ABSTRACT
In this review, different extraction techniques of bioactive protein gluten from wheat and their association in the pathogenesis of celiac disease have been updated. Wheat gluten is an important constituent of whole-grain wheat. It comprises two subunits named as alpha-subunit and beta-subunit. Due to its importance in nutritional and pharmaceutical biochemistry, it gains much attention in molecular biology. This study compares different techniques used for isolation, purification, and identification of gluten protein from wheat. Different conventional approaches had been used to characterize gluten, but due to certain limitations and risk assessment, recent and advanced techniques have taken their place. Moreover, gluten seeks its intention as it may cause different allergic responses e.g., gluten intolerance and celiac disease in humans due to absence or low level of enzymes required for is proper digestion. Certain treatments based on different modification techniques cure these allergic reactions. These modifications include genetic modifications and generation of different immune responses in body to combat these diseases. Intestinal permeability and cytokine-based modifications in T-cells are essential to become resistant against gluten intolerance and celiac disease. However, vaccine development is on the way to treat patients suffering from these diseases. In conclusion, being a vital part of an important staple food, gluten characterization and disease treatment is a significant step. It will help in improving quality of wheat gluten and patients to fight against certain health disorders.
Introduction
All wheat end products, including baked breads, noodles, paste, cakes, and cookies, must consider wheat protein content. Wheat variety, growth area, soil type and quality, fertilizer input (amount and timing), notably nitrogen, all significantly impact wheat protein concentration.[Citation1] Flour from higher wheat protein has a better capacity to absorb water and, therefore, a bigger bread volume potential, if all other elements are equivalent.[Citation2] Wet gluten and wheat protein content have been utilized as quality indicators when selling and buying wheat (because of their strong correlations). The environmental impact is much bigger than what breeders can regulate, even though protein content is an intrinsic genetic feature and a selection criterion in breeding programmes.[Citation3–5] However, factors such as protein quality, processing, and the interaction between protein and starch in particular food systems further complicate wheat and flour quality.[Citation6] Following more than two and a half centuries of investigation into the quality of wheat and bread in the baking industry, many conventional methodologies were employed to characterize various wheat types. However, there is still a need for cutting-edge strategies and procedures to be applied for the efficient and convenient extraction, purification, and quantification of wheat proteins.Although the various proteins found in Wheat are crucial for establishing the nutritional and superior quality of bread dough, extraction and purification of all proteins in wheat grain had been extremely difficult to understand their functional characterization by traversing the dynamic nature of wheat grain components. Because of the flour’s propensity to ferment when added in its milled powdered condition, as well as its durability, flexibility, and capability to make dough better, gluten plays a variety of fascinating functions in the baking business. Due to its elastic and stable properties, gluten is the protein that contributes the most to bread manufacturing.[Citation7] The various cutting-edge methods for separating gluten from wheat and identifying it are compiled in this paper. Furthermore, the body’s production of various allergic reactions was examined using pure gluten. Such techniques must to be practical, straightforward, and economical while minimizing the possibility of cross-contamination and effectively recovering proteins. Celiac disease is a well-known condition associated with the term “gluten intolerance” among allergy reactions. Due to a lack of the enzymes needed for efficient wheat digestion, patients with gluten intolerance experience a recognized allergic reaction.[Citation8] Some people develop immunological problems as a result of wheat proteins. While glutenins, gliadins, or trypsin/amylase inhibitors increase wheat allergies, celiac disease is brought on by gliadin peptides, and it is uncertain what antigen causes non-celiac wheat sensitivity.[Citation9] Several strategies could be employed to prevent T cell presentation or activation to lessen the immunogenicity of proline- and glutamine-rich wheat proteins found in food. One of the methods is trans-amidating amino acids, amine compounds, or short peptides to glutamine to create a steric bulk. Moreover, enzymatic hydrolysis employing native or recombinant proteases from various sources or sourdough fermentation are some other techniques. An acceptable equilibrium with sensory features is necessary because protein modification impacts technical properties. For patients on a wheat-free diet, the advantages of detoxified wheat may include better nutritional quality, taste, and scent that they may be lacking.[Citation7] This review highlights the complication due to gluten. However, on the other hand, different extraction techniques of bioactive protein gluten from wheat and their association with the pathogenesis of celiac disease have been updated.
Wheat gluten
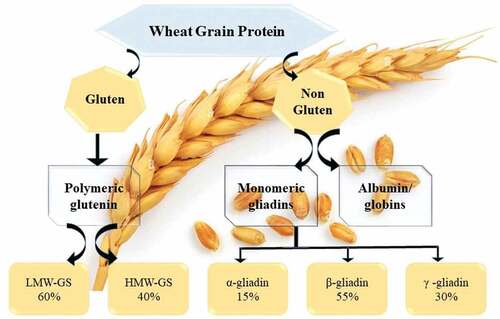
In many nations, Triticum aestivum (wheat) is regarded as a staple diet and is an important crop. Wheat proteins have been divided into four main categories based on how soluble they are. Salt-soluble globulins, water-soluble albumins, the monomers of alcohol-soluble gliadins, and HMMGS (high-molecular-weight-gluten-subunits) and LMM-GS (low-molecular-weight-subunits) are the main types of polypeptides found in flour.[Citation7] In wheat, monomeric gliadins and polymeric glutenin make up the gluten protein. The gluten was further separated into “HMMGS and LMW-GS” based on its molecular mass. The range of LMW-GS (low-molecular-weight-gluten) is 50 kDa, while the range of HMW-GS (high-molecular-weight gluten) is 120 kDa ().[Citation7]
Cross linkages between GLU-3 to GLU-1 loci of chromosome encodes LMWG (low-molecular-weight-glutenin-subunits). On the other hand, the high molecular weight gluten is encoded by the Glu-1 gene, which is located on chromosomal number 1 with specificity of its extended arm region. Both genetic and environmental variables can affect gluten’s physical and functional characteristics.[Citation7] Growing grains’ healthy embryo cells store gluten proteins. These create a matrix rich in proteins in the mature grain cells.[Citation9] In the vicinity of the starch granules, the gluten proteins form insoluble aggregates held together by hydrophobic forces, intermolecular disulfide bonds, and other forces. Alcohol solubility differs between gliadins and glutenin.[Citation7] For glutenin to be soluble in alcohol, the bi-sulfide bond must be reduced. It might be anything between 500,000 to more than 10 million.[Citation10]
Techniques for extraction and purification of proteins
Cell disruption is the study’s first significant accomplishment. There are numerous methods available for disrupting the cells in various plant tissues.[Citation11] Cell disruption is required before protein solubilization and extraction. For cell disruption, a variety of mechanical and chemical techniques are available. Homogenization methods are the general terms for these cell disruption approaches.[Citation12]
Each homogenization method has benefits and drawbacks of its own. Still, wet milling, a form of mechanical homogenization, is the most effective and sophisticated process for extracting wheat protein. In plant tissues, where robust cell walls surround cells, mechanical homogenization effectively disrupts cells.[Citation5] With this technique, wheat protein is separated based on its physiochemical characteristics.[Citation13] In this process, water is combined with a mixture of chemicals and enzymes that are crucial for separating wheat’s constituent parts and starch recovery.[Citation14] Proteins are divided using this technique according to their density, solubility in water, and particle size. When gluten proteins are moistened, they transform into larger, less dense starch granule-like particles. The four techniques now used in industry to separate wheat proteins effectively. First one is Martin process, second one includes alfa-laval/raisio process, the third is called hydrocyclone separator and the last is (HD- processes). The wet-milling quality of flours had been wet-processed by these four processes can be assessed using small-scale studies.[Citation15] After disruption, we acquire the required product using extraction and purification methods, which are further dried and optimized to create a powder.[Citation15]
Because tissues include many proteases and additional interfering substances, plants are typically more challenging to extract protein from.[Citation10] Proteolysis, which efficiently and swiftly disrupts cells to remove protein, may be used to extract the protein.[Citation16] We are concentrating on methods with high specificity and sensitivity to quickly and effectively identify gluten proteins. Scientists are worried about the emergence of new approaches in the future. In this regard, several novel gluten detection approaches, based on recent advancements, have been published, including aptamers, magnetic beads, microarrays, or multi-analytic profiling.
As our desired component is gluten protein, extracting wheat’s protein content is essential before proteome analysis effectively. We remove the wheat’s proteins, then purify them to make gluten. Precipitation follows tissue disruption as the next phase.[Citation16] To avoid protein denaturation, the optimum extraction process should extract the greatest amount of protein while causing the least amount of non-protein contamination. Additionally, the precipitation method should be reliable, quick, and reasonably priced.[Citation16] There are various extraction techniques, but our goal is to draw attention to the unique precipitation that isolates the protein with the highest gluten concentration. The precipitation of proteins prevents the extraction of non-protein cellular components from plants since they include secondary compounds like terpenes and phenols, among others.[Citation17]
Ammonium sulfate is employed because of its exceptional ability to stabilize proteins and high solubility.[Citation12] The most popular salt for protein precipitation is ammonium sulfate. Since protein solubility depends on ionic strength, ammonium sulfate’s presence of strongly charged ions makes it possible to precipitate protein by changing its ability. As ionic strength increases, beginning with a low concentration of ammonium salt, the protein solubility increases. Proteins are protected from the charged groups of other proteins by water molecules or ions that bind to the charged protein group. This result is seasoning.[Citation18] However, a high salt concentration makes proteins less soluble, which causes them to precipitate. We refer to this as salting out. Ammonium sulfate’s charged groups compete with water for binding to proteins at high concentrations, reducing solubility and causing the protein to precipitate. Centrifugation is then used to remove this protein.[Citation18]
Recently, bio-magnetic separation has become a popular method for protein isolation since it is affordable, often only requires one step, can be used with unprocessed samples, and effectively isolates HMW protein complexes that chromatographic methods can break down. It uses magnetic carriers, typically strong permanent magnets immobilized with affinities compounds that have an affinity for the targeted proteins.[Citation19] Magnetic carriers – typically coated with antibodies – attract the desired protein in protein solutions. Then, the bound proteins are released from the magnets by utilizing various elution conditions such as a change in pH, an increase in ionic strength, or affinity elution.[Citation19]
Proteins precipitate at a specified isoelectric point where there is no net charge on the protein. Isoelectric precipitation is carried out at extremely low or no salt since proteins are likewise less soluble at very low ionic strength. Using this technique, protein mixtures are separated into protein fractions.[Citation20] Trichloroacetic acid TCA/acetone precipitation offers effective extraction since it inactivates contaminants that could degrade our desired protein. It applies to large samples, can extract all the protein in the total cell, and is particularly effective for enriching basic charged proteins.[Citation21] These techniques separate proteins on the charge-to-pH ratio, killing proteolytic enzymes and protecting all other components from denaturing. Even more denaturation activity is induced by acetone.
Utilizing chloroform and methanol to concentrate proteins from various sources produces a dry protein without the need of a detergent like SDS or triton X-100. This technique takes advantage of the phase separation of two solvents, the protein liquid interface being further precipitated and pelleted once the aqueous top layer (a water-methanol mixture) is removed.[Citation22] Protein needs to be fully dissolved in order to prevent protein loss. Buffers are employed for this reason. These buffers are designed to dissolve as many proteins as possible, stop proteins from aggregating, and sever non-covalent bonds that bind proteins together into smaller units. For maximal solubilization, buffer often contains a chaotropic agent, a zwitter ionic detergent, proteolytic enzymes, and a reducing agent.[Citation12]
Purification is a crucial step after removing gluten from wheat since the extracted gluten sample still contains minute amounts of the original grain. Our primary focus in this review is on cutting-edge purification techniques for gluten-free diets. A very completive technique pure gluten protein and its various types had been assessed by RM for clinical studies and gluten free compliance tests. All isolated GPT (1 AND 2-gliadins, 5-gliadins, -gliadins, and (HMWGS and LMWGS from wheat) had been thoroughly characterized using N-terminal sequencing and SDS-PAGE, untargeted LC-MS/MS of chymotryptic hydrolyzes of the single GPT. All GPT had been reliably separated in high purity from the flours, according to the combined results of the analytical methods, and they may be used as a reference material, for example, to calibrate (ELISA) and LC-MS/MS methods.[Citation23]
This had been based on the principle of dry fractionation, which is more suitable than wet fractionation.[Citation15] Elect electrostatic separation is used based on triboelectric charge to separate the small gluten particles based on their conductivity differences during dry fractionation.[Citation24] To conduct the triboelectric charging, the gluten particles are introduced into a gas flow in a channel and allowed to collide with the channel wall. Then, these particles will be separated under the influence of an external electric field.[Citation24] The Bech-Scale Electrostatic Separator is divided into four components. The feeding system comes first, specifically made to regulate the gas flow rate and gluten content. The second is a charging slit, where particles charge up due to interactions with other particles and the wall.[Citation24] The third one is a separation chamber made up of two electrodes with opposing charges that produce electric flies. The fourth is a collecting chamber made up of two filter bags that separate the positively and negatively charged portions. To determine the charge, the electrometer is linked to the device. Gluten will be removed from the reaming sample since it has a low positive charge. The particle concentration, gas flow rate, applied voltage, and separation between the particles with opposing charges influence the charge separation.[Citation24]
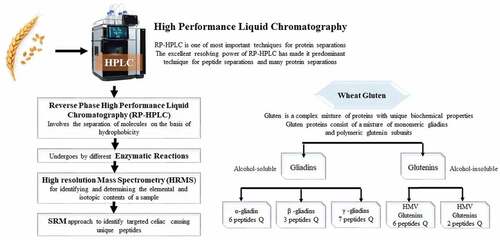
A unique variation of capillary electrophoresis called capillary isoelectric focusing separates gluten based on its charge homogeneity and isoelectric point (pI). The capillaries are coated with linear polyacrylamide (LPA) and polyvinyl alcohol (PVA). A combination of ampholytes with pH ranges between 3.10 and 5.8 along with a significant amount of urea and detergents are utilized. The protein sample that creates the steady pH gradient also contains amino acids. Numerous gluten isoforms can be distinguished.[Citation25] For the investigation of protein mixtures, capillary zone electrophoresis (CZE), which separates gluten protein with UV detection, was created. The separation was carried out using an acidic H3PO4/-ala (pH 2.5) buffer contained (HPMC), acetonitrile, and urea. It takes 6 minutes to complete this analysis.[Citation26] Nano-HPLC is the most modern and cutting-edge liquid chromatography method employed for the gluten separation. It includes all of the elements found in traditional liquid chromatography but in a scaled-down form.[Citation27] It is superior to conventional LC in several ways, including decreased mobile and stationary phase small sample greater sensitivity and ease of coupling with (MLDI-TOF) for improved protein analysis.[Citation25] Using the hydrophobicity of the gluten as a basis, it was separated. The hydrophobic stationary phase and the gluten sample are in the 10–100 nm-long column’s mobile phase.
In the past, competitive ELISA and antibodies in the classic sandwich have been the two most important approaches for detecting gluten protein. Larger antigens, including complete gluten proteins, are the only ones for which the sandwich ELISA is appropriate. It is improper for gluten analysis because of this need. The R5, G12, and a 20 mAbs as well as a number of pAbs are the antibodies used in these ELISA experiments.[Citation10] We are working to build immunological sensors for the quick and accurate detection of the gluten protein in light of this analytical strategy since they provide user-friendly, affordable, quick, miniaturized, and on-site analysis.[Citation28] Today, ELISA tests are used to quickly detect gluten.[Citation29]
“PCR” is an effective method in the genomic approach. RNA and DNA markers are used when determining if a grain of wheat has gluten. The highly sensitive approach to detect the presence of wheat protein gluten is ELISA and PCR.[Citation28] Real-time PCR tests employing TaqMan probes were used to identify cereals containing gluten.[Citation30] MS and one- and two-dimensional methods are used in the proteomics-based approach to identify gluten proteins. Several analytical techniques, including, 2-DGE, SDS-PAGE, RP-HPLC, HPCE and MALDI-TOF-MS, had been developed to measure the amount of variation in allele during wheat advanced wheat breeding programmers. Although SDS-PAGE is straight forward and simple to use, it has inferior resolution and reproducibility compared to other techniques. Similar mobilities of HMW-GS are difficult to distinguish by SDS-PAGE, and laboratory results can vary. Based on their mobility in SDS-PAGE, wheat grains glutenins can be divided into (high-molecular-weight and low-molecular-weight-gluten) HMWG and LMWG respectively. The second most common type of storage proteins after gliadins is the (low-molecular-weight-gluten) LMW subunits of glutenin, which make up around 1/3rd of the total seed proteins in wheat. However, they have only lately been studied in terms of allelic variation, chromosomal placement of genes, and impact on functional characteristics. The lack of appropriate methods for isolating the LMW subunits from gliadins, which have similar extract abilities and electrophoretic mobilities, is mostly to blame for the delay in getting this information. Using 2-D-electrophoretic methods, the initial chromosomal locus of the genes encoding LMW subunits was identified. However, these methods are difficult and time-consuming, allowing only one or two samples on each gel, making them unsuitable for screening numerous samples.[Citation31]
Although MALDI-TOF-MS offers good resolution and repeatability, its apparatus costs are substantially greater than those of the other methods.[Citation32] The great sensitivity and specificity are absent from one-dimensional approaches. To address this issue, we are integrating one-dimensional and two-dimensional techniques, specifically isoelectric focusing, SDS, and mass spectrometry, particularly its advanced variants, MALDI-TOF and ESI. Gluten was present, and MALDI-TOF MS analysis supported R5 (ELISA).[Citation28] Only semi-quantitative measurements can be used with (MALDI-TOF MS). These restrictions might be removed by combining HPLC separation with ESI LC-MS/MS ().
Table 1. Common analytical methods for gluten detection in food.
Quantitative determination
Allelic research has been conducted using RP-HPLC since the 1980s. In RP-HPLC, subunits are differentiated based on their hydrophobicity and had been separated on a straight gradient of organic solvent. For categorizing and measuring individual HMW-GS, RP-HPLC departure, similar to SDS-PAGE, is very accurate, fully programmed, and highly reproducible. However, this approach cannot tell apart HMW-GS with parallel hydrophobicity. Recent results show that many 1Dx and 1By subunits have significant overlap. HPCE analyses HMW-GS more quickly than RP-HPLC. However, several peaks are found for some HMW-GS subunits, making it more difficult to interpret the findings. To measure the levels of the various gluten protein types (5-, 1,2-, – and – gliadins; H-M-W-G and L-M-W-G glutenin subunits in wheat flour, a combined (extraction-HPLC technique) was created on a microscale. With the help of 3 mL of sixty percent of aqueous ethanol, gliadins were extracted. Then, at 60°C and under nitrogen, the glutenin subunits were extracted using fifty percent aqueous 1-CH3-CH2-OH, urea (2 mol per liter), Tris-HCl (0.05 mol per Liter, pH 7.5), and dithioerythritol (one percent). Afterward, gliadins and glutenin subunits were separated and quantitatively determined using reversed-phase HPLC on C8 silica gel at 50°C with a gradient of increasing acetonitrile concentration and 0.1% trifluoroacetic acid. The detecting wavelength was 210 nm, and the flow rate was 1.0 M per min. For the quantification of individual, underivatized H-M-W-S, temperature and flow rate were changed. Different protein standards, gliadins peptides, (low-molecular-weight-gluten-subunits), BSA with known protein contents were compared to HPLC absorbance regions to ascertain the absolute amounts of various protein types. Over a wide range (20–220 g), the calibrated curves had been essentially equal and straight. The quality of cereals used as raw materials can be assessed using this extraction-HPLC process, which enables an exact, repeatable, sensitive, and reasonably quick quantitative measurement of whole gluten proteins kinds in wheat. The relative levels of the all gluten protein types, in wheat flour had been quickly and accurately determined using the combination extraction-HPLC approach described in this article. When a protein standard such as gliadin or BSA had been used for calibration, the absolute amounts in flour could be determined. The technique is sensitive enough to analyses just one kernel. A specific protein standard like gliadin or BSA might be used to calibrated the exact quantities in flour. It is sensitive enough to analyses a single kernel with this method. The method applies to different cereals in theory and had been used to quantitatively characterize the proteins of the gluten molecule in flours and processed commodities (such as vital gluten and baked goods). The acquired results offer beneficial information for cereal breeding programmers and genetic development, as well as for raw material and finished product quality control.[Citation10] The optimum water and oil absorption qualities were found in gluten that had been freeze-dried.[Citation40] Three different drying techniques did not significantly alter the thermal characteristics of gluten. Therefore, gluten should be dried using a freeze drying process in baking since the reconstitution properties were likewise optimal for freeze dried gluten ().[Citation23]
Gluten: as diseased ingredient
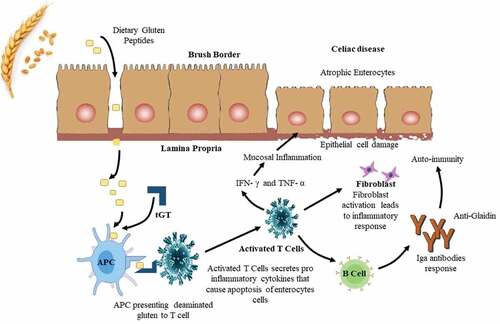
Celiac disease, had been affected 1% of the general population, is the most prevalent genetically based food intolerance in the world and has been brought on by the absorption of gluten in genetically susceptible individuals. This enteropathy can be manifested at any age and recognized by a wide range of clinical symptoms and signs that is extended far beyond the digestive system. Although extra intestinal manifestations had been becoming more frequent in children, GIT symptoms might be like abdominal distentions are quite common. Numerous disorders have included in their scope, including dental disorders, skin disorders, blood disorders, bones disorders, nervous disorder, unexplained proteins disorders, and reproductive organ disorder. As a result, diagnosis of celiac disease required a high level of suspicion, accurate screening, and a confirmation test that had included an intestinal biopsy.[Citation41] The fine, hair-like projections (villi) that line the small intestine had been destroyed, when the body’s immune system overreacted to gluten in food.[Citation42,Citation43] To be soluble as individual subunits in alcohol-water mixtures, such as fifty % (voleum by voleum) aqueous CH3CH2OH or sixty to seventy percent (v/v) aqueous ethanol, the wheat proteins that are involved in celiac syndrome and other gluten-related disorders must be classified as prolamins. However, some of these subunits are presented as polymers in wheat grain and flour. These polymers showed insolubility in alcohol-water solutions unless the disulfide bonds between the component subunits had been reduced using a substance like 2-ME or DTT. The accompanying alcohol-soluble monomers are known as gliadins, while the corresponding alcohol-insoluble polymers are called glutenins; combined, these two categories of proteins make up a sizable portion of the gluten fraction. By washing the dough to remove most of the starch, cell wall, and soluble ingredients, gluten could be made from wheat.[Citation44] In addition to the gluten proteins, it is a cohesive viscoelastic mass that includes tiny amounts of other proteins, residual starch (approximately 25% dry weight), and lipid Tatham.[Citation45,Citation46] These proteins have brought hypersensitivity reactions such as celiac disease, non-celiac gluten sensitivity, and wheat allergy. To confirm the safety of gluten-free diets, clinical research, diagnostics, the clarification of disease causes, and food analyses require well-defined reference materials. Various RM are currently in use; however, it frequently lacked a full description of the gluten source, content, and composition. Due to the complexity and heterogeneity of gluten, this characterization is essential to prevent confusing results brought on by variations in the RM used.[Citation23] Numerous food allergies had been associated with wheat proteins that are soluble in chloroform-methanol mixtures ().[Citation26]
Treatment of gluten intolerance
A currently known treatment for gluten intolerance is only “gluten-free diet” which has many drawbacks for CD sufferers. Dietary mistakes are, therefore frequent, suggesting intestine injury and the potential for long-term consequences. Undoubtedly, non-dietary alternatives are necessary to prevent injury from unintentional contamination or willful dietary violations. The degradation of gluten in the intestinal lumen, the control of the immune response, the modification of intestinal permeability, and the creation of immunological tolerance have all been explored and addressed by various CD therapies and treatments in recent years.
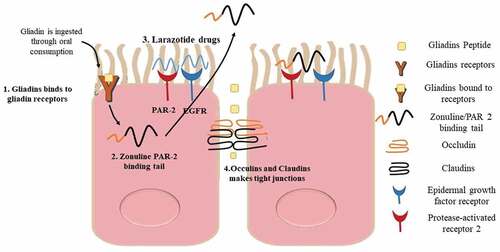
In managing CD, the anti-zonulin AT-1001, also known as larazotide acetate, functions as a regulator of gut permeability. Hoilat et al., 2022 researched the effectiveness and safety of AT-1001 by conducting randomized control trials in CD patients. Innovative and synthetic 8-a.a polypeptides are called larazotide acetate, also known as AT-1001. It shares structural similarities with the zonula occludens toxin (ZOT), which was previously identified and made by the Vibrio cholera bacterium. To lessen the zonulin-mediated increase in intestinal barrier permeability, larazotide acetate drugs blocks zonulin receptor.[Citation26] Larazotide acetate drug disrupts the arrangements of tight junctions across cells as well as cytoskeletal filaments ().[Citation47]
Gluten modification
Studies have been done on genetically modified wheat as a potential alternative to reduce gluten toxicity. Low-gluten wheat strains may be less immunogenic, although other gluten epitopes may stimulate an immunological response. It has been demonstrated that replacing or deleting the gene locus that translated into gliadin protein could stop T-cell stimulatory response epitopes ().
Table 2. Gluten modification.
Active proteases selectively break down gluten into minute, non-immunogenic particles before it can pass the small intestinal mucosa. Cysteine EP-B2 or ALV001 and SC PEP or ALV002, produced by bacteria and barley, are combined to form the active oral protease ALV003. This works better to decrease gluten than either enzyme working separately. Tye-din et al., researched that ALV003 would be effective in CD patients by administrating drugs for three day trial and concluded the effect of inflammatory cytokine.[Citation52] Alternative therapies at the pre-clinical stage, such as probiotic preparations by lactobacilli, as well as advanced glutenase therapy at the early clinical stage, such as AN-PEP and additional commercially available food-grade enzymes (STAN1), had been resulted in the development of additional secure and well-tolerated detoxification of dietary gluten techniques.
[Citation50] attempted to clarify issue by proving that silencing WAD-associated proteins did not influence wheat total protein and starch content, but the effect of gliadin knock down on wheat nutritional qualities remained ambiguous. The researchers covered most of WAD’s key components, including low molecular weight glutenin subunits and gliadins, using a mix of seven plasmids containing RNAi fragments. Two of these combinations resulted in a > 90% reduction in gluten content compared to the wild-type when tested with an anti-gliadin 33-mer monoclonal antibody. However, none of the combinations impacted the overall protein and starch amounts.
Gel electrophoresis, reversed-phase high-performance liquid chromatography, and liquid chromatography-mass spectrometry were used to determine the degree of silencing. Altenbach et al. concluded encouraging results, but unless preclinical and clinical trials show equally convincing outcomes, 54 questions will remain unsolved.
Generation of immune response
It might be crucial for the management of celiac disease in the future. Numerous studies have examined the onset of tolerance following vaccination in celiac, autoimmune, and allergy cases.[Citation53] Combining three peptides (secalin, hordein and gliadin) that T-cells in patients with the specific (HLA-DQ2 genotype) normally recognize, Nexvax 2 is a gluten-specific therapeutic vaccination that will eventually rewire T-cells to recognize gluten. After weekly injections for three weeks in celiac disease patients following a GFD, Nexvax 2 was found to be safe and well-tolerated in a phase-I randomized experimental trial (Clinical Trials registration number NCT00879749). A biological response to Nexvax 2 was observed, and future studies are anticipated to determine how effectively vaccinations might restore immunological tolerance to gluten.
Intestinal permeability modulation
The gut epithelium is the body’s main defense against internal and external stressors.[Citation54] There are two possible pathways for immunogenic antigens to breach the mucosal barrier in a healthy state: trans-cellular and para-cellular. The latter involves complex tight junction control. Recent insights into the complex process that regulates intestinal epithelial paracellular pathways have led to the discovery of (zonulin) a protein often observed in several clinical disorders, including celiac disease.
(Larazotide acetate) formerly AT-001, an 8-mer peptide, is a (tight junction regulator) TG that controls cellular changes by gliadin and cytokines. Larazotide inhibits the translocation of the gliadin 13-mer peptide, which has been strongly linked to celiac disease, as shown by in vitro studies utilizing Caco-2 cell monolayers. In contrast, in vivo studies have demonstrated that gliadin protects the structure of TG and prevents the development of macrophages in the small intestine.After three stages II human clinical studies, larazotide has shown to be a suitable and promising option for the treatment of celiac disease. All randomized, placebo-controlled studies appeared to be risk-free, well-tolerated, and effective in reducing gastrointestinal symptoms after a gluten challenge. According to early results from the most recent phase-II b study, larazotide acetate significantly improved both gastrointestinal and non-gastrointestinal symptoms clinically in celiac disease patients on a GFD lasting 12 months at the lowest dose (0.5 mg), in comparison to placebo (Clinical Trials registration number NCT01396213). A rising body of research demonstrating the safety and efficacy of larazotide acetate suggests that it may be a potential therapeutic option for the treatment of celiac disease.
Gluten-free diet
A rigorous gluten-free diet is necessary after diagnosis, which in severe cases will cause a noticeable worsening of symptoms. However, there are considerable problems with compliance and quality of life, especially in adolescents.[Citation41,Citation55] The only approach to treat celiac disease is to consume a gluten-free diet. When gluten is removed from the diet, the small intestine recovers and complete recovery is anticipated. Undiagnosed celiac disease can cause long-term problems like starvation, cancer,[Citation56] osteoporosis, neurological issues, and miscarriage.[Citation57] Gluten-free foods are utilized for celiac disease patients, and they can be tested for gluten intolerance using a test kit and real-time polymerase chain reaction detection (PCR).[Citation58]
Conclusion
The initial stage in precisely extracting and purifying our preferred protein, protein precipitation and purification technologies are reviewed in this article for proteomic and genomic studies and gluten-free diets. This review discusses gluten extraction since gluten is a more potent wheat protein that gives dough its elasticity and flexibility while also giving certain people who are gluten allergic celiac disease. To determine gluten’s rheological characteristics to enhance wheat quality and research its immune-stimulating effects in illnesses, gluten was isolated for proteome analysis. Because it is a cheap way to precipitate proteins by altering their solubility and consequently their ionic strength, ammonium sulfate precipitation was covered in this article. Since gluten is a more potent wheat protein that gives dough its elasticity and flexibility, it also causes celiac disease, particularly in people who are gluten intolerant. This article examines gluten extraction. Gluten was extracted for proteome analysis to evaluate its rheological properties, improve wheat quality, and explore its immune-stimulating effects in celiac diseases. An Ammonium sulfate precipitation was discussed in this article because it is an inexpensive method of precipitating proteins by changing their ionic strength and, subsequently their solubility.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cato, L.; Mullan, D. Wheat Quality: Wheat Breeding and Quality Testing in Australia, in Breadmaking; Elsevier, 2020; pp 221–259.
- Boita, E. R.; Oro, T.; Bressiani, J.; Santetti, G. S.; Bertolin, T. E.; Gutkoski, L. C. Rheological Properties of Wheat Flour Dough and Pan Bread with Wheat Bran. J. Cereal Sci. 2016, 71, 177–182. DOI: 10.1016/j.jcs.2016.08.015.
- Maiorano, A. M.; Lourenco, D. L.; Tsuruta, S.; Ospina, A. M. T.; Stafuzza, N. B.; Masuda, Y.; Filho, A. E. V.; Cyrillo, J. N. D. S. G.; Curi, R. A.; Silva, J. A. I. I. D. V., et al. Assessing Genetic Architecture and Signatures of Selection of Dual Purpose Gir Cattle Populations Using Genomic Information. PLoS One. 2018, 13(8), e0200694.
- Riaz, S.; Kabir, A.; Haroon, A.; Ali, A.; Manzoor, M. F. Food Dehydration Recent Advances and Approaches; 2022.
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M. K.; Koraqi, H.; Ali, A.; Lima, C. M. G.; Alansari, W. S.; Eskandrani, A. A., et al. Extraction of Bioactive Compounds from Different Vegetable Sprouts and Their Potential Role in the Formulation of Functional Foods against Various Disorders: A Literature-Based Review. Molecules. 2022, 27(21), 7320.
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure− Quality Relationships− a Review. Chem. Rev. 2010, 110(10), 6144–6168. DOI: 10.1021/cr100090k.
- DuPont, F. M.; Chan, R.; Lopez, R.; Vensel, W. H. Sequential Extraction and Quantitative Recovery of Gliadins, Glutenins, and Other Proteins from Small Samples of Wheat Flour. J. Agric. Food Chem. 2005, 53(5), 1575–1584.
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on Dietary Fibre by Fourier transform-infrared Spectroscopy and Chemometric Methods. Food Chem. 2016, 196, 114–122. DOI: 10.1016/j.foodchem.2015.09.029.
- Li, X.; Qin, H.; Anwar, A.; Zhang, X.; Yu, F.; Tan, Z.; Tang, Z. Molecular Mechanism Analysis of m6A modification-related lncRNA-miRNA-mRNA Network in Regulating Autophagy in Acute Pancreatitis. Islets. 2022, 14(1), 184–199.
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24(2), 115–119. DOI: 10.1016/j.fm.2006.07.004.
- Ahmed, N.; Ali, A.; Riaz, S.; Ahmad, A.; Aqib, M. Vegetable Proteins: Nutritional Value, Sustainability, and Future Perspectives, in Vegetable Crops-Health Benefits and Cultivation; IntechOpen, 2021.
- Burgess, R. R. Protein Precipitation Techniques. Methods Enzymol. 2009, 463, 331–342.
- Khalid, W.; Maqbool, Z.; Arshad, M. S.; Kousar, S.; Akram, R.; Siddeeg, A.; Ali, A.; Qin, H.; Aziz, A.; Saeed, A., et al. Plant-derived Functional Components: Prevent from Various Disorders by Regulating the Endocrine Glands. Int. J. Food Prop. 2022, 25(1), 976–995.
- Khalid, W.; Gill, P.; Arshad, M. S.; Ali, A.; Ranjha, M. M. A. N.; Mukhtar, S.; Afzal, F.; Maqbool, Z. Functional Behavior of DHA and EPA in the Formation of Babies Brain at Different Stages of Age, and Protect from Different brain-related Diseases. Int. J. Food Prop. 2022, 25(1), 1021–1044.
- Sayaslan, A. Wet-milling of Wheat Flour: Industrial Processes and small-scale Test Methods. LWT Food Sci. Technol. 2004, 37(5), 499–515. DOI: 10.1016/j.lwt.2004.01.009.
- Grabski, A. C. Advances in Preparation of Biological Extracts for Protein Purification. Methods Enzymol. 2009, 463, 285–303.
- Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M. W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M. F. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes. 2022, 10(10), 2014.
- Duong-Ly, K.; Gabelli, S. Methods in Enzymology, Chapter 7; Elsevier Inc.: San Diego, USA, 2014; Vol. 541.
- Safarik, I.; Safarikova, M. Magnetic Techniques for the Isolation and Purification of Proteins and Peptides. Biomagnetic Research and Technology. 2004, 2(1), 1–17. DOI: 10.1186/1477-044X-2-7.
- Englard, S.; Seifter, S. 22] Precipitation Techniques, in Methods in Enzymology; Elsevier, 1990; pp 285–300.
- Jiang, L.; He, L.; Fountoulakis, M. Comparison of Protein Precipitation Methods for Sample Preparation Prior to Proteomic Analysis. J. Chromatogr. A. 2004, 1023(2), 317–320. DOI: 10.1016/j.chroma.2003.10.029.
- Glenn, W. S.; Stone, S. E.; Ho, S. H.; Sweredoski, M. J.; Moradian, A.; Hess, S.; Bailey-Serres, J.; Tirrell, D. A. Bioorthogonal Noncanonical Amino Acid Tagging (BONCAT) Enables time-resolved Analysis of Protein Synthesis in Native Plant Tissue. Plant Physiol. 2017, 173(3), 1543–1553.
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K. A. Isolation and Characterization of Gluten Protein Types from Wheat, Rye, Barley and Oats for Use as Reference Materials. PloS one. 2017, 12(2), e0172819.
- Dwari, R.; Mohanta, S. K.; Rout, B.; Soni, R. K.; Reddy, P. S. R.; Mishra, B. K. Studies on the Effect of Electrode Plate Position and Feed Temperature on the tribo-electrostatic Separation of High Ash Indian Coking Coal. Adv. Powder Tech. 2015, 26(1), 31–41.
- Mazzeo, M. F.; Bonavita, R.; Maurano, F.; Bergamo, P.; Siciliano, R. A.; Rossi, M. Biochemical Modifications of Gliadins Induced by Microbial Transglutaminase on Wheat Flour. Biochimica Et Biophysica Acta (Bba)-general Subjects. 2013, 1830(11), 5166–5174.
- Piergiovanni, A. R. Minor Wheat Protein Fractions Analysis by Using Capillary Zone Electrophoresis. Separations. 2016, 3(2), 17. DOI: 10.3390/separations3020017.
- Gama, M. R.; Collins, C. H.; Bottoli, C. B. Nano-liquid Chromatography in Pharmaceutical and Biomedical Research. J. Chromatogr. Sci. 2013, 51(7), 694–703. DOI: 10.1093/chromsci/bmt023.
- Scherf, K. A.; Poms, R. E. Recent Developments in Analytical Methods for Tracing Gluten. J. Cereal Sci. 2016, 67, 112–122. DOI: 10.1016/j.jcs.2015.08.006.
- Haraszi, R.; Chassaigne, H.; Maquet, A.; Ulberth, F. Analytical Methods for Detection of Gluten in food—method Developments in Support of Food Labeling Legislation. J. AOAC Int. 2011, 94(4), 1006–1025.
- Oszmiański, J.; Wojdyło, A. Comparative Study of Phenolic Content and Antioxidant Activity of Strawberry Puree, Clear, and Cloudy Juices. Eur. Food Res. Technol. 2009, 228(4), 623–631. DOI: 10.1007/s00217-008-0971-2.
- Joppa, L.; Khan, K.; Williams, N. Chromosomal Location of Genes for Gliadin Polypeptides in Durum Wheat Triticum Turgidum L. Theor. Appl. Genet. 1983, 64(4), 289–293. DOI: 10.1007/BF00274164.
- Jang, Y.-R.; Beom, H.-R.; Altenbach, S.; Lee, M.-K.; Lim, S.-H.; Lee, J.-Y. Improved Method for Reliable HMW-GS Identification by RP-HPLC and SDS-PAGE in Common Wheat Cultivars. Molecules. 2017, 22(7), 1055.
- Wang, W.; Li, J.; Fan, B.; Zhang, X.; Guo, R.; Zhao, Y.; Zhou, J.; Zhou, J.; Sun, D.; Li, B., et al. Development of a Novel Double Antibody Sandwich ELISA for Quantitative Detection of Porcine Deltacoronavirus Antigen. Viruses. 2021, 13(12), 2403.
- Li, Y.-F.; Lin, -Z.-Z.; Hong, C.-Y.; Huang, Z.-Y. Histamine Detection in Fish Samples Based on Indirect Competitive ELISA Method Using iron-cobalt co-doped Carbon Dots Labeled Histamine Antibody. Food Chem. 2021, 345, 128812. DOI: 10.1016/j.foodchem.2020.128812.
- Panwar, S.; Duggirala, K. S.; Yadav, P.; Debnath, N.; Yadav, A. K.; Kumar, A. Advanced Diagnostic Methods for Identification of Bacterial Foodborne Pathogens: Contemporary and Upcoming Challenges. Crit. Rev. Biotechnol. 2022, 1–19. DOI:10.1080/07388551.2022.2095253.
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S. S.; Bhaskara Rao, K. V. Protease Inhibitors from Marine Actinobacteria as a Potential Source for Antimalarial Compound. PloS one. 2014, 9(3), e90972.
- Gautheron, C.; Pinna-Jamme, R.; Derycke, A.; Ahadi, F.; Sanchez, C.; Haurine, F.; Monvoisin, G.; Barbosa, D.; Delpech, G.; Maltese, J., et al. Analytical Protocols and Performance for Apatite and Zircon (U–Th)∕ He Analysis on Quadrupole and Magnetic Sector Mass Spectrometer Systems between 2007 and 2020. Geochronology. 2021, 3(1), 351–370.
- Výrostková, J.; Regecová, I.; Zigo, F.; Marcinčák, S.; Kožárová, I.; Kováčová, M.; Bertová, D. Detection of Gluten in Gluten-Free Foods of Plant Origin. Foods. 2022, 11(14), 2011.
- Pla, L.; Martínez-Bisbal, M. C.; Aznar, E.; Sancenón, F.; Martínez-Máñez, R.; Santiago-Felipe, S. A Fluorogenic Capped Mesoporous Aptasensor for Gluten Detection. Anal. Chim. Acta. 2021, 1147, 178–186. DOI: 10.1016/j.aca.2020.12.060.
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022.
- Guandalini, S.; Assiri, A. Celiac Disease: A Review. JAMA Pediatrics. 2014, 168(3), 272–278. DOI: 10.1001/jamapediatrics.2013.3858.
- Babar, Q.; Ali, A.; Saeed, A.; Tahir, M. F. Novel Treatment Strategy against COVID-19 through Anti-Inflammatory, Antioxidant and Immunostimulatory Properties of the B Vitamin Complex, in B-Complex Vitamins-Sources, Intakes and Novel Applications; Intechopen,2021.
- Rubio–Tapia, A.; Van Dyke, C. T.; Lahr, B. D.; Zinsmeister, A. R.; El–Youssef, M.; Moore, S. B; Bowman, M.; Burgart, L. J.; Melton III, L. J.; Murray, J. A. Predictors of Family Risk for Celiac Disease: A population-based Study. Clinical Gastroenterology and Hepatology. 2008, 6(9), 983–987.
- Ahmad, N.; Riaz, S.; Ali, A. Ingredients for Food Products, in Palm Trees and Fruits Residues; Elsevier, 2023; pp 115–153.
- Wang, L.; Zheng, W.; Yang, J.; Ali, A.; Qin, H. Mechanism of Astragalus Membranaceus Alleviating Acquired Hyperlipidemia Induced by High-Fat Diet through Regulating Lipid Metabolism. Nutrients. 2022, 14(5), 954.
- Tatham, A. S.; Gilbert, S. M.; Fido, R. J.; Shewry, P. R. Extraction, Separation, and Purification of Wheat Gluten Proteins and Related Proteins of Barley, Rye, and Oats, in Celiac Disease. 2000, Springer, p. 55–73.
- Jauregi-Miguel, A. The Tight Junction and the Epithelial Barrier in Coeliac Disease. International Review of Cell and Molecular Biology. 2021, 358, 105–132.
- Gil-Humanes, J.; Pistón, F.; Barro, F.; Rosell, C. M. The Shutdown of Celiac disease-related Gliadin Epitopes in Bread Wheat by RNAi Provides Flours with Increased Stability and Better Tolerance to over-mixing. PLoS One. 2014, 9(3), e91931.
- Altenbach, S. B.; Tanaka, C. K.; Seabourn, B. W. Silencing of Omega-5 Gliadins in Transgenic Wheat Eliminates a Major Source of Environmental Variability and Improves Dough Mixing Properties of Flour. BMC Plant Biol. 2014, 14(1), 1–13. DOI: 10.1186/s12870-014-0393-1.
- Barro, F.; Giménez, M. J.; García-Molina, M. D.; Ozuna, C. V.; Comino, I.; Sousa, C.; Gil-Humanes, J. Targeting of Prolamins by RNA I in Bread Wheat: Effectiveness of Seven Silencing‐fragment Combinations for Obtaining Lines Devoid of Coeliac Disease Epitopes from Highly Immunogenic Gliadins. Plant Biotechnol. J. 2016, 14(3), 986–996.
- Sasaki, M.; Koplin, J. J.; Dharmage, S. C.; Field, M. J.; Sawyer, S. M.; McWilliam, V.; Peters, R. L.; Gurrin, L. C.; Vuillermin, P. J.; Douglass, J., et al. Prevalence of clinic-defined Food Allergy in Early Adolescence: The SchoolNuts Study. J. Allergy Clin. Immunol. 2018, 141(1), e4, 391–398. doi:10.1016/j.jaci.2017.05.041.
- Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; Ahmed, M.; Bostani, A. Bio-Molecular Characteristics of Whey Proteins with Relation to Inflammation; 2021.
- Qin, H.; Song, Z.; Zhao, C.; Yang, J.; Xia, F.; Wang, L.; Ali, A.; Zheng, W. Liquiritigenin Inhibits Lipid Accumulation in 3T3-L1 Cells via mTOR-Mediated Regulation of the Autophagy Mechanism. Nutrients. 2022, 14(6), 1287.
- Manzoor, M.; Arif, Z.; Kabir, A.; Mehmood, I.; Munir, D.; Razzaq, A.; Ali, A.; Goksen, G.; Coşier, V.; Ahmad, N., et al. Oxidative Stress and Metabolic Diseases: Relevance and Therapeutic Strategies. Front. Nutrit. 2022, 9.
- Khalid, W.; Ali, A.; Arshad, M. S.; Afzal, F.; Akram, R.; Siddeeg, A.; Kousar, S.; Rahim, M. A.; Aziz, A.; Maqbool, Z., et al. Nutrients and Bioactive Compounds of Sorghum Bicolor L. Used to Prepare Functional Foods: A Review on the Efficacy against Different Chronic Disorders. Int. J. Food Prop. 2022, 25(1), 1045–1062.
- Ali, A.; Mughal, H.; Ahmad, N.; Babar, Q.; Saeed, A.; Khalid, W.; Raza, H.; Liu, A. Novel Therapeutic Drug Strategies to Tackle immune-oncological Challenges Faced by Cancer Patients during COVID-19. Expert Rev. Anticancer Ther. 2021, 21(12), 1371–1383.
- Ali, A.; Manzoor, M. F.; Ahmad, N.; Aadil, R. M.; Qin, H.; Siddique, R.; Riaz, S.; Ahmad, A.; Korma, S. A.; Khalid, W., et al. The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review. Front. Nutrit. 2022, 1553.
- Astapova, M.; Tikhomirova, T.; Asadi, E. Study of Food Products Containing Gluten by Using the Method of Polymerase Chain Reaction. Voprosy Pitaniia. 2010, 79(5), 66–71.