ABSTRACT
Cosmos caudatus (Asteraceae), is known as Ulam raja in Malaysia or kenikir in Indonesia also sometimes referred to as “King’s salad.” It is usually consumed as a salad and the leaves have been widely used as a traditional medicine due to their pharmacological activities and beneficial effects on human health. The leaves have been reported to contain several phytoconstituents such as flavonoids and their derivatives, other phenolics, and essential oil, while the roots only contain non-flavonoids. Furthermore, the leaves have been reported to contain a high total phenolic content (TPC) which is attributable for various activities including antidiabetes, antioxidant, anti-inflammation, antibacterial, antifungal, anti-osteoporosis, anti-hyperlipidemic, anticancer, antihypertensive, anti-hepatoprotective, and to manage fertility problems. However, further research needs to be done in order to determine whether C. caudatus is effective in treating thrombolytic and leishmanial disorder. Clinical study regarding the use of C. caudatus as an antidiabetic agent has been reported as a supplement to improve insulin resistance and sensitivity in type 2 diabetes patients [NCT02322268]. The findings of the toxicity tests revealed that the leaves are nontoxic and that they can be taken without risk. It is still need to conduct an additional in vitro and in vivo investigations to confirm various traditional claims about the therapeutic potential of this plant in the treatment of various ailments. This traditional medicinal plant’s genuine medicinal benefit will be further confirmed by additional clinical trials and toxicity assessments.
Introduction
Cosmos caudatus is a herbal plant that has beneficial effects on human health. It is traditionally used as an antihypertensive, antidiabetic, antioxidant, antiosteoporosis, antifungal, and antibacterial agent.[1] It has been reported to demonstrate high total antioxidant capacity.[Citation2] Furthermore, C. caudatus belongs to Asteraceae that has 20–26 species worldwide.[Citation3] It is also traditionally consumed as a salad. This plant is known as Ulam raja in Malaysia[Citation3,Citation4] or Kenikir in Indonesia.[Citation5] Several scientific studies have validated the traditional health benefits attributed to this plant.
In Latin America and Southeast Asia, the leaves are used to delay the aging process, rigidify bone as well as treat several cardiovascular-associated problems.[Citation6] The leaf extract has been reported to reduce blood glucose levels and total cholesterol as well as to regenerate pancreatic tissue of hypercholesterolemic male white mice (Rattus norvegicus).[Citation5] C. caudatus has been demonstrated to possess antioxidant capabilities using a variety of in-vitro tests including 2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and 2,2-Diphenyl-1-picrylhydrazil (DPPH) radical assays.[Citation7,Citation8] Moreover, it has been observed that it inhibits lipid peroxidation[Citation9] and pathogenic bacterial growth including Salmonella sp., Proteus mirabilis, Proteus mirabilis, Staphylococcus aureus, Listeria monocytogenes, and Vibrio cholerae.[Citation7,Citation10] As a result, C. caudatus has been claimed that this plant might be used in the treatment of a variety of ailments.[Citation11]
It has been well documented that this herb can be consumed in fresh, powdered form, or extract form. Even, Clinical tests have been done to ensure the fresh leaf of C. caudatus is effective as an anti-diabetic drug. A previous study reported that C. caudatus leaves contain several active compounds including flavonoids and their derivatives and also non-flavonoid compounds. Therefore, this review is an attempt to explain the bioactive compounds of C. caudatus which contribute to responsible for preventing and treating several diseases. Based on this fact, the application of C. caudatus in various herbal products including food ingredients or products and nutrition can be explored. Therefore, this paper explains the advantages of C. caudatus in treating various diseases, as well as its toxicity.
Research materials and methodology
An online literature search utilizing phrases such as roots and leaves of C. caudatus Kunth or Kenikir or Ulam Raja, profiling of active compounds, total phenolic content, antioxidant activity and other pharmacological activities, toxicological evaluation, and isolation techniques. The online databases to gather information related to the C. caudatus used were PubMed, Wiley Online, Science Direct, Google Scholar, Springer Link, and the International Islamic University Malaysia (IIUM) Discovery Service. The online search was tailored between the years 1990 to 2022 for the published articles and the accepted ones were based on the inclusion and exclusion criteria. The inclusion criteria include (1) all published papers between 1990–2022, (2) study-based primary papers, and (3) written in English language. The exclusion criteria include (1) all studies that had been cited and (2) had no commentaries, and reviews of editorials. Based on the inclusion and exclusion criteria, only 41 primary papers were selected from more than 100 published between 1990–2022. As a result, the current review accounts for previous and most recent information on C. caudatus’s traditional uses, pharmacognosy, phytochemistry, pharmacological activities, and toxicity.
Taxonomic of the C. caudatus
This plant is usually consumed as a salad and belongs to the family Asteraceae It has been used to reduce blood glucose in East Java, Indonesia.[Citation12] Cosmos spp. are originally from tropical and subtropical regions including southern Mexico, Central America, or the Antilles. C. caudatus (Kunth), is one of the Cosmos species that was brought by the Spanish to Southeast Asia via the Philippines from Mexico.
It is also known as Wild Cosmos and is an indigenous plant with pale pink flowers (). The synonyms of C. caudatus include Bidens caudatus (Kunth) Sch. Bip.,[Citation10] Bidens berteriana Spreng, Bidens carnea Heer, Cosmos caudatus var. Exaristatus Sherff, Cosmea caudata (Kunth) Spreng, Bidens artemisiifolia subsp. Caudata (Kunth) Kuntze.[Citation1,Citation13,Citation14]
The taxonomy of this plant comprises Kingdom: Plantae, Subkingdom: Viridiplantae, Infrakingdom: Streptophyta, Superdivision: Embryophyta, Division: Tracheophyta, Subdivision: Spermatophytina, Class: Magnoliopsida, Superorder: Asteranae, Order: Asterales, Family Asteraceae, Genus: Cosmos Cav. (Cosmos), Species: Cosmos caudatus Kunth (Wild Cosmos).[Citation6] It is an annual herb that has a height of about 0.3–3 m, and the flowers are yellow or purple.[Citation15] In addition, it has weak roots, branched stems with a purplish-green color, and long petioles of about 10–70 mm.[Citation1]
Nowadays, the classification of the plant is not merely based on traditional taxonomy but relies on the molecular systematic. This plant is classified within one species but contains different chromosomes (). The chromosome numbers of C. caudatus in Indonesia (West Java areas) observed are 2 n = 2x+2 = 22; 2 n = 3x = 30; 2 n = 3x+2 = 32; 2 n = 3x+6 = 36; and 2 n = 4x = 40, however, the previous study carried out by Jose and Matthew (1995) reported that it contains two chromosomes namely n = 24 and 2 n = 48.[Citation16] Consequently, this plant within the same family may possess several chromosomes. An herbal plant’s molecular structure is utilized to identify it in this categorization.
Figure 2. Chromosome numbers of Cosmos caudatus: a) 22; b) 30; c) 32; d) 36; and e) 40. (adapted from Salamah et al., [Citation16]
![Figure 2. Chromosome numbers of Cosmos caudatus: a) 22; b) 30; c) 32; d) 36; and e) 40. (adapted from Salamah et al., [Citation16]](/cms/asset/b95dd7b6-c901-4f98-b710-376b767fd38e/ljfp_a_2158862_f0002_b.gif)
Phytochemical contents of C. caudatus
C. caudatus contains important bioactive compounds that support various pharmacological activities. Previous studies have reported that both its leaf and root parts contain several bioactive compounds as illustrated in . The leaves contain 3 major groups of active metabolites such as flavonoids and derivatives, non-flavonoids, and essential oils. However, there are no flavonoid compounds in the roots (). The reported flavonoids and their derivatives include quercetin, kaempferol, myricetin, catechin, luteolin, apigenin, quercetin 3-O-rhamnoside (quercitrin), quercetin 3-O-glucoside, quercetin 3-O-xyloside, quercetin 3-O-arabinofuranoside, and rutin. Quercetin content was reported around 51.3 ± 4.1 mg/100 g of fresh leaves as dominant flavonoid groups from a total of 52.2 ± 4.1 mg/100 g.[Citation9] Another flavonoid and its derivatives content such as quercitrin, catechin, and rutin were around 36.9, 25.0, and 8.2 mg/g of C. caudatus extract, respectively.[Citation17]
Figure 3. The summary of the reported active compounds from Cosmos caudatus based on the profiling and isolation techniques.
Figure 4. The chemical structure of active compounds in the Cosmos caudatus plant. (1) Quercetin; (2) Kaemferol; (3) Qyercitrin; (4) Rutin; (5) Chlorogenic acid; (6) Stigmasterol; (7) Costunoloide; (8) Lutein; (9) α-Copaine; (10) Bergamotene; (11) ɣ-Muurolene.
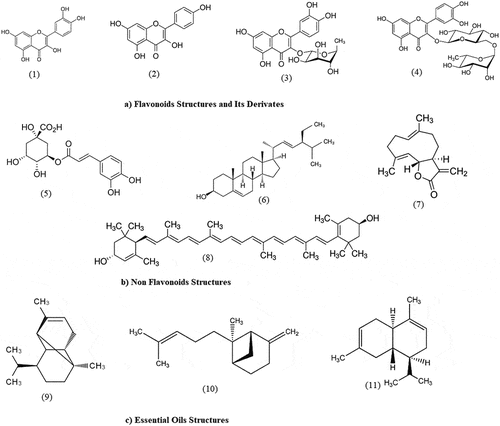
Furthermore, the reported non-flavonoid compounds from C. caudatus leaves were phenolics, benzoic acid derivatives, cyclohexene-1-carboxylic, chlorogenic, α-Linolenic, and ascorbic acids, as well as α-tocopherol, Myo-inositol, α-D-glucopyranoside, 4,4′ bipyridine, diterpenoids, costunolide, stigmasterol, lycopene, and lutein. These active compounds were identified in the leaves by HPLC and HPLC-MS analyses. Moreover, several individual compounds have also been isolated 4 active compounds which include (1) 4,4′ bipyridine, (2) costunolide, (3) stigmasterol, and (4) lutein which is applied as antimutagen and antifungal agents.[Citation18] 13 distinct bioactive components were found in the GC-MS study of C. caudatus fresh leaves, including essential oil which are) α-Cadinene, (E)-Ocimene, 2,6-Dimethyl-1,3,5,7-octatetraene, α-Copaene, β-Elemene, caryophyllene, α-Humulene, γ-Muurolene, bergamotene, β-Selinene, bicyclogermacrene, α-Farnesene, and δ-Cadinene .[Citation7]
. shows that the roots of C. caudatus roots contain only non-flavonoid compounds. Fuzzati et al. (1995) successfully isolated 6 active compounds such as (1) Z-coniferyl alcohol- 3’-acetyl-4-isobutyrate, (2) l’,2’-dihydroxy-coniferyl alcohol-3’-isobutyryl-4-isobutyrate, (3) l’-acetoxy-eugenol-4-isobutyrate, (4) l’,2’-epoxy-Z-coniferyi alcohol-3’-(2-methylbutyryl)-4- isobutyrate, (5) l’,2’-epoxy-Z-coniferyl alcohol-3’-acetyl-4-isobutyrate and (6) l’,2’-epoxy-Z-coniferyl alcohol-3’-isobutyryi- 4-isobutyrate. The presence of these phytoconstituents in the C. caudatus leaves may help to predict the potential medicinal effect in pharmaceutical applications. Traditionally, C. caudatus has been used as an antioxidant agent and gives beneficial effects on human health, including anti-diabetic, anti-hypertensive, anti-inflammatory, bone-protective, anti-microbial,[Citation19] anti-quorum sensing, antifungal, hepatoprotective, detoxification, and anti-hyperlipidemic activities.[Citation11] . shows the chemical structure of several bioactive compounds present in this plant.
Medicinal uses of C. caudatus Studies
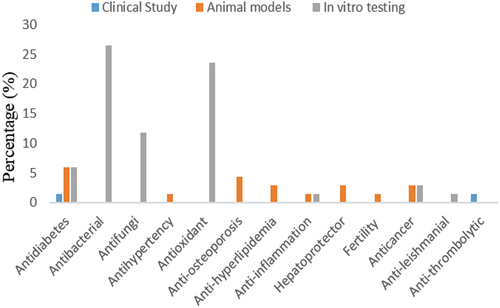
Traditionally, its leaves are used as a blood circulation booster, bone strengthener, body-cooling agent, and anti-aging agent[Citation20] Numerous in vitro and in vivo investigations have therefore been done to support the traditional usage, efficacy, and efficiency of C. caudatus in the treatment of a variety of ailments, including diabetes, bacterial and fungal infections, hypertension, oxidation, etc. depicts the documented scientific research of its many traditional usage as a medicinal plant.
. shows that many studies have been conducted to confirm C. caudatus antibacterial and antioxidant potentials using in vitro assays. These activities were also found to be correlated with the bioactive compounds contained in the plant. Seyedreihani et al.[Citation17] reported that this plant contains bioactive compounds such as quercitrin, catechin, and rutin which are well-known for their antioxidant effects. Hence, C. caudatus is a natural source of antioxidants in food and medicinal applications, which is a priority in several countries.[Citation21] . also shows that C. caudatus exerts many pharmacological activities confirming its beneficial effects on humans. Human clinical investigations have confirmed that the fresh leaves of C. caudatus have anti-diabetic benefits via lowering blood glucose. However, clinical trials are required to substantiate its other purported pharmacological properties and traditional claims. The clinical trial’s anti-diabetic results demonstrate the safety and efficacy of the fresh leaves of C. caudatus, as well as their anti-diabetic properties.[Citation2,Citation22]
C. caudatus has been the subject of a number of research that have profiled its active chemicals,[Citation21,Citation23,Citation24] metabolomics approaches,[Citation22,Citation23,Citation25] and the isolation of pure active compounds from the leaves and roots[Citation18,Citation26] to further confirm its beneficial role in the traditional medicinal systems. The profiling technique was conducted to ascertain the active compounds contained in the herb, while the metabolomic approach was carried out to look for potential bioactive compounds that play a role in pharmacological activities. Based on the metabolomic approach, it is possible to find active compounds that provide pharmacological effects. Meanwhile, the isolation technique was conducted to identify potential bioactive compounds from the C. caudatus that had not earlier been reported using the metabolomic approach. This plant was previously subjected to the isolation of pure active principle on the leaves and roots parts which were considered to possess antioxidants, antimutagen, and antifungal properties. However, more phytochemical studies are still warranted to isolate other active principles present in the leaves and roots of C. caudatus which may be responsible for a variety of different pharmacological effects. Moreover, the application of C. caudatus is not only traditionally consumed, but it can be processed into several commercial products such as herbal tea, cream ingredients, and herb products in capsule forms for various medicinal uses.
Pharmacological activities
The traditional uses of C. caudatus and various scientific studies have been reported to confirm the beneficial effects of this plant on human health owing to the fact that it contains active compounds that play a role in treating different diseases. These active compounds are grouped into flavonoids and their derivatives, non-flavonoid compounds, and essential oil (). displays the in vitro tests, in vivo experiments, and clinical trials were undertaken on C. caudatus efficacy. According to , the varied pharmacological activities of C. caudatus depend mostly on the extract types, since the different solvents employed in the extraction process are believed to produce variations in the extraction of bioactive metabolites from the plant’s material of interest). Therefore, this review discusses and highlights the predicted active compounds of C. caudatus which have great potential for pharmacological activities.
Table 1. Potency of Cosmos caudatus on its pharmacological activities.
Antidiabetic activity
Diabetes mellitus (DM) is a general class of heterogeneous metabolism disorder associated with chronic hyperglycemia caused by insulin secretion, its activity, or both.[Citation38] In general, DM is classified into type 1 DM and type 2 DM. Type 1 DM can be traced through insulin secretion, where the body does not produce enough insulin. Meanwhile, type 2 DM is caused by insulin resistance or relative deficiency and is normally known as non-insulin-dependent diabetes mellitus.[Citation39] Both types 1 and 2 DM require different treatments. Type 1 DM can be treated using insulin injection to control blood glucose levels in the body, while type 2 DM can be treated with drugs to increase the secreted insulin, improve sensitivity, or decrease the absorbed glucose rate.[Citation40]
Based on an in vitro testing, the C. caudatus leaves ethanol extract inhibited α-glucosidase with IC50 values ranging 12.6–40.9 μg/mL (). Besides, n-hexane extract of the leaves also inhibited α-glucosidase and α-amylase, but dichloromethane extract did not show inhibitory activity against both the enzymes.[Citation41] Both ethanol and n-hexane extracts are candidate drugs which may be applied as anti-diabetic agents to treat postprandial hyperglycemia. These results have been supported by in vivo and clinical testing in humans. C. caudatus extract at 200 mg/kg and 400 mg/kg body weight (BW) of male rats was effective in reducing blood glucose levels on different days, viz. 35 and 49 days, respectively. Meanwhile, a dose of 100 mg/kg BW showed no significant effect on the reduction of blood glucose levels.[Citation5] Furthermore, individuals with type 2 diabetes who were given fresh C. caudatus leaves (15 grammes per day for eight weeks) shows significant improvements in their insulin sensitivity.[Citation2] According to the findings of this research study, C. caudatus is possible to be used as an adjuvant therapy in patients who are registered with ClinicalTrials.gov. Identifier: NCT02322268.[Citation42] The high potency of C. caudatus leaves as an anti-diabetic agent is ascribed to several active phytoconstituents such as quercetin, kaempferol, myricetin, catechin, luteolin, apigenin, quercetin 3-O-rhamnoside (quercitrin), quercetin 3-O-glucoside, quercetin 3-O-arabinofuranoside, rutin, phenolic acid, chlorogenic acid, α-Linolenic acid, Myo-inositol, diterpenoids, costunolide, and stigmasterol identified based on the profiling techniques and metabolomic approach. Wan-nadilah et al.[Citation22] reported that quercetin derivatives are potential active compounds that play a role in the treatment of diabetes. However, the isolation of active compounds from C. caudatus as an anti-diabetic agent has not been reported yet. Therefore, isolation and identification of active compounds as antidiabetic agents is an important part of drug discovery in future studies.
Antibacterial activity
The active compounds contained in C. caudatus leaves have been reported to be applied as antibacterial agents. These active compounds are essential oils extracted from fresh leaves, while flavonoid and their derivatives are obtained from the ethanolic extract. According to research conducted by Lee and Vairappan,[Citation7] the bacterial activity of the essential oil that is extracted from fresh leaves can inhibit Salmonella sp. However, the ethanolic extract of the plant produced a higher level of inhibitory activity on other bacteria, including Proteus mirabilis, Salmonella typhimurium, Staphylococcus aureus, Listeria monocytogenes, and Vibrio cholerae. Additionally, C. caudatus leaves methanolic extract was reported to inhibit the growth of microflora in oyster mushrooms, such as E. coli and S. aureus, when applied at a concentration of 0.05% for ten minutes and 0.05% for fifteen minutes.[Citation3]
Other extracts including n-hexane, ethanol, and diethyl ether extracts of C. caudatus also found to inhibit S. aureus growth that can be expressed by minimum inhibitory concentrations (MIC) values of 25, 6.25, 6.25 mg/ml, respectively.[Citation10] These MIC values indicated that the leaf extracts of C. caudatus could be a useful source of antibacterial chemicals due to the presence of multiple possible antimicrobial components. Several compounds present in the essential oil such as α-Cadinene, (E)-Ocimene, 2,6-Dimethyl-1,3,5,7-octatetraene, α-Copaene, β-Elemene, caryophyllene, α-Humulene, γ-Muurolene, Bergamotene, β-Selinene, Bicyclogermacrene, α-Farnesene, and δ-Cadinene have the potential to be employed as antibacterial agents).[Citation7] According to the profiling method, the leaf extract possesses compounds such as quercetin, catechin, luteolin, quercetin 3-O-rhamnoside (quercitrin), quercetin 3-O-glucoside, quercetin 3-O-arabinofuranoside, benzoic acid, chlorogenic acid, 4,4’ bipyridine, diterpenoids, and stigmasterol, all of which have the potential to inhibit the growth of bacteria.[Citation7] Moreover, Ragasa et al.[Citation18] isolated stigmasterol, lutein, 4,4’ bipyridine and costunolide from the chloroform extract of C. caudatus as antibacterial compounds.
Antifungal activity
C. caudatus leaf extracts viz. n-hexane, ethanol, and diethyl ether extracts showed a great antifungal potential as it inhibited C. albicans growth with a minimum inhibitory concentrations (MIC) values of 25, 6.25, 6.25 mg/ml, respectively.[Citation10] Moreover, the ethyl acetate (EtOAc) fraction exhibited inhibition against the several fungal strains found in the trees namely P. palmivora (found in Theobroma cacao), C. gloeosporioides (found in Carica papaya), C. gloeosporioides (found in Mangifera indica) with 52%, 23.5%, and 18% growth inhibition, respectively.[Citation43] C. caudatus leaves and roots were shown to contain hydroxyeugenol and oniferyl alcohol as antifungal agents.[Citation26] In addition, Ragasa et al.[Citation18] reported that chloroform extract of C. caudatus leaves contained active compounds such as 4,4′ bipyridine, costunolide, stigmasterol, and lutein that can be applied as antimutagenic, antimicrobial, and antifungal agents.
Effect of antihypertensive
Several active metabolites from C. caudatus, including flavonoids, phenolic acids, and diterpenoids, have been reported to reduce hypertension).[Citation11] A previous study demonstrated that aqueous extract of C. caudatus leaves at doses of 500 and 1000 mg/kg decreased heart rate and amplitude of stroke volume, where these parameters are harmful indications of hypertension.[Citation28] Besides, both n-hexane and dichloromethane extracts had a positive effect on hypertension through ACE inhibition.[Citation41] Therefore, this plant has been suggested to be effective in reducing hypertension related problems and controlling heart rate.
Antioxidant activity
Oxidation reactions are responsible for the damage that is done to tissue biomolecules such as proteins, lipids, and deoxyribonucleic acid (DNA). The long-term effects can lead to chronic diseases such as cancer, aging, inflammation, diabetes, and rheumatoid arthritis.[Citation44,Citation45] Therefore, the exploitation of natural antioxidant sources is one of the solutions to prevent oxidation reactions and they are safer for consumption. C. caudatus is a herbal plant that has high total phenolic content (TPC) and antioxidant activities. Several active compounds in this plant presenting as antioxidant agents are flavonol and flavoneglycosides,[Citation28] as well as proanthocyanidins including quercetin glycosides, chlorogenic, neo-chlorogenic, crypto-chlorogenic, etc..[Citation24]
The TPC of C. caudatus leaves ranged from 36.09 to 37.76 mg gallic acid equivalent (GAE) per gram of dried plant material.[Citation17] TPC is affected by the solvent used in the extraction process. Mediani et al.[Citation21] observed that 80% of methanol and 80% of ethanol extracts of C. caudatus leaves contained less total phenolic content (TPC) than water extract. In addition, butanol fractionation was shown to rise by more than 1.5 times.[Citation46]
In most cases, a higher level of TPC will result in increased antioxidant activity. The IC50 value for the ethanol extract’s activity in inhibiting DPPH radicals ranged from 32 to 55 ug/ml.[Citation8,Citation21,Citation29] In addition to this, it was discovered that it had the ability to reduce ABTS with an IC50 value of 31.97 ± 1.42 g/mL[Citation8] and that it possessed the antioxidant power to reduce ferric (Fe3+) by approximately 172 ± 1 mol TE/fresh weight[Citation9] or 50.08 ± 0.71 mg ascorbic acid equivalent (AAE)/g.[Citation7]
According to these findings, the ethanol extract of this plant could be used as an anti-aging agent.[Citation25] Due to its high antioxidant activity, this plant is also recommended for use in herbal tea which was demonstrated by ferric reducing antioxidant power (FRAP) and DPPH inhibitory activity of 502.21 21.18 M Trolox equivalent (TE)/ml and 1055.36 42.38 g/ml of tea samples, respectively.[Citation31] However, the pro-oxidant activities from the aqueous extract of C. caudatus leaves need to be tested at high doses (1000 mg/kg body weight), as it was found that low doses did not have any effect.[Citation30]
Effect of anti-osteoporotic
The antioxidant and mineral contents in C. caudatus leaves have been suggested to be used to treat osteoporosis. Aqueous leaves extract of C. caudatus at a dose of 500 mg/kg was found to protect against bone damage in post-menopausal osteoporosis problems.[Citation32,Citation33] There were no negative effects caused by leaves extract on osteoclasts, even though it increased dynamic and cellular histomorphometry parameters,[Citation33] as well as increased biomechanical features of the healed bone.[Citation47] Furthermore, the use of C. caudatus leaves extract with 1% calcium was reported to increase bone resorption, which is shown by an increase in serum interleukin-1 (IL-1) and pyridinoline levels.[Citation48]
Anti-hyperlipidemic activity
The ethanol leaves extract of C. caudatus produced anti-obesity activity through pancreatic lipase inhibition (21.7 ± 1.3%) at a dose of 1000 ppm. This is because of the fact that pancreatic lipase inhibitors are one of the parameters that are generally taken into account to evaluate the use of herbs as anti-obesity agents.[Citation29] Besides, C. caudatus extract was found to be more effective in controlling weight gain, visceral fat mass, plasma total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-c), leptin, and insulin. Moreover, C. caudatus was also found to increase high-density lipoprotein cholesterol (HDL-c) on rats.[Citation49 Perumal et al.[Citation34] also reported that the ethanolic extract of C. caudatus leaves at a dose of 200 mg/kg body for 4 weeks can reduce plasma triglycerides, total cholesterol, low-density lipoprotein-cholesterol, and glucose. C. caudatus significantly increased the high-density lipoprotein-cholesterol and atherogenic index values in rats. Tandi et al.[Citation5] reported that C. caudatus extract was effective in lowering total cholesterol and had a better effect on regenerating the pancreatic tissue at a dose of 400 mg/kg BW. The capacity of C. caudatus leaves ethanol extract to demonstrate an anti-obesity action can be attributed to the fact that the extract includes a number of potential active components including catechin, quercetin, rutin, kaempferol, and chlorogenic acid which have all been earlier reported for their anti-hyperlipidemic or hypolipidemia activity.[Citation49]
Anti-inflammatory activity
The leaves of C. caudatus have been found to contain a number of possible bioactive chemicals, some of which have been hypothesized to have an anti-inflammatory effect. These constituents consist of, among others, ascorbic acid, quercetin, and chlorogenic acid.[Citation19] A previous study reported that the methanol and aqueous extracts of C. caudatus leaves at a dose of 200 mg/kg body weight were found to reduce the inflammatory process, whereas both extracts suppressed Carrageenan induced paw edema in mice for 4 hours.[Citation36] The ethanol extract of C. caudatus leaves was able to prevent the platelet aggregation that was caused by adenosine diphosphate (ADP) with an IC50 value of 87.26 mg/ml. Platelet aggregation is generally thought to be the cause of strokes because it leads to persistent inflammation of the arterial walls.[Citation35]
Hepatoprotective activity
The potent phytotherapeutic modalities against hepatotoxicity have prompted many natural product scientists to investigate several plants and multi-herbal preparations due to the fact that conventional drug discovery has been considered more expensive and tiresome. In this regard, the hepatoprotective effect of plant-based herbal products has been evaluated through an animal model in which key enzymes of the liver such as alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST), as well as total bilirubin in plasma were taken into consideration to confirm the hepatoprotective potential of traditional medicinal plants.[Citation50] The use of the C. caudatus leaves as a hepatoprotective agent was evaluated by Abdullah et al.[Citation30] In this study, an aqueous leaf extract at a dose of 1000 mg/kg body weight was found to exhibit protection toward several mice vital organs including lungs, kidneys, and stomach. Therefore, C. caudatus leaves were suggested to be used as a potential hepatoprotective agent because of their hepatoprotective characteristic demonstrated in a mice model.
Fertility effect
The use of C. caudatus leaves to treat fertility related problems has been reported. C. caudatus ethanol extract (CCEE) was found to increase quality of sperm indices such as its motility, count, and morphology.[Citation51] However, aqueous extract at 500 mg/kg body weight for 28 days had no significant effect on fertility and the body weight of female mice compared to the control.[Citation37] A higher concentration of C. caudatus leaf extract may be more efficient than a lower concentration in maintaining sperm quality.[Citation52] In addition, CCEE was found to enhance urine output in rats and decrease prostate volume, suggesting that it may be effective for controlling the reproductive health of rats with benign prostatic hyperplasia (BPH) or as an anti-BPH agent.[Citation53] In fact, both extracts did not show any negative effect on fertility in female mice and were considered to be safer for consumption.
Anticancer activity
DNA damage has been long recognized as a pivotal factor for cancer occurrence. When flawed DNA repair leads to mutations or chromosomal abnormalities affecting oncogenes and tumor suppressor genes, cells go through malignant transformation resulting in cancerous growth. As a result, DNA damage and unchecked cell proliferation are generally thought to be the driving forces behind the development of a cancer disease. It has been claimed that the extract of C. caudatus leaves has significant antioxidant qualities, which may explain why it has been shown to be effective in preventing cancer. Evaluations of the in vitro cytotoxicity of herbal-based products, including traditional medicinal plants used to treat cancers, are typically conducted against various cancer cell lines, such as Hella cells. These evaluations are carried out in order to confirm the anticancer potential of herbal-based products.[Citation8,Citation54] C. caudatus leaves ethanol extract demonstrated an IC50 value of 89.90 ± 1.30 ug/mL against the Hella cell, indicating the leaves’ potential to inhibit cancer growth.[Citation8] In addition, the ethyl acetate extract of C. caudatus leaves resulted in cytotoxic activity with an IC50 value of 6.31 ppm. This demonstrates that the ethyl acetate extract is more effective as an anticancer agent than the ethanol extract.[Citation54]
Other biological activities
This medicinal plant is also employed in the treatment of leishmaniasis and thrombolysis. Leishmaniasis is a disease of humans caused by protozoan parasites of the genus Leishmania,[Citation55] whereas thrombolysis is a treatment for blood loss in the event of an accident. According to Topcu and Goren,[Citation56] diterpenoids are one of the active chemical classes that are thought to be antileishmanial agents. Several active chemicals including oleanolic acid, polysaccharides, gentiopicroside, sweroside, swertiamarin, shikonin derivatives (deoxyshikonin, acetyl shikonin, 3-hydroxy-isovaleryl shikonin, and 5,8-Odimethyl acetyl shikonin), asiaticoside, asiatic acid, madecassic, quercetin, isorhamnetin, kaempferol, curcumin, sesamol (3,4 methylenedioxyphenol), coluteol, colutequinone B, hyperforin, catechins, and isoflavonoids have been discovered to have a substantial part in the process of limiting the amount of blood loss that is occurring.[Citation57] There have been reports that this medicinal plant does constitute of these active chemicals, hence C. caudatus leaves have the potential to be utilized in the treatment of several disorders.[Citation11,Citation58] Nonetheless, Salim et al.[Citation59] demonstrated that the methanol extract of C. caudatus leaves may not be an effective treatment for leishmania, as its IC50 was greater than 200 g/mL. Therefore, its usefulness as an anti-leishmanial drug needs to be established with further research in order to move forward with clinical trials. Additionally, an investigation along these lines ought to be carried out in order to provide additional evidence that the leaves of C. caudatus might in fact be useful as a thrombolytic agent.
Toxicological evaluation and future recommendation
Consumption of fresh C. caudatus leaves as much as 15 g have been proven and recommended safe as a supplementation agent in the treatment of diabetes .[Citation2,Citation11] C. caudatus extract has also been shown to be safe, and it has the potential to improve kidney function when administered at a dose of 50 mg extract/kg of body weight in rats.[Citation60] According to the findings of another investigation, C. caudatus extract is efficacious and safe on brine shrimp nauplii at concentrations below 100 g/mL. On the other hand, it was discovered that it caused a death rate of one hundred percent at 400 and 800 g/mL, and the Lethal concentration doses (LC50) were discovered to be between 100 and 200 g/mL.[Citation61]
The administration of a high dose of 2,000 mg/kg of aqueous extract has the potential to induce acute hepatotoxicity in male rats. This was observed in the post-treatment data as a reduction in serum albumin levels.[Citation60] Therefore, C. caudatus extracts can affect human health and is considered safe to be consumed at lower doses. However, it has been reported to demonstrate acute hepatotoxicity at higher doses.[Citation1] The limitation of the toxicological evaluation of this herb still needs to be tested using different in-vitro assays and animal models. Moreover, the previously isolated active compounds from this plant should also be thoroughly evaluated for their pharmacological effects and toxicity, and safe nature to ensure the safety of all the compounds to be consumed by humans. Hence, it is suggested to follow the steps of toxicological evaluation as represented in by Sharwan et al.[Citation62]
Figure 6. Proposed toxicological evaluations of herbal extracts and isolated bioactive compounds (Adapted from Sharwan et al., [Citation62]).
![Figure 6. Proposed toxicological evaluations of herbal extracts and isolated bioactive compounds (Adapted from Sharwan et al., [Citation62]).](/cms/asset/8db23234-1b90-4dd7-8889-58b06a83621c/ljfp_a_2158862_f0006_b.gif)
In point of fact, the research and development of herbal remedies derived from C. caudatus to treat a variety of illnesses are still in its infancy. Tea preparations that are based on this plant have been offered as potential antioxidants, and the fresh leaves and extracts of the C. caudatus plant have been suggested as a potential alternative treatment for diabetes. In addition, the use of C. caudatus for the treatment of other disorders is still done in the form of an extract. As a result, this article presents the most recent findings from research on C. caudatus so that the use of this plant can be expanded to include other herbal products in addition to its extract.
Conclusion
The leaves of C. caudatus have been reported in the treatment of various illnesses, particularly by functioning as an antihypertensive, antidiabetic, antioxidant, antiosteoporosis, antifungal, or antibacterial agent, among other potential uses. However, additional focus and research are required on this plant in order to have a deeper understanding of the role it plays as an anti-leishmanial and anti-thrombolytic agent. Its medicinal potential has been studied in vivo in a variety of ways, but just one human clinical trial (Identifier number: NCT02322268) has provided sufficient evidence of its actual medicinal efficacy in treating an ailment as diabetes. As a result, the great potency of this herb ought to be further verified, along with its efficiency and security, by conducting additional clinical tests on humans to examine its other pharmacological actions and any adverse effects. This review demonstrates that the uses and pharmacological research of C. caudatus are currently restricted to fresh leaves and the crude extract of the leaves. Additional research is required to establish whether the particular chemicals and other components of this plant play the role of pharmacological actions as hypothesized. In the event that the findings are positive, they will contribute to the development of more herbal-based pharmaceutical treatments that cure various ailments more effectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bunawan, H.; Baharum, S. N.; Bunawan, S. N.; Amin, N. M.; Noor, N. M. Cosmos Caudatus Kunth: A Traditional Medicinal Herb. Gjp. 2014, 8(3), 420–426.
- Cheng, S. H.; Ismail, A.; Anthony, J.; Ng, O. C.; Hamid, A. A.; Barakatun-Nisak, M. Y. Eight Weeks of Cosmos Caudatus (Ulam Raja) Supplementation Improves Glycemic Status in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Evid. Based Complement. Alternat. Med. 2015, 1–7. DOI: 10.1155/2015/405615.
- Yosoff, N. A. H.; Sanuan, F. M.; Rukayadi, Y. Cosmos Caudatus Kunth. Extract Reduced Number of Microflora in Oyster Mushroom (Pleurotus Ostreatus). IFRJ. 2015, 22(5), 1837–1842.
- Javadi, N.; Abas, F.; Mediani, A.; Hamid, A. A.; Khatib, A.; Simoh, S.; Shaari, K. Effect of Storage Time on Metabolite Profile and alpha-glucosidase Inhibitory Activity of Cosmos Caudatus leaves-GCMS Based Metabolomics Approach. JFDA. 2015, 23(3), 433–441. DOI: 10.1016/j.jfda.2015.01.005.
- Tandi, J.; Claresta, J. A.; Ayu, G.; Irwan, I. Effect of Ethanol Extract of Kenikir (Cosmos Caudatus Kunth.) Leaves in Blood Glucose, Cholesterol and Histopathology Pancreas of Male White Rats (Rattus Norvegicus). IJPST. 2018, 5(1), 1–7.
- Moshawih, S.; Cheema, M. S.; Ahmad, Z.; Zakaria, Z. A.; Hakim, M. N. A Comprehensive Review on Cosmos Caudatus (Ulam Raja): Pharmacology, Ethnopharmacology, and Phytochemistry. IRJES. 2017b, 1(1), 14–31.
- Lee, T. K.; Vairappan, C. S. Antioxidant, Antibacterial and Cytotoxic Activities of Essential Oils and Ethanol Extracts of Selected South East Asian Herbs. J. Med. Plant Res. 2011, 5(21), 5284–5290.
- Nurhayati, B.; Rahayu, I. G.; Rinaldi, S. F.; Zaini, W. S.; Afifah, E.; Arumwardana, S.; Kusuma, H. S. W.; Rizal,; Widowati, W.; Rizal, R. The Antioxidant and Cytotoxic Effects of Cosmos Caudatus Ethanolic Extract on Cervical Cancer. Indones Biomed. J. 2018, 10(3), 243–249. DOI: 10.18585/inabj.v10i3.441.
- Andarwulan, N.; Batari, R.; Sandrasari, D. A.; Bolling, B.; Wijaya, H. Flavonoid Content and Antioxidant Activity of Vegetables from Indonesia. Foodchem. 2010, 121(4), 1231–1235.
- Rasdi, N. H. M.; Abd. Samah, O.; Sule, A.; Ahmed, Q. U. Antimicrobial Studies of Cosmos Caudatus Kunth. (Compositae). JMPR. 2010, 4(8), 669–673.
- Chan, E. W. C.; Wong, S. K.; Chan, H. T. Ulam Herbs of Oenanthe Javanica and Cosmos Caudatus: An Overview on Their Medicinal Properties. JNR. 2016, 16(4), 137–147. DOI: 10.18311/jnr/2016/8370.
- Sukarso, S.; Fidrany, I.; Anggadireja, K.; Handayani, W. A.; Anam, K. Influence of Drying Method on Flavonoid Content of Cosmos Caudatus (Kunth) Leaves. Rjmp. 2011, 5, 189–195.
- Wiart, C.;. Medicinal Plants in Asia for Metabolic Syndrome: Natural Products and Molecular Basis; CRC press Taylor and Francis Group: Boca Raton London New York, 2018.
- Quattrocchi, U.;. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press, Taylor and Francis Group: Boca Raton London New York. Page, 2012; pp 1149.
- Cheng, S. H.; Khoo, H. E.; Ismail, A.; Abdul-Hamid, A.; Barakatun-Nisak, M. Y. Influence of Extraction Solvents on Cosmos Caudatus Leaf Antioxidant Properties. Iran. J. Sci. Technol. Trans. A: Sci. 2016, 40(1), 51–58.
- Salamah, A.; Oktarina, R.; Ambarwati, E. A.; Putri, D. F.; Dwiranti, A.; Andayani, Chromosome Numbers of Some Asteraceae Species from Universitas Indonesia Campus, Depok, Indonesia,Biodiv. 2018, 19(6), 2079–2087.
- Seyedreihani, S. F.; Tan, T.-C.; Alkarkhi, A. F. M.; Easa, A. M. Total Phenolic Content and Antioxidant Activity of Ulam Raja (Cosmos Caudatus) and Quantification of Its Selected Marker Compounds: Effect of Extraction. Int. J. Food Prop. 2016, 20(2), 260–270. DOI: 10.1080/10942912.2016.1155055.
- Ragasa, C. Y.; Nacpil, Z. D.; Penalosa, B. A.; Coll, J. C.; Rideout, J. A. Antimutagen and Antifungal Conpounds from Cosmos Caudatus. Philipp. J. Sci. 1997, 126(3), 199–206.
- Cheng, S. H.; Barakatun-Nisak, M. Y.; Anthony, J.; Ismail, A. Potential Medicinal Benefits of Cosmos Caudatus (Ulam Raja): A Scoping Review. J. Res. Med. Sci. 2015a, 20(10), 1000–1006. DOI: 10.4103/1735-1995.172796.
- Mohd Bukhari, D. A.; Siddiqui, M. J.; Shamsudin, S. H.; Rahman, M. M.; Z, S. S. α-Glucosidase Inhibitory Activity of Selected Malaysian Plants. J. Pharm. Bioall. Sci. 2017, 9(3), 164–170. DOI: 10.4103/jpbs.JPBS_35_17.
- Mediani, A.; Abas, F.; Khatib, A.; Tan, C. P. Cosmos Caudatus as a Potential Source of Polyphenolic Compounds: Optimisation of Oven Drying Conditions and Characterisation of Its Functional Properties. Molecules. 2013, 18(9), 10452–10464. DOI: 10.3390/molecules180910452.
- Wan-Nadilah, W. A.; Akhtar, M. T.; Shaari, K.; Khatib, A.; Hamid, A. A.; Hamid, M. Variation in the Metabolites and α-glucosidase Inhibitory Activity of Cosmos Caudatus at Different Growth Stages. BMC Complement. Altern. Med. 2019, 19(1), 1–15. DOI: 10.1186/s12906-019-2655-9.
- Javadi, N.; Abas, F.; Hamid, A. A.; Simoh, S.; Shaari, K.; Ismail, I. S.; Mediani, A.; Khatib, A. GC-MS-based Metabolite Profiling of Cosmos Caudatus Leaves Possessing alpha-glucosidase Inhibitory Activity. J. Food Sci. 2014, 79(6), C1130–C1136. DOI: 10.1111/1750-3841.12491.
- Shui, G.; Leong, L. P.; Wong, S. P. Rapid Screening and Characterisation of Antioxidants of Cosmos Caudatus Using Liquid Chromatography Coupled with Mass Spectrometry. J. Chromb. 2005, 827(1), 127–138. DOI: 10.1016/j.jchromb.2005.07.029.
- Hussin, M.; Abdul Hamid, A.; Abas, F.; Ramli, N. S.; Jaafar, A. H.; Roowi, S.; Majid, N. A.; Pak Dek, M. S. NMR-based Metabolomics Profiling for Radical Scavenging and anti-aging Properties of Selected Herbs. Molecules. 2019, 24(17), 3208. DOI: 10.3390/molecules24173208.
- Fuzzati, N.; Sutarjadi, Dyatmiko, W.; Rahman, A.; Hostettmann, K. Phenylpropane Derivatives from Roots of Cosmos Caudatus. Phytochemistry. 1995, 39(2), 409–412. DOI: 10.1016/0031-9422(95)00031-2.
- Wan-Nadilah, W. A.; Khozirah, S.; Khatib, A.; Hamid, A. A.; Hamid, M. Evaluation of the α-glucosidase Inhibitory and Free Radical Scavenging Activities of Selected Traditional Medicine Plant Species Used in Treating Diabetes. IFRJ. 2019, 26(1), 75–85.
- Amalia, L.; Anggadireja, K.; Sukrasno, Fidrianny, I.; Inggriani, R. Antihypertensive Potency of Wild Cosmos (Cosmos Caudatus Kunth, Asteraceae) Leaf Extract. JPT. 2012, 7(8), 359–368.
- Rahman, H. A.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M. W.; Hamid , A. A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017a, 20(11), 2616–2629.
- Abdullah, A.; Dhaliwal, K. K.; Roslan, N. N. F; Lee, C. H., Kalaiselvam, M., Radman, H. M., Saad, Q. H. M., Jaarin, K. The Effects of Cosmos Caudatus (Ulam Raja) on Detoxifying Enzymes in Extrahepatic Organs in Mice. J. App. Pharm. Sci. 2015, 5(1), 2616–2629.
- Dian-Nashiela, F.; Noriham, A.; Nooraain, H.; Azizah, A. A. Antioxidant activity of herbal tea prepared from Cosmos caudatus leaves at different maturity stages. IFRJ 2015, 22(3), 1189–1194. DOI:.
- Mohamed, A.; Sahhugi, K. K.; Ramli, N. N. F.; Muhammad, C. H. The effects of Cosmos caudatus (ulam raja) on dynamic and cellular bone histomorphometry in ovariectomized rats. BMC Res. Notes 2013, 6(1), 239.
- Mohamed, N.; Khee, S. G. S.; Shuid, A. N.; Muhammad, N.; Suhaimi, F.; Othman, F.; Babji, A. S.; Soelaiman, I. N. The Effects of Cosmos Caudatus on Structural Bone Histomorphometry in Ovariectomized Rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–6. DOI: 10.1155/2012/817814.
- Perumal, V.; Hamid, A. A; Ismail, A.; Saari, N.; Abas, F.; Ismail, I.; Maulidiani, Lajis, N. H, Khatib, A. Effect of Cosmos Caudatus Kunth leaves on the lipid profile of A hyperlipidemia-Induced animal model. J. Food Chem. Nutr. 2014, 02(1), 43–51. DOI: .
- Sandhiutami, N. M. D.; Desmiaty, Y.; Noviyanti. Inhibitory Effect of Lantana camara L., Eclipta prostrata (L.) L. and Cosmos caudatus Kunth. leaf extracts on ADP-induced platelet aggregation. Pharmacog J. 2018, 10(3), 581–5.
- Ajaykumar, T. V.; Anandarajagopal, K.; Sunilson, J. A. J.; Ar`shad, A.; Jainaf, R. A. M.; Venkateshan, H. Anti-inflammation activity of Cosmos Caudatus. IJUPBS. 2012, 1(2), 40–48.
- Daud, D.; Azahar, A.; Abidin, S. S. F. Z., Tawang, A., Ross, E. E. R., Hashim, N. Comparative effects of Cosmos caudatus and Piper sarmentosum aqueous extracts on estrous cycle and fertility in female mice. J Pharm Adv Res. 2018, 1 7 , 346–351.
- Kerner, W.; Brückel, J. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes. 2014, 122(7), 384–386. DOI: 10.1055/s-0034-1366278.
- Baynes, H. W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes. Metab. 2015, 6(5), 1–9.
- Jabir, K. V.; Jayalakshmi, B.; Hashim, K. M. In-vitro Anti Diabetic Studies and Phytochemical Evaluation of Heracleum Candolleanum. Asian J. Plant Sci. Res. 2014, 4(4), 31–36.
- Loh, S. P.; Hadira, O. In Vitro Inhibitory Potential of Selected Malaysian Plants against Key Enzymes Involved in Hyperglycemia and Hypertension. Malays. J. Nutr. 2011, 17(1), 77–86.
- Cheng, S. H.; Ismail, A.; Anthony, J.; Ng, O. C.; Hamid, A. A.; Yusof, B. N. M. Effect of Cosmos Caudatus (Ulam Raja) Supplementation in Patients with Type 2 Diabetes: Study Protocol for a Randomized Controlled Trial. BMC Complement. Altern. Med. 2016, 16(1), 1–8. DOI: 10.1186/s12906-016-1047-7.
- Salehan, N. M.; Meon, S.; Ismail, I. S. Antifungal Activity of Cosmos Caudatus Extracts against Seven Economically Important Plant Pathogens. Int. J. Agric. Biol. 2013, 15, 864–870.
- Ayoub, Z.; Mehta, A. Medicinal Plants as Potential Souece of Antioxidant Agents: A Review. AJPCR. 2018, 11(6), 50–56.
- Chirag, J. P.; Tyagi, S.; Halligudi, N.; Yadav, J.; Pathak, S.; Singh, S. P.; Pandey, A.; Kamboj, D. S.; Shankar, P. Antioxidant Activity of Herbal Plants: A Recent Review. Jddt. 2013, 1(8), 1–8.
- Moshawih, S.; Cheema, M. S.; Ibraheem, Z. O.; Tailan, N. D., Hakim, M. N. Cosmos caudatus extract/fractions reduce smooth muscle cells migration and invasion in vitro: A potential benefit of suppressing atherosclerosis. PJB. 2017a, 2(6), 293–300 .
- Godspower, P. R.; Mohamed, N.; Shuid, A. N. Cosmos Caudatus Enhances Fracture Healing in Ovariectomised Rats: A Preliminary Biomechanical Evaluation. Int. J. Appl. Res. Nat. Prod. 2015, 8(1), 12–19.
- Mohamed, N.; Yin, C. M.; Shuid, A. N.; Muhammad, N.; Babji, A. S.; Soelaiman, I. N. The Effects of Cosmos Caudatus (Ulam Raja) Supplementation on Bone Biochemical Parameters in Ovariectomized Rats. Pak. J. Pharm. Sci. 2013b, 26(5), 1027–1031.
- Rahman, H. A.; Sahib, N. G.; Saari, N., Abas, F., Ismail, A., Mumtaz, M. W., Hamid, A. A. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement Altern Med 2017b, 17(1), 1–17.
- Khan, F. S.; Akram, M.; Aslam, N.; Zaheer, J.; Mustafa, S. B.; Kausar, S.; Khan, A. H.; Khan, I. A.; Munir, N.; Ali Shah, S. M., et al. Phytochemical Analysis and Hepatoprotective Effect of Polyherbal Formulation on CCl4 Induced Hepatotoxicity in Mice. Pak. J. Pharm. Sci. 2018, 31(6(Suppl), 2719–2723.
- Sarpin, N.; Daud, D.; Hashim, S. N. Comparative effects of Cosmos caudatus, Piper sarmentosum and Premna cordifolia ethanolic extracts on mice (Mus musculus) sperm parameters. Malays Appl Biol 2017, 46, 9–14.
- Booh, M. J.; Hashim, H.; Ismail, H. H.; Daud, D.; Samsulrizal, N.; Yahya, M. F. Z. R. Effects of Cosmos Caudatus on Sperm Quality of Mice, Mus Musculus. Malays. Appl. Biol. 2015, 44(1), 89–93.
- Daud, D.; Fekery, N. F. M.; Hashim, N. Reproductive Health of Rats with Benign Prostatic Hyperplasia following Cosmos Caudatus Ethanolic Extract Consumption. J. App. Pharm. Sci. 2017, 7(6), 202–205.
- Dwira, S.; Fadhillah, M. R.; Fadilah, F.; Azizah, N. N.; Putrianingsih, R.; Kusmardi, K. Cytotoxic Activity of Ethanol and Ethyl Acetate Extract of Kenikir (Cosmos Caudatus) against Cervical Cancer Cell Line (HELA). Research J. Pharm. Technol. 2019, 12(3), 1225–1229. DOI: 10.5958/0974-360X.2019.00203.8.
- Colares, A. V.; Almeida-Souza, F.; Taniwaki, N. N.; Souza, C. D. S. F.; da Costa, J. G. M.; Calabrese, K. D. S.; Abreu-Silva, A. L. In Vitro Antileishmanial Activity of Essential Oil of Vanillosmopsis Arborea (Asteraceae) Baker. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–7. DOI: 10.1155/2013/727042.
- Topçu, G.; Gören, A. C. Bological Activity of Diterpenoids Isolated from Anatolia Lamiaceae Plants, Rec. Nat. Prod. 2007, 1(1), 1–16.
- Thakur, R.; Jain, N.; Pathak, R.; Sandhu, S. S. Practices in Wound Healing Studies of Plants. Evid. Based Complement. Alternat. Med. 2011:438056, 2011, 1–17 doi:10.1155/2011/438056.
- Nawi, L.; Musa, N. L. W.; Zain, W. Z. W. M.; Kassim, J.; Karim, S. A. Premilinary Studies on Phytochemical Screening of Ulam and Fruit from Malaysia. E- J. Chem. 2011, 8(s1), S285–S288. DOI: 10.1155/2011/464595.
- Salim, R. J. M.; Krishnasamy, G.; Adenan, M. I.; Norhayati, I.; Jauri, M. H.; Sain, A. A. In Vitro anti-leismanial Activity of Malaysian Medicinal and Forest Plant Species. JTFS. 2018, 30(2), 234–241. DOI: 10.26525/jtfs2018.30.2.234241.
- Norazlina, M.; Ehsan, S. Z.; Adilah, K. N.; Lee, C. P.; Farhana, E.; Derick, P.; Nirwana, S. I.; Nazrun, A. S.; Norliza, M. Acute Toxicity Study of Cosmos Caudatus on Biochemical Parameters in Male Rats. Sains Malays. 2013, 42(9), 1247–1251.
- Rameli, N. M.; Kader, M. A.; Aznan, A. S.; Musa, N. Effect of Cosmos Caudatus Extract on Antibacterial Activity and Lethality Activity of Brine Shrimp. AACL Bioflux. 2018, 11(3), 606–612.
- Sharwan, G.; Jain, P.; Pandey, R.; Shukla, S. S. Toxicity Profile of Traditional Herbal Medicine. Int. J. Ayurvedic Herb. Med. 2015, 1(3), 81–90. DOI: 10.31254/jahm.2015.1306.