?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Herbal medication developed from natural products has antibacterial, antioxidant, and antidiabetic effects on human health. This research aimed at the investigation of the biological activities, estimation of total phenolic and flavonoid content, and Gas Chromatography and Mass Spectrometry (GC-MS) profiling of chemical compounds in Artemisia vulgaris root and leaf grown at three different altitudes of Nepal. The radical scavenging property of the extracts was studied by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, antidiabetic activity was performed by α-amylase inhibition activity, and the total phenolic and flavonoid content was estimated by Folin–Ciocalteu phenol reagent and aluminium chloride colorimetric methods. The IC50 against the DPPH activity shown by the root extract of A. vulgaris grown in Kathmandu was evaluated to be 67.31 ± 2.80 µg/mL, whereas the lowest activity was shown by the leaf extract of A. vulgaris grown in Chitwan of IC50 149.62 ± 2.40 µg/mL. The α-amylase enzyme inhibition activity shown by the root extract grown in Gorkha was found to be 39.43% as compared to the leaf extract grown in Gorkha of 8.69%. The total phenolic content was found to be maximum in root grown in Kathmandu of 96.30 ± 0.52 mg.GAE/g, whereas the least was found in leaf grown in Chitwan of 26.04 ± 1.66 mg.GAE/g. The flavonoid content was found to be maximum in leaf extracts of A. vulgaris grown in Kathmandu of 71.15 ± 2.07 mg.QE/g, whereas the least was found in leaf grown in Gorkha of 31.54 ± 0.70 mg.QE/g. The essential oils extracted from the A. vulgaris growing in Gorkha (862 m altitude) showed the effective antibacterial property against Klebsiella pneumonia and Acinetobacter baumannii with zone of inhibition (ZOI) 12 and 15 mm, respectively. Similarly, the essential oils of A. vulgaris growing in Chitwan (208 m altitude) showed the ZOI 12 and 11 mm against Klebsiella pneumonia and Acinetobacter baumannii, respectively. The positive control used was ampicillin and polymyxin with ZOI of 22 mm. However, the extracts from plants growing in all these altitudes are found to be inactive against these organisms. From the results of this study, it can be concluded that the biological activities of leaf and root extracts of A. vulgaris increased with an increase in altitudes, and the plants grown in high altitudes are the rich sources of secondary metabolites that play a significant role against infectious diseases and diabetes in human beings.
Introduction
The free radicals, generally reactive oxygen species (ROS) and reactive nitrogen species (RNS), are the entire class of highly active species derived from the metabolic activity of oxygen in the human body. These free radicals can cause extensive damage to cells and tissues by oxidative stress. The oxidative damage ultimately caused various degenerative disorders, such as cardiovascular disease, aging, and neurodegenerative diseases such as Alzheimer's disease, mutation, and cancer.[Citation1] The radicals generated in the human body are destroyed by many antioxidants such as superoxide dismutase, catalase and glutathione, α-tocopherol, and ascorbic acid and protect from the damage by ROS and RNS. These antioxidants do not provide complete protection from the attack of free radicals under the conditions of severe oxidative stress.[Citation2] Several compounds showed high antioxidant activities by scavenging the free radicals formed in the human body, such as quercetin, rebamipide, and trolox, as shown in . The mechanism of antioxidant activity is shown in .
Diabetes mellitus is a chronic disease that increases the blood glucose level in the human being due to the disruption of carbohydrate and protein metabolism. The rise in blood glucose level in the human body, decreasing the elasticity of blood vessels and narrowing them, lowers the flow of blood, and it causes a decrease in the oxygen supply to the body organs. The mechanism of α-amylase enzyme inhibition activity is shown in . The low level of oxygen in blood increases the risk of hypertension, macro and microvascular illnesses, heart attack, stroke, peripheral arterial disease, retinopathy, and neuropathy.[Citation3,Citation4]
In recent decades, diabetes mellitus is an emerging health burden due to the lack of production of insulin and resistance to insulin in the human body. Oxidative stress and inflammation contribute to the development of diabetes-associated complications. Therefore, there is an urgent need to develop new antidiabetic agents targeting diabetes and associated complications. The commonly used antidiabetic agents are biguanides, sulfonylureas, meglitinides, α-glucosidase inhibitors, α-amylase inhibitors, and dipeptidyl peptidase-IV inhibitors.[Citation5]
The use of medicinal plant extracts and the isolated pure compounds for the cure of diabetes mellitus has been widely increased in recent decades. The plants’ secondary metabolites play a significant role for the treatment of different kinds of human diseases. Plant-derived secondary metabolites such as steroids, terpenes, flavonoids, alkaloids, essential oils, and phenolic compounds act as antioxidants and anticancer, antiallergic, antiinflammatory, antibacterial, antifungal, and antiaging agents in the human health.[Citation6]
The variation in altitudes of plant growing plays an important role for the production of plant secondary metabolites. The formation of different plant metabolites is due to the difference in wind speed, snowfall, oxygen content, drying soils, frozen soil, atmospheric pressure, and some others of the regions where the plant is growing.[Citation7] Plants growing in high altitudes cope with such situations by producing different chemical compounds like polyphenols, alkaloids, flavonoids, tannins, and terpenoids as specialized metabolites.[Citation8] These secondary metabolites perform certain physiological and ecological functions, which help the plants to adapt to different environmental conditions.[Citation9]
The genera Artemisia is one of the largest of family Asteraceae and consists of shrubby herbs with about 500 species reported throughout the globe.[Citation10] The plant Artemisia vulgaris belonging to family Compositae contains several bioactive secondary metabolites including essential oils, flavonoids, triterpenes, and coumarin as major constituents. Traditionally, A. vulgaris have been extensively used by the people in different communities as antihelmintic, antiseptic, antidiabetic, antidepressant, and vermicides. The root of A. vulgaris has different uses as a tonic for psychoneuroses, depression, autonomic, neurosis, irritability, restlessness, insomnia, and anxiety.[Citation11,Citation12]
The study on the biological activities and quantitative analysis of total phenolic and flavonoid content in methanolic extracts of A. vulgaris growing in different altitudes of Nepal has not yet been well studied. Hence, this research aims to perform a comparative altitudinal impact of A. vulgaris growing in different geographical regions of Nepal for their antioxidant, antidiabetic, and antimicrobial effects along with the estimation of total phenolic and flavonoid content of the plant extracts and GC-MS profiling of chemical compounds in the essential oils extracted from the leaf of A. vulgaris.
Materials and methods
Equipment
The major equipment used in the study are digital weighing machine (GT 210), grinder, rotatory evaporator (Buchi RE 111), water bath (Buchi 461), micropipette, 96-well plate reader, vertex, incubator, hot air oven (Griffin-Grundy), UV–visible spectrophotometer (WPA, supplied by Philip Harris Shenstone, England), hot air oven (Griffin-Grundy), electric grinder, mortar and pestle, and distillation flask (Borosil).
Chemicals
The chemicals and reagents used in this research are of analytical grade. Methanol (Fisher Scientific), acetone (Fischer Scientific), and dimethyl sulfoxide (DMSO, Merck) were purchased from the local vendor of Kathmandu, Nepal. Folin–Ciocalteu phenol reagent (FCR; Sigma Aldrich), α-amylase enzyme, and acarbose (Sigma-Aldrich, St. Louis) were also purchased from Kathmandu, Nepal. Chemicals and reagents such as gallic acid, quercetin, ascorbic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), NaNO2, AlCl3, KOH, and NaOH were of analytical grade (Sigma Aldrich, St. Louis) and purchased from local city Kathmandu, Nepal.
Plant materials and extraction
The plant materials were collected during the mature vegetative stage in February 2019, growing in different altitudes (Chitwan 208 m height with geographic coordinate 27.5291° N, 84.3542° E; Gorkha 862 m height with geographic coordinate 28.2964° N, 84.8568° E; and Kathmandu 1324 m height with geographic coordinate 27.7293° N, 85.3343° E) of Nepal. The herbarium of the plant sample was deposited to the Central Department of Botany, Tribhuvan University, Nepal, for its identification no. 1214. The collected plant parts (leaf and root) were washed, shade dried, and weighed. The dry powder (100 g each) was percolated in methanol for the first 72 h with continuous agitation (). After 72 h of percolation, it was first filtered, and the filtrate was concentrated in the rotatory evaporator at 40°C. The concentrated extracts were kept for air dry and collected in the air-tight vial and preserved at 4°C until use. The yield percentage of the extracts was calculated as follows:
Extraction of essential oils
The essential oils were extracted from the fresh leaves of A. vulgaris (100 g each) collected from the different altitudes by hydro-distillation using the Clevenger type of apparatus for 6 h reflux. The oil was collected and dried over anhydrous sodium sulfate and stored at 4°C until the GC-MS analysis was done.
Total phenolic content
Total phenolic content (TPC) of the extracts was estimated by using FCR using 96-well plate reader, which was slightly modified from colorimetric method.[Citation13] At first, 20 µL of different concentrations of standard 10, 20, 30, 40, 50, 60, 70, and 80 µg/mL gallic acid was loaded on 96-well plate in triplicate by diluting the stock solution of 1000 ppm with distilled water. Then, 20 µL of plant sample of 5000 ppm was loaded on 96-well plate in triplicate. After that in each well plate containing the standard and sample, 100 µL F-C phenol reagents was added followed by 80 µL Na2CO3 solution separately. Then, it was left in dark for 15 min, and the absorbance was taken at 765 nm using micro-plate reader (Synergy LX, Bio-Tek, Instruments, Inc., USA). A calibration curve was constructed by using gallic acid as standard in triplicate reading from which TPC was analyzed and expressed as milligram gallic acid equivalent per gram (mg.GAE/g) of dry extracts. The TPC was calculated using the equation as follows:
where C = total content of the phenolic compounds in mg.GAE/g, c = concentration of gallic acid established from the calibration curve (mg/mL), V = volume of extract (mL), and m = weight of the plant extract (mg).
Total flavonoid content (TFC)
The total flavonoid content of each plant extract was estimated by adopting the procedure by Chang et al. (2002).[Citation14] In brief, 130 μL of different concentrations such as 10, 20, 30, 40, 50, 60, 70, and 80 μg/mL standard quercetin was loaded in 96-well plates in triplicate by diluting a stock solution of 0.1 mg/mL with distilled water. Then, 20 μL of plant extract of 5 mg/mL was loaded on 96-well plates in triplicate, and then, 110 μL of distilled water was added to each well for maintaining the final volume of 130 μL. Then to each well containing standard quercetin solution and plant extract, 60 μL ethanol, 5 mL AlCl3, and 5 μL potassium acetate were added separately. Then, it was left in dark for 30 min, and the absorbance was taken at 415 nm using a microplate reader (Synergy LX, Bio-Tek, Instruments, Inc., USA). The total flavonoid content was estimated using the equation as follows:
where C = total flavonoid content in mg.QE/g, c = concentration of quercetin established from calibration curve (mg/mL), V = volume of the extract in mL, and m = weight of the plant extract in mg. Data were recorded as a mean of determinations of absorbance for each concentration, from which linear correlation coefficient (R2) value was calculated. The regression equation is given as follows:
where y = absorbance of the extract, m = slope from the calibration curve, x = concentration of the extract, and c = intercept.
Antioxidant activity (DPPH assay)
The 0.1 mM DPPH solution was prepared by dissolving 3.9 mg DPPH in 100 mL methanol in a volumetric flask covered with aluminum foil. The stock solution of quercetin was prepared by dissolving 1.0 mg of quercetin in 1 mL of methanol. Then, the final concentration of 20, 10, 5, 2.5, and 1.25 μg/mL was prepared by serially diluting the stock solution. A stock solution of plant extract was prepared by dissolving 50 mg of plant extracts in 1 mL of DMSO using a vortex. The final concentrations of plant extracts were prepared such as 1000, 500, 250, 125, and 62.5 μg/mL in 50% DMSO. The antioxidant activity was evaluated by following the procedure by Subedi et al. 2014.[Citation15] In brief, quercetin was used as a positive control and 50% DMSO was used as a negative control. The positive control quercetin, negative control DMSO, and plant samples were loaded in 96-well plates in triplicate. Then, the DPPH reagent was added to each well, and it was incubated for 30 min in dark. After 30 min, the absorbance was taken at 517 nm using a microplate reader.
where A (control) = absorbance reported for the control and A (sample) = absorbance reported for the sample.
α-Amylase inhibition assay
The 100 mM of sodium dihydrogen orthophosphate and disodium hydrogen orthophosphate was prepared, and both buffers were mixed for 50 mL, and pH was maintained at 7. Then, an equal volume of distilled water was added to make buffer 50 mM of 100 mL volume. The plant extracts were prepared 5 mg/mL in 50% DMSO by the serial dilution of the stock solution. The plant extracts which show the α-amylase enzyme inhibition more than 50%, was further diluted as 500, 250, 125, 62.5, and 31.25 μg/mL for IC50 calculation.
The α-amylase enzyme inhibition was studied using 50 mM phosphate buffer of pH 7.0 and 0.9% sodium chloride solution. 80 μL porcine pancreatic α-amylase at the final concentration of 1.5 units/mL (prepared in buffer mentioned above) with the various concentrations of 20 μL of the test compounds (prepared in DMSO) was incubated at 37°C for 15 min. Their action was started by the addition of 100 μL substrate, CNPG 3, at 375 μM final concentrations prepared in the buffer. The change in absorbance by released p-nitroaniline was continuously monitored at 405 nm. The DMSO tolerance was tested and found no effect up to 5% for the inhibition of α-amylase, and therefore, 5% DMSO was taken as control. All the experiments were performed in triplicate in a final volume of 200 µL, by using a microplate reader (SynergyLX, Bio Tek, Instruments, Inc., USA).[Citation16]
where Abs (control) =absorbance reported for the positive control and Abs (sample) = absorbance reported for the plant extracts.
Antibacterial activity
Antibacterial assay of extracts and essential oils was performed by the agar well diffusion method in Mueller–Hinton Agar (MHA) plates.[Citation17] The test organisms were inoculated in Mueller–Hinton broth and incubated at 37°C to adjust the turbidity to 0.5 McFarland standards giving a final inoculum of 1.5 × 108 CFU/mL. The MHA plates were lawn cultured with the above-maintained microbial inoculum. Plant extracts of 5 mg/mL (in 50% DMSO) and 5% concentration of essential oil (100% DMSO) were prepared. Five wells of 8 mm were bored in the cultured lawn media with the help of a sterile cork-borer (8 mm). Each well was filled with 75 μL extracts with a positive control (neomycin 1 mg/mL) and a negative control (50% DMSO for plant extracts and 100% for essential oil) in each plate, respectively. It was allowed to diffuse for about 15 min at room temperature and incubated for 18–24 h at 37°C. After incubation, plates were observed a clear zone of inhibition (ZOI) around the well and measured in mm.
Antifungal activity
Antifungal activity of plant extracts and essential oils was performed by the agar well diffusion method in potato dextrose agar (PDA) plates. The test organisms were inoculated in Potato Dextrose Broth at 37°C to adjust the turbidity to 0.5 McFarland standards giving final inoculum of 1.5 × 108 CFU/mL. The PDA plates were lawn cultured with Candida ablicans in which the plant extracts of 5 mg/mL (in 50% DMSO) and 5% concentration of essential oils (in 100% DMSO) were prepared. Five wells of 4 mm were bored in the cultured lawn media with the help of a sterile cork-borer (4 mm). Each well was filled with 75 μL extract solution and the solution of essential oils prepared from the different samples with a positive control (Fluconazole (IP 2018) 0.5 mg/mL) and a negative control (50% DMSO for plant extracts and 100% DMSO for essential oils) in each plate, respectively. It was allowed to diffuse for about 15 min at room temperature and incubated for 18–24 h at 37°C. After incubation, plates were observed a clear ZOI around the well, and the ZOI was measured in mm.[Citation18,Citation19]
GC-MS analysis of the essential oils
The analysis of the essential oils of A. vulgaris leaf and root growing in different altitudes was performed by using a gas chromatogram of Hewlett-Packard 5890 series II. Helium was the carrier gas at a flow rate of 1 mL/min with a variation of temperature in sample injector and the oven.
Statistical analysis
All the experiments were performed in triplicate, and data were presented in mean ± standard error of the mean. The TPC, TFC, antioxidant activity, and enzyme inhibition results were processed by using Gen5Microplate Data Collection and Analysis Software and then by MS excel 2007. The IC50 value was calculated using GraphPad prism software version 8. The results obtained were compared with the control group, and p < .05 was considered statistically significant.
Results and discussion
Yield value of extracts and essential oils
To know the richness of plant secondary metabolites, the yield percentage of the essential oils and the plant extracts was calculated. The yield percentage of methanolic root extracts and the essential oils of A. vulgaris aerial parts growing in different altitudes of Nepal is shown in .
Table 1. Yield percentage of extracts and essential oils for root and leaf of A. vulgaris growing in different altitudes.
The essential oil yield percentage is lower than that of the root extracts of A. vulgaris. A. vulgaris growing at the altitudes of 208 m is found to be the rich sources of plant secondary metabolites as compared to the plants growing at 862 m and 1324 m, respectively. The production of the plant secondary metabolites depends on the environment of the plant grown and the harvesting time.
Total phenolic and flavonoid content (TPC and TFC)
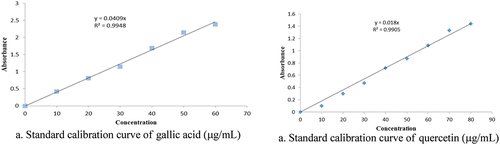
The results of the total phenolic and flavonoid content were estimated with the help of calibration curve as shown in . For the gallic acid (the regression equation y = mx + c, which is displayed as (y = 0.0409x and R2 = 0.9948), whereas for the standard quercetin, it is y = 0.018x and R2 = 0.9905).
The results showed () that the amount of TPC and TFC was found to be highest in extracts of A. vulgaris root and leaf of plants growing in Kathmandu (1324 m) of 96.30 ± 0.52 mg.GAE/g and 71.15 ± 2.07 mg.QE/g, respectively. The A. vulgaris leaf growing in Chitwan (208 m) showed the least TPC of 26.04 ± 1.66 mg.GAE/g, whereas the A.vulgaris leaf growing in Gorkha (862 m) showed the least TFC of 31.54 ± 0.80 mg.QE/g, respectively. The rest of the extracts showed moderate amount of TPC and TFC. The results showed that plants grown in high altitudes are found to be the rich sources of phenolic and flavonoid compounds.
Table 2. Total phenolic and flavonoid contents in the leaf and root extracts of A. vulgaris growing in different altitudes.
Antioxidant activity
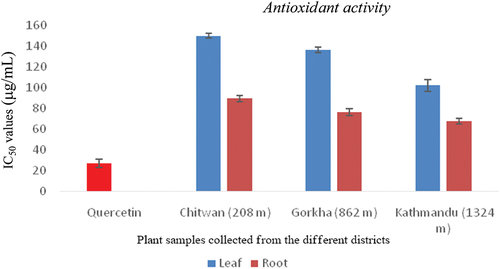
The results of the DPPH free radical scavenging activities are displayed in bar diagram (). The free radical scavenging potential of the plant extracts growing in different altitudes is expressed as IC50 in µg/mL. The A. vulgaris root extract grown in Kathmandu (1324 m) exhibited the strongest free radical scavenging activity with an IC50 of 67.31 ± 2.80 µg/mL followed by the root extract of A. vulgaris grown in Gorkha (862 m) of 75.10 ± 3.3 µg/mL to leaf extract grown in Chitwan (208 m) 149.62 ± 2.40 µg/mL. The results showed that the plant extracts act as moderate free radical scavengers as compared to the positive control quercetin (IC50 = 26.94 ± 3.90 µg/mL).
Antibacterial activity
The results of the antibacterial activity of the root and leaf extracts of A. vulgaris grown in different altitudes are given in . Among the tested six strains of bacteria, the essential oils of A. vulgaris grown in Gorkha (862 m) showed the potential against the Staphylococcus aureus ATCC 43300 with ZOI (11 mm), Klebsiella pneumonia ATCC 700603 (12 mm), and Acinetobacter baumannii ATCC 19606 (15 mm) followed by the essential oils of A. vulgaris grown in Kathmandu (1324 m) showing the same inhibition (15 mm) against Acinetobacter baumannii ATCC 19606, same 12 mm against the Klebsiella pneumonia ATCC 700603, and 10 mm against the Shigella sonnei ATCC 25931. The essential oils of A. vulgaris grown in Chitwan (208 m) showed ZOI 12 mm against the Acinetobacter baumannii ATCC 19606. The results showed that the plants grown in high altitudes are found to be the potent sources of the secondary metabolites and could be used as the source of antibacterial drug candidates in the future drug development process. The root and leaf extracts of A. vulgaris growing in different altitudes were not found to be active against fungus Candida albicans.
Table 3. Zone of inhibition (ZOI) shown by the plant extracts and essential oils growing at different altitudes against six strains of bacteria in agar diffusion test.
α-Amylase inhibitory activity
The preliminary screening of α-amylase enzyme inhibition was performed for 500 µg/µL of plant extracts. All the plant extracts showed inhibition below 50%, due to which the further experiment was not conducted to study the inhibitory activity of the plant extracts for α-amylase. The inhibitory concentration (IC50) was not calculated due to their percentage inhibition less than 50%. The percentage inhibition of plant extract toward α-amylase is shown in .
Table 4. α-Amylase inhibition shown by A. vugaris root and leaf extracts.
The results of α-amylase enzyme inhibition are expressed in percentage. Although all the percentage inhibitions are found less than 50%, the A. vulgaris root extract growing in Gorkha (862 m) showed the most potent α-amylase inhibitory activity of 39.43%, whereas the leaf extract growing in Chitwan (208 m) showed the least inhibitory activity of 8.69%. The rest of the extracts of plants growing in different altitudes showed the moderate inhibitory activity. The plant growing in high altitude showed the high percentage inhibition as compared to the plant growing in low land region. It is due to the formation of different bioactive secondary metabolites affected by the local environment and the conditions required for the plant growth as well as the harvesting period of the plant samples.
GC-MS profiling of compounds in the plant extracts
The essential oils of the different extracts were continued in a Shimadzu GCMS-QP 2010 Plus for the qualitative analysis. The chromatogram of the GC-MS is shown in . The MS Library used for the comparison was FFNSC 1.3, NIST 2017, and from it, the oil components were identified based on their retention indices (RI) and by comparison of their mass spectra fragmentation patterns. The major components present in the samples were different. The results of GC-MS analysis of the A. vulgaris essential oils growing in different altitudes, Chitwan (208 m), Gorkha (862 m), and Kathmandu (1324 m), are given in .
Figure 7. Chromatograms of components of essential oils of A. vulgaris growing in different altitudes such as Chitwan (208 m), Gorkha (862 m), and Kathmandu (1324 m).
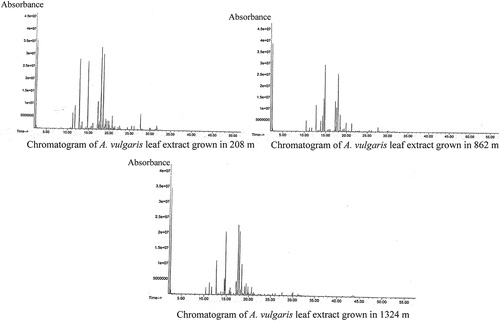
Table 5. Chemical composition of essential oils of A. vulgaris leaf growing in Chitwan (208 m) and Gorkha (862 m).
Table 6. Chemical composition of essential oils of A. vulgaris leaf growing in Kathmandu (1324 m).
The GC-MS analysis showed that many of the compounds like camphene, cyclohexane, eucalyptus, borneol, and caryophyllene are detected in A. vulgaris grown in all three altitudes of Nepal. The compounds carene, thujone, and D-limonene are only detected in the A. vulgaris grown in high-altitude regions. The compounds like α-pinene and α-tocopherol are detected only in the plant grown in low-altitude region. The compounds like β-phellandrene and eugenol are found in plant sample grown in Chitwan (208 m) and Gorkha (862 m). The compound vitamin E is detected in the plant grown at low- and high-altitude regions. This study shows that the altitudes play a significant role in the production of the components of essential oils in A. vulgaris.
Discussion
The present study was conducted to compare the antioxidant, antimicrobial, total phenolic, and flavonoid contents and the α-amylase enzyme inhibition property of root and leaf extracts of A. vulgaris growing in different altitudes of Nepal. Numerous plant extracts showed the free radical scavenging or antioxidant activity. Plants growing in the different environments are the sources of diverse bioactive chemical compounds, also known as secondary metabolites, that play a significant role against the different human diseases as antioxidant, antidiabetic, anti-inflammatory, and so on. The extraction of plant secondary metabolites growing in different altitudes depends on the environmental factors that play a significant role to adapt the plant in particular altitudes. A. vulgaris growing in high altitude is the rich source of secondary metabolites, mainly alkaloids, flavonoids, terpenes, steroids, essential oils, and so on.[Citation20] Phenolic compounds are widely distributed in A. vulgaris and are included as an important part of the human diet owing to their antioxidant and various medicinal values against different human diseases.[Citation21] The results of the present study showed that A. vulgaris grown in different altitudes are the rich source of phenolic and flavonoid contents. The concentration of these constituents varies with the altitudes in which the plant is growing. In our present study, A. vulgaris root and leaf extracts showed the high scavenging activity of DPPH radical. The antioxidant property of A. vulgaris could be attributed to its flavonoid and phenolic contents. A. vulgaris growing in the different altitudes of Nepal showed the moderate antioxidant property in DPPH radical scavenging activity. However, A. vulgaris grown in Nepal at high altitudes showed the highest antioxidant potential of IC50 67.31 ± 2.8 µg/mL, which is found very weak as compared to the IC50 11.4 µg/mL reported by Temraz and Tantawy.[Citation22] The plant A. vulgaris showed the highest phenolic and flavonoid contents in leaf and root extracts growing in Kathmandu (1324 m) of 66.38 ± 3.25 mg.GAE/g and 96.30 ± 0.52 mg.GAE/g and the flavonoid content of 71.15 ± 2.07 mg.QE/g and 63.78 ± 1.59 mg.QE/g, respectively. The results showed that A. vulgaris growing in Nepal is found to be rich in TPC and TFC contents as reported by Temraz and Tantawy, which were 19.0 ± 0.16 mg.GAE/g and 7.96 ± 0.76 mg.QE/g.[Citation22]
The amount of phenolic and flavonoid compounds is high in the plant that is growing at higher altitudes, indicating that the altitude is the major driving force causing the variation in phytochemicals in plants.[Citation23] It has been reported that volatile organic compounds are more abundant in the plants growing at lower altitudes. The volatile organic compounds such as alpha-thujone, camphor, 2-hexadecanol, and geranyl isovalerate protect the human body from oxidative stress.[Citation23] The terpenoids are the major class of compounds consisting of volatile organic compounds that are known to provide an adaptive strategy against heat and light stress in plants.[Citation24,Citation25] The harmful ROS formed in the plants are scavenged by the phenolic acids when the plant is experiencing a combination of abiotic stresses.[Citation26,Citation27] Previously, it has been reported that A. vulgaris extract increases the bioactivity of paraoxonase-1 enzyme that prevents the lipid peroxidation by which the serum concentration is decreased during the inflammation.[Citation28] The secondary metabolites such as flavonoids, sesquiterpenoids, and essential oils such as tannins, phenols, and saponins produced in A. vulgaris are responsible for inhibiting the activity of prostaglandin-synthesizing enzymes.[Citation29] The specific research work has not been conducted to study the variation in the concentration of secondary metabolites and the biological activities of A. vulgaris growing in different altitudes of Nepal. In our study, a higher activity was observed from the plant growing in high altitudes. Various factors such as genetic phase of growth of plants, soil condition, organogenesis, and anatomical parts may influence the quantitative composition of the plant secondary metabolites, which ultimately shows the different pharmacological activities.[Citation30,Citation31] The concentrations and the types of phytochemicals found in the plant sample depend on the variation in the weather conditions and the nature of the soil in which the plant is growing.[Citation31] The phenolic and flavonoid compounds produced in plant have antioxidant and antidiabetic activities. The results of the present study support the fact that A. vulgaris growing in high altitudes showed the highest α-amylase enzyme inhibition activity. It is due to the high concentration of chemical compounds and the nature of the components produced in plant to adapt in high-altitude regions.[Citation32] The plant A. vulgaris collected from Kathmandu (1324 m) and Gorkha (862 m) showed the major compounds eucalyptus, but the beta-phellandrene is the major compound in the plant growing in Chitwan (208 m).
Essential oils are the secondary metabolites and act as a major bioactive component found in all the species of genus Artemisia. The amount and the biological properties of essential oils present in the genus Artemisia are always influenced by altitudes and the geographical conditions in which the plants are grown.[Citation33] It has been reported that the plant Artemisia nilagirica grown at different altitudes of Himachal Pradesh India, contains the different concentrations of essential oils showing the significant variation with change in altitudes.[Citation33] The total amount of plant secondary metabolites varied from one altitude to another due to which the biological importance of such plant depends upon the altitudes in which plants are grown. From all these facts, we can reach to the conclusion that in A. vulgaris the concentration of antioxidant, antidiabetic, phenolic, and flavonoid compounds and antibacterial compounds increases with altitudes that ultimately showed the higher activity as compared to the plant grown in low altitudes.
Conclusion
This study suggested that the A. vulgaris leaf and root extracts possess antioxidant property, which might be helpful in stopping or slowing down the oxidative damage to prevent oxidative stress in the human body. In this research, the quantitative analysis of phenolics and flavonoids, antioxidant activity, α-amylase inhibition by the root and leaf extracts, and GC-MS profiling of chemical constituents present in essential oils of A. vulgaris growing in the different altitudes of Nepal were justified by adopting the valid procedure. The plants growing in different regions showed different biological activities due to the production of different phytochemicals in the plants. The A. vulgaris is easy to collect and found enough quantity in all regions of Nepal. Hence, the plant be the appropriate source for the development of the potent antioxidant and antidiabetic drug candidate in the future drug development process. However, extensive research is needed to be performed to confirm the effect through the isolation of target compounds. This research showed that the altitude variation plays a significant role in essential oil yield and its constituents and the biological activities shown by A. vulgaris. Further research can be done in this plant growing in high altitudes to investigate the isolation and identification of potent antioxidant, antidiabetic, and antibacterial components, which may be the potential drug candidates in the future drug development process.
Authors’ contributions
SA: Study deign and experimental analysis. KRS: Preparation of the manuscript draft, original idea presentation, study supervision, and final approval of the version to be published.
Data availability
Access to raw data is possible upon justifiable request.
Ethical approval
All the experimental works were carried out in accordance with the valid protocol with ethical approval.
Acknowledgments
The authors are thankful to the Central Department of Botany, Tribhuvan University, Nepal, for the identification of the plant. The authors are grateful to the Central Department of Chemistry, Tribhuvan University for providing the laboratory facilities.
Disclosure statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Ardeshirlarijani, E., Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R. Effect of Probiotics Supplementation on Glucose and Oxidative Stress in Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Trials. DARU J. Pharm. Sci. 2019, 27(2), 827–837.
- Birben, E.; Sahiner, U. M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5(1), 9–19. DOI: 10.1097/WOX.0b013e3182439613.
- Cervino, G.; Terranova, A.; Briguglio, F.; De Stefano, R.; Famà, F.; D’Amico, C.; Amoroso, G.; Marino, S.; Gorassini, F.; Mastroieni, R., et al. Diabetes: Oral Health Related Quality of Life and Oral Alterations. Biomed Res. Int. 2019. DOI:10.1155/2019/5907195.
- Jeong, S.; Min Cho, J.; Kwon, Y.-I.; Kim, S.-C.; Yeob Shin, D.; Ho Lee, J. Chitosan Oligosaccharide (GO2KA1) Improves Postprandial Glycemic Response in Subjects with Impaired Glucose Tolerance and Impaired Fasting Glucose and in Healthy Subjects: A Crossover, Randomized Controlled Trial. Nutr. Diabetes. 2019, 9(1), 1–9.
- Piyumi Wasana, K. G.; Attanayake, A. P.; Jayatilaka, P. W.; Weerarathna, K. A.; Antidiabetic, T. P. Activity of Widely Used Medicinal Plants in the Sri Lankan Traditional Healthcare System: New Insight to Medicinal Flora in Sri Lanka. Evid. Based Complementary Altern. Med. 2021, 12. DOI: 10.1155/2021/6644004.
- Saba, Z.; Iqra, S.; Azhar, R.; Muhammad, S.; Ghulam, A. H.; Muhammad Kashif, Z.; Nusrat, S.; Ammara, R.; Zeliha, S.; Satyajit, S., et al. Compound with Potential Anticancer, Antioxidant and Anti-inflammatory Activities. Mini-Rev. Med. Chem. 2021, 21(18), 2747–2763.
- Körner, C.;. Alpine Plant Life; Springer Berlin Heidelberg: Berlin, 2003. DOI: 10.1007/978-3-642-18970-8.
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction. Front. Physiol. 2018, 9(364). DOI: 10.3389/fphys.2018.00364.
- Holopainen, J. K.; Virjamo, V.; Ghimire, R. P.; Blande, J. D.; Ulkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9(1445). DOI: 10.3389/fpls.2018.01445.
- Nigam, M.; Atanassova, M.; Mishra, A. P.; Pezzani, R.; Devkota, H. P.; Plygun, S.; Salehi, B.; Setzer, W. N.; SharifiRad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14(7). DOI:10.1177/1934578X19850354.
- Pandey, B. P.; Thapa, R.; Upreti, A. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oil and Methanol Extract of Artemisia vulgaris and Gaultheria fragrantissima Collected from Nepal. Asian Pac. J. Trop. Med. 2017, 10(10), 952–959. DOI: 10.1016/j.apjtm.2017.09.005.
- Gurmet, R.; Bharti, U.; Mir, G. J.; Sharma, N. Cytological Variability in Artemisia L. Inhabiting North-West Himalayas: B Chromosomes in Artemisia gmelini Weber Ex Stechm. Cytol. Genet. 2018, 52(3), 231–235. DOI: 10.3103/S0095452718030052.
- Lu, X.; Ross, C. F.; Powers, J. R.; Aston, D. E.; Rasco, B. A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance–Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59(10), 5215–5221. DOI: 10.1021/jf201254f.
- Chang, C. C.; Yang, M. H.; Wen, H. M.; Chern, J. C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. Food Drug Anal. 2002, 10(3), 178-182.
- Subedi, L.; Timalsena, S.; Duwadi, P. R.; Thapa, R.; Paudel, A.; Parajuli, K. Antioxidant Activity and Phenol and Flavonoid Contents of Eight Medicinal Plants from Western Nepal. J. Tradit. Chin. Med. 2014, 34(5), 584–590. DOI: 10.1016/S0254-6272(15)30067-4.
- Rahhal, B. M.; Jaradat, N.; Hawash, M.; Qadi, M.; Issa, L.; Yahya, A.; Sanyora, S.; Saed, M.; Al-Rimawi, F. Phytochemical Screening, Antioxidative, Antiobesity, Antidiabetic and Antimicrobial Investigations of Artemisia scoparia Grown in Palestine. Processes. 2022, 10(10), 2050. DOI: 10.3390/pr10102050.
- Bolouri Moghaddam, M. R.; Vilcinskas, A.; Rahnamaeian, M. Cooperative Interaction of Antimicrobial Peptides with the Interrelated Immune Pathways in Plants. Mol. Plant Pathol. 2016, 17(3), 464–471. DOI: 10.1111/mpp.12299.
- Saleh, A. M.; Aljada, A.; Rizvi, S. A.; Nasr, A.; Alaskar, A. S.; Williams, J. D. In Vitro Cytotoxicity of Artemisia vulgaris L. Essential Oil Is Mediated by a Mitochondria-Dependent Apoptosis in HL-60 Leukemic Cell Line. BMC Complement. Altern. Med. 2014, 14(1), 226. DOI: 10.1186/1472-6882-14-226.
- Puškárová, A.; Čková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7(1), 8211.
- Flora, H.; Narendra, K. S.; Banerjee, S.; Naqvi, A. A.; Bagchi, G. D. Effect of Altitude on the Essential Oil Constituents of Artemisia roxburghiana Besser Var. Purpurascens (Jacq.) Hook. J. Essent. Oil Res. 2009, 21(4), 303–304. DOI: 10.1080/10412905.2009.9700177.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-industrial By-products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. DOI: 10.1016/j.foodchem.2005.07.042.
- Temraz, A.; El-Tantawy, W. H. Characterization of Antioxidant Activity of Extract from Artemisia vulgaris. Pak. J. Pharm. Sci. 2008, 21(4), 321–326.
- Nataraj, N.; Hussain, M.; Ibrahim, M.; Hausmann, A. E.; Rao, S.; Kaur, S.; Khazir, J.; Mir, B. A.; Olsson, S. B. Effect of Altitude on Volatile Organic and Phenolic Compounds of Artemisia brevifolia Wall Ex Dc. From the Western Himalayas. Front. Ecol. Evol. 2022, 10, 864728. DOI: 10.3389/fevo.2022.864728.
- Vickers, C. E.; Gershenzon, J.; Lerdau, M. T.; Loreto, F. A. Unified Mechanism of Action for Volatile Isoprenoids in Plant Abiotic Stress. Nat. Chem. Biol. 2009, 5, 283–291. DOI: 10.1038/nchembio.158.
- Haberstroh, S.; Kreuzwieser, J.; Lobo-Do-Vale, J. R.; Caldeira, M. C.; Dubbert, M.; Werner, C. Terpenoid Emissions of Two Mediterranean Woody Species in Response to Drought Stress. Front. Plant Sci. 2018, 9, 1071. DOI: 10.3389/fpls.2018.01071.
- Bistgani, Z. E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M. R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. andThymus daenensis Celak. Ind. Crop Prod. 2019, 135, 311–320. DOI: 10.1016/J.INDCROP.2019.04.055.
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of Exogenous Ca2+ on Phenolic Accumulation and Physiological Changes in Germinated Wheat (Triticum aestivum L.) under UV-B Radiation. Food Chem. 2019, 288, 368–376. DOI: 10.1016/J.FOODCHEM.2019.02.131.
- El-Tantawy, W. H. Biochemical Effects, Hypolipidemic and Anti-inflammatory Activities of Artemisia vulgaris Extract in Hypercholesterolemic Rats. J. Clin. Biochem. Nutr. 2015, 57(1), 33–38. DOI: 10.3164/jcbn.14-141.
- Ashok, P. K.; Upadhyaya, K. Evaluation of Analgesic and Anti-inflammatory Activities of Aerial Parts of Artemisia vulgaris L. in Experimental Animal Models. J. Biol. Act. Prod. Nat. 2013, 3(1), 101–105. DOI: 10.1080/22311866.2013.782761.
- Nemeth, E. Essential Oil Composition of Species in the Genus Achillea. J. Essent. Oil Res. 2005, 17(5), 501–512. DOI: 10.1080/10412905.2005.9698978.
- Gudaityte, O.; Venskutonis, P. R. Chemotypes of Achillea millefolium Transferred from 14 Different Locations in Lithuania to the Controlled Environment. Biochem. Syst. Ecol. 2007, 35(9), 582–592. DOI: 10.1016/j.bse.2007.03.016.
- Abad, M. J.; Bedoya, L. M.; Apaza, L.; Bermejo, P. Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules. 2012, 17(3), 2542–2566. DOI: 10.3390/molecules17032542.
- Jitendra, P. B.; Sushma, B.; Laxman, N.; Maya, K.; Rasmita, R.; Himal, B.; Parasmani, G.; Dhakaraj, P.; Pramod, A.; Rabindrakumar, R., et al. Anti-Inflammatory Activity of Artemisia vulgaris Leaves, Originating from Three Different Altitudes of Nepal. Sci. World J. 2021, 2021, 8. DOI: 10.1155/2021/6678059.