin Flour. J. Food Sci. 2009, 74(7), 328–333.
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Elrasheid Tahir, H.; Taiye Mustapha, A.; Ma, H. Effects of Ultrasound, Osmotic Dehydration, and Osmosonication Pretreatments on Bioactive Compounds, Chemical Characterization, Enzyme Inactivation, Color, and Antioxidant Activity of Dried Ginger Slices. J. Food Biochem. 2019, 43(5), 1–14.
- Fedha, M. S. Physicochemical Characterization and Food Application Potential of Pumpkin (Cucurbita Sp.) Fruit and Seed Kernel Flours. Kenya: Jomo Kenyatta University of Agriculture and Technology. 2008. http://hdl.handle.net/123456789/1429.
- Fellows, P. J. Food Processing Technology: Principles and Practice: Third Edition. Food Process. Technol. Princ. Pract. Third Ed 2009, 1–913.
- Van Toan, N.; Thi , N. Production of high-quality Flour and the Made Biscuits from Pumpkin. International Journal of Food Science and Nutrition. 2018, 3(5), 157–166.
- El-Deremy, M. Evaluation of physico-chemical Properties of Toast Breads Fortified with pumpkin(Cucurbita Moschata) Flour. The 6th Arab and 3rd International Annual Scientific Conference Report, 2146-2157:Development of Higher Specific Education Programs in Egypt and the Arab World in the Light of Knowledge Era Requirements, Egypt. Mansoura Univer. 2011.
- Khandpur, P.; Gogate, P. R. Effect of Novel Ultrasound Based Processing on the Nutrition Quality of Different Fruit and Vegetable Juices. Ultrason. Sonochem. 2015, 27, 125–136.
- Marques, L. G.; Silveira, A. M.; Freire, J. T. Freeze-drying Characteristics of Tropical Fruits. Dry. Technol. 2006, 24(4), 457–463.
- Saini, R. K.; Shetty, N. P.; Prakash, M.; Giridhar, P. Effect of Dehydration Methods on Retention of Carotenoids, Tocopherols, Ascorbic Acid and Antioxidant Activity in Moringa Oleifera Leaves and Preparation of a RTE Product. J. Food Sci. Technol. 2014, 51(9), 2176–2182.
- Morris, A.; Barnett, A.; Burrows, O. Effect of Processing on Nutrient Content of Foods: A Handbook of Vegetables and Vegetable Processing. Asian J. Biochem. 2004, 37(3), 160–164.
- Sun, X.; Ohanenye, I. C.; Ahmed, T.; Udenigwe, C. C. Microwave Treatment Increased Protein Digestibility of Pigeon Pea (Cajanus Cajan) Flour: Elucidation of Underlying Mechanisms. Food Chem. 2020, 329(May), 127196.
- Verma, A.; Sharma, A.; Rai, P. K.; Kumar, D. Effect of Microwave pre-treatment on Quality Parameters in Indian Mustard. J. Food Sci. Technol. 2019, 56(11), 4956–4965.
- Kaseke, T.; Opara, U. L.; Fawole, O. A. Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil. Foods. 2020, 9(9), 1287. DOI: 10.3390/foods9091287.
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of Microwave Treatment on Phenolic Content and Antioxidant Activity of Citrus Mandarin Pomace. Food Chem. 2010, 123(2), 423–429.
- April, M.; Muchirah, P. N.; Waihenya, R.; Muya, S.; Abubakar, L.; Ozwara, H. Characterization and anti-oxidant Activity of Cucurbita Maxima Duchesne Pulp and Seed Extracts Characterization and anti-oxidant Activity of Cucurbita Maxima Duchesne Pulp and Seed Extracts. J Phytopharmacol . 2018, 7(2), 134–140.
- Roongruangsri, W.; Bronlund, J. E. Effect of air-drying Temperature on physico-chemical, Powder Properties and Sorption Characteristics of Pumpkin Powders. Int. Food Res. J. 2016, 23(3), 962–972.
- Horuz, E.; Jaafar, H. J.; Maskan, M. Ultrasonication as Pretreatment for Drying of Tomato Slices in a Hot air–microwave Hybrid Oven. Dry. Technol. 2017, 35(7), 849–859.
- Opalic, M.; Domitranl, Z.; Komes, D.; Belšcak, A.; Horžic, D.; Karlović, D. The Effect of Ultrasound Pretreatment and air-drying on the Quality of Dried Apples. Czech J. Food Sci. 2009, 27, 297–300.
- Dini, I.; Tenore, G. C.; Dini, A. Effect of Industrial and Domestic Processing on Antioxidant Properties of Pumpkin Pulp. LWT - Food Sci. Technol. 2013, 53(1), 382–385.
- Ren, F.; Perussello, C. A.; Zhang, Z.; Kerry, J. P.; Tiwari, B. K. 2018. Impact of ultrasound and blanching on functional properties of hot-air dried and freeze dried onions. 87, 102–111. DOI: 10.1016/j.lwt.2017.08.053.
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Molecular Aspects of Medicine. 2004, 24, 345–351. DOI: 10.1016/S0098-2997(03)00030-X.
- Asif, M.; Ali , S.; Sherazi, T. A.; Ahmad, M.; Fawad, A.; Anjum, S.; Hussain, Z.; Mahmood, H.; Mahmood, N. Antioxidant, Antibacterial & Antiproliferative Activities of Pumpkin (Cucurbit) Peel & Puree Extracts -an in Vitro Study. Pak. J. Pharm. Sci. 2017, 30(4), 1327–1334.
- Rodríguez, Ó.; Santacatalina, J. V.; Simal, S.; Garcia-Perez, J. V.; Femenia, A.; Rosselló, C. Influence of Power Ultrasound Application on Drying Kinetics of Apple and Its Antioxidant and Microstructural Properties. J. Food Eng. 2014, 129, 21–29.
- Titikan, L.; Rungarun, S. Effect of Thermal Treatments on Antioxidant Properties of Pumpkin Flesh and Their Stability during in-vitro Gastrointestinal Digestion. Food and Applied Bioscience Journal. 2019, 7(3), 118–130.
- Lim, J.; Saleena , F.; Ibrahim, M. N.; Abdul , N.; Rezaul, M. Effect of Pre-Treatment on the Physical Properties of Pumpkin Powder. Australian Journal of Basic and Applied Sciences. 2016, 10(7), 146–151.
- Mirhosseini, H.; Amid, B. T. Effect of Different Drying Techniques on Flowability Characteristics and Chemical Properties of Natural carbohydrate-protein Gum from Durian Fruit Seed. Chem. Cent. J. 2013, 7(1), DOI: 10.1186/1752-153X-7-1.
- Asif-Ul-Alam, S. M.; Islam, M. Z.; Hoque, M. M.; Monalisa, K. Effects of Drying on the Physicochemical and Functional Properties of Green Banana (Musa Sapientum) Flour and Development of Baked Product. Am. J. Food Sci. Technol 2014, 2(4), 128–133.
- Osundahunsi, O. F.; Fagbemi, T. N.; Kesselman, E.; Shimoni, E. Comparison of the Physicochemical Properties and Pasting Characteristics of Flour and Starch from Red and White Sweet Potato Cultivars. J. Agric. Food Chem. 2003, 51(8), 2232–2236.
- Traynham, T. L.; Myers, D. J.; Carriquiry, A. L.; Johnson, L. A. Evaluation of water-holding Capacity for wheat-soy Flour Blends. JAOCS, J. Am. Oil Chem. Soc. 2007, 84(2), 151–155.
