ABSTRACT
Lentinan (LNT) is extracted from the fruiting body of Lentinus edodes. Its active ingredient is β−1,3-dextran and has many biological activities, but its rheological characteristics and antibacterial mechanism when mixed with saliva are rarely reported. In this study, the LNT and artificial saliva (AS) mixture was used to investigate the rheological analysis and antibacterial mechanism against Streptococcus mutans (S. mutans). The results showed that the apparent viscosity of the mixture was positively correlated with the concentration, showing a shear thinning behavior and a “non-Newtonian fluid” with a pseudoplastic behavior with the increase of shear rate. In dynamic rheology, the 8% and 6% mixtures became a gel trend and viscoelastic properties, showing elasticity of weak gel at low frequencies and liquid-like viscosity at high frequencies. Moreover, the compound could damage the structures of S. mutans and increased the leakage of intracellular macromolecules, electrical conductivity, and activities of alkaline phosphatase (AKP) and β-galactoside but inhibited acid production, fluorescence intensity, and ATP synthesis by decreasing the levels of glucokinase (glk), acetate kinase (ackA), pyruvate kinase (pykf), phosphoglycerate kinase (pgk), 6-phosphofructokinase (pfk) in the glycolysis pathway. These results indicate that LNT plays a dominant role in the oral processing and antibacterial mechanism against S. mutans. It can provide an excellent theoretical basis for studying LNT as a functional food raw material.
Introduction
Oral processing is the first stage of food decomposition and is necessary for absorbing nutrition to obtain energy and sensory pleasure.[Citation1] Saliva, as a vital component in food oral processing, can moisten oral tissues, relieve xerostomia, and promote a smooth swallowing process.[Citation2] Moreover, saliva is the first line of defense for teeth and oral health because of its antibacterial effects on oral pathogenic bacteria, especially Streptococcus mutans (S. mutans).[Citation3] Thus, saliva is important when conducting in vitro simulated oral processing studies. However, artificial saliva (AS) is often used for oral processing due to the complexity of natural saliva composition.[Citation4] Saliva plays a vital role in protecting the surface of teeth and the mouth.
Polysaccharides are widely used as thickeners and gel agents in food processing due to their ability to retain water and hydrogel.[Citation5,Citation6] Thus, it is necessary to develop new polysaccharide products based on polysaccharide rheology to meet the needs of swallowing safety. Moreover, polysaccharides from Paeonia sinjiangensis have inhibitory effects on the growth and acid production of S. mutants.[Citation7] Thus, it is necessary to study the rheological information and antibacterial function of the mixture of polysaccharides with saliva.
Lentinan (LNT) is extracted from the fruiting body of Lentinus edodes. Its active ingredient is β−1,3-dextran and has many biological activities such as antibacterial, antitumor, and immune regulation.[Citation8–10] For example, LNT can cure phenol induced oral ulceration by significantly increasing the activities of antioxidant enzymes in rat serum.[Citation11] Moreover, LNT can inhibit oral pathogens that cause tooth decay and gingivitis.[Citation12] However, the rheological characteristics of the mixture of LNT and AS and its antibacterial mechanism against S. mutants in vitro simulated oral processing are relatively limited.
Therefore, in this study, the effects of LNT and AS mixture on rheological and antibacterial characteristics on S. mutants in vitro oral processing were investigated to reveal the perception of food texture in xerostomia patients and the possible mechanisms against oral bacteria. It provides a theoretical basis for effectively preventing dental caries and developing new oral products.
Materials and methods
Materials
LNT (98% purity) (Solarbio Life Science, Beijing, China). AS (NaCl, KCl, Na2SO4, NH4Cl, CaCl2·2 H2O, NaH2PO4·2 H2O, CN2H4O, NaF, deionized water) (Shanghai Yuanye Biotechnology Co., Ltd., China). 4,’6’-diamidino−2-phenylindole (DAPI) (Beijing Simejie Technology Co., Ltd.); brain heart infusion (BHI) medium, TSA + 5% defibrotic sheep blood, Sand Glucosin (PDA) medium (Beijing Solebao Technology Co., Ltd., China); the kits of soluble protein content, alkaline phosphatase (AKP) and β-galacsidase activity, adenosine triphosphate (ATP) concentration (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China); the total RNA extraction kit, StarScript II First-strand cDNA synthesis mix kit, 2×M5 Hi Per SYBR Premix Es Taq kit (Beijing Jumei Biotechnology Co., Ltd., China). Ultra-thin slicer (Leica RM2265).
Streptococcus mutans. S. mutans ATCC 25,175, Streptococcus salivarius. S. salivarius ATCC 49,295, and Candida albicans were purchased from Shanghai Conservation Biotechnology Center. Actinomyces viscosus. A. viscosus ATCC 27,044 and Candida albicans. S. salivarius ATCC 13,419 were purchased from Shanghai Jiachu Biological Engineering Co., Ltd.
Mixture of LNT and AS
A 16% LNT solution was prepared by mixing LNT and distilled water. Then, the 16% LNT solution was autoclaved, serially diluted, and combined with AS (1:1, v/v) to obtain a mixture of LNT and AS with the final LNT content of 0%, 0.125%, 0.25%, 0.5%, 1%, 2%, 3%, 4%, 5%, 6%, 7% and 8%.
Preparation of bacterial suspension
Under sterile conditions, S. mutans and A. viscosus were picked into BHI liquid medium, and S. salivalis and S. sanguinis were picked into TSA + 5% defibrated sheep blood agar solid media. After inoculation under anaerobic conditions (5% CO2, 5% H2 and 90% N2) at 37°C or 28°C for 18 h, they were identified and confirmed. Their growth curves were recorded at OD630 nm or OD500 nm to determine the number of colonies by the dilution-coated plate method. The concentration of bacterial suspension was adjusted to 10 iequation1 > −104 CFU/mL during logarithmic growth period.
Shear rheology
Steady rheology: The steady rheology was determined using a DHR−3 rheom (TA, USA). The measurement mode was set as steady-state shear measurement, and the equipment used 40 mm, 1° lamina. The samples were set in shear rate range of 1–100 rad/s, and the deformation condition was below 0.1%. The point time variable was selected as the linear rule changes, and the flow curve was fitted by Power Law model. All the rheological experiments were performed at 25°C to accommodate the oral processing temperature.
Dynamic rheology: For dynamic rheology, the determination mode was amplitude scanning. The device was equipped with CP60–0.5 conical plates with two surfaces of a 0.101 μm-set gap. The angular frequency of the sample was set to 1–100 rad/s, and the frequency was set to 1 Hz. The point time variable was selected as the regular linear change, and the deformation was controlled at 2%. The experiment was carried out at 25°C, during which the storage modulus G’ and the loss modulus G” with the angular frequency were measured.
Particle size distribution
The particle size and particle size distribution were measured by Zeta potential particle size analyzer (Malvern UK Co., Ltd.). The conditions were as follows: the temperature at 25°C, measurement type of size, dispersant of water, one test cycle, three times of measurement, and 2 min of operation time. Since the solutions less than 1% had too weak particle size signals, the 1–8% mixture was determined. Three replicates were performed for each sample.
Surface tension
The surface tension was measured with a DWK tensiometer (Harke, Beijing, China) based on a previous method.[Citation6]
Extensional rheology
The refinement and fracture of fluid filaments were analyzed by an MCR 302 tensile rheometer (Shanghai Antonpa Co., Ltd.). The device was equipped with a 20 mm x 10 mm flat plate with an initial tensile height of 0.25 mm and a maximum tensile height of 10 mm. The deformation condition was below 1% and the value was recorded every 0.01 s. The tensile fracture time, the tensile fracture height, and the variation of tensile stress with tensile height were recorded at the tensile speeds of 0.04, 0.2, 0.5, and 1 mm/s.
Antibacterial activity
Before the experiment, AS was determined to have no inhibitory effects on the strains used in this study. Mixtures of different concentrations (0%, 2%, 4%, 8%) were mixed with each bacterial suspension with an initial concentration of 104−105 CFU/mL at 1:9 (v:v) in the experimental groups. AS was mixed with the same proportion of bacteria suspension in the control group. The initial pH value was adjusted to 7.20 ± 0.01, and the bacteria were cultivated in an anaerobic incubator (YQX-II, Shanghai Yuejin Medical Device Col., Ltd., China) at 37°C. After incubation for 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 min, the absorbance and pH value were measured to calculate the antibacterial rate. The antibacterial rate was calculated based on the formula: antibacterial rate (%)= OD1-(OD1-OD2)/OD1 × 100, where the OD1 is the absorbance of 0 h, OD2 was the absorbance of experiment groups, OD3 was the absorbance of the mixture of LNT and AS.[Citation13] In addition, 5 mL of bacterial solution was collected each time and centrifuged at 4°C and 5000 r/min for 10 min to obtain the supernatant and precipitate for the subsequent experiment.
Intracellular biomacromolecule leakage, the activities of AKP and β-galactosidase, and conductivity
The obtained supernatant was used to determine the concentration of nucleic acid and soluble protein, the activities of AKP and β-galactosidase, and conductivity based on the corresponding kits.[Citation14]
ATP concentration of S. mutans
The obtained precipitate was immediately put into an ice bath and washed twice with normal saline. After centrifugation, the deposit was re-suspended with 1 mL of normal saline and placed in a water bath at 100°C for 10 min to determine ATP concentration using the ATP Assay Kit. Each experiment was repeated three times.
Fluorescence intensity of S. mutans
After anaerobic incubation at 37°C for 120 min, the residue was treated with 10% formaldehyde for 20 min, and treated with DAPI. After being cultured in the dark for 20 min, the fluorescence intensity was observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Morphological observation of S. mutans
The morphology of S. mutans was observed by scanning electron microscope (SEM) and transmission electron microscope (TEM). After anaerobic culture at 37°C for 2 h, the bacteria were centrifuged at 4°C, 5000 r/min for 5 min. The precipitates were fixed with 2.5% glutaraldehyde at 4°C overnight and dehydrated in ascending series of ethanol. Then, the samples were soaked in ethanol-terbutanol solution (1:1, V:V) for 20 min and then in 100% terbutanol twice for 15 min each time. Afterwards, they were freeze-dried by vacuum freeze-dryer (PL3000, Thermo Fisher Thermo Co., Ltd.), sprayed by an ion sputter (MSP−1S, Shanghai Huishang Instrument Co., Ltd.) and observed by a scanning electron microscope (SEM, FEI Company, Holland).[Citation15]
The obtained precipitate was fixed with 2.5% glutaraldehyde overnight and then fixed with 1% osmium acid at 4°C for 1 h. Furthermore, they were dehydrated with acetone solution, soaked and embedded with epoxy resin overnight. After being dried at 70°C for 48 h, the samples were sliced in an ultra-thin slicer, stained with uranyl dioxide acetate and lead citrate, and observed by transmission electron microscope (TEM).
Gene expression analysis
After anaerobic incubation at 37°C for 120 min, total RNA was extracted using the bacteria according to the instructions of the total RNA extraction kit. After anaerobic culture at 37°C for 120 min, the bacteria were used to extract total RNA according to the instructions of total RNA extraction kit. The concentration and purity of total RNA was determined by Nanodrop−2000 spectrophotometer (Thermo Electron Corporation, USA). Reverse transcription was performed to obtain cDNA according to the instructions of StarScript II First-strand cDNA synthesis mix kit. The reaction of qPCR was performed using a CFX 96 Real-Time PCR Detection System (Bio-rad Corporation, Hercules, USA) according to the 2 × M5 Hi Per SYBR Premix Es Taq kit instructions. The reaction conditions were pre-denaturation reaction at 95°C for 3 min, 40 cycles of 95°C for 5 s, 60°C for 34 s, 95°C for 1 min, and dissolution curve analysis at 60°C for 30 s and 95°C for 30 s. The specific primers of acetate kinase (ackA), pyruvate kinase (pykf), phosphoglycerate kinase (pgk), 6-phosphofructokinase (pfk), and glucokinase (glk) are shown in . The 16S ribosomal RNA (16S rRNA) was used as an internal reference. After the reaction, the CT value was determined to be valid and derived. The relative expression levels of target genes were calculated by the 2−△△Ct method.[Citation16]
Table 1. Nucleotide sequences of primers used for qPCR and product sizes.
Statistical analysis
All data were expressed as means ± standard deviations (SD). They were statistically analyzed using one-way ANOVA in SPSS 20.0 software and Duncan multiple comparisons, before applying Graph Pad Prism 8.0.1 for drawing (GraphPad Software Inc., San Diego, CA, USA).
Results
Steady rheology
The sample concentration had some influences on the viscosity coefficient (R2 = 0.96138–0.99945). The rheological characteristics curve of the LNT solution and the LNT/AS mixture was in accordance with the power-law model in the concentration range of 0.125%−8% (). The 0.125%−4% LNT solution was a shear shinning pseudoplastic fluid (n < 1). The apparent viscosity of LNT solution gradually decreased until stable with the increase of shear rate but raised with the increase of LNT concentration at the same shear rate ().
Figure 1. Effects of the shear rate on the shear stress and apparent viscosity of LNT and AS mixtures at different concentrations. (a, c) LNT; (b, d) LNT + AS (1:1/v:v).
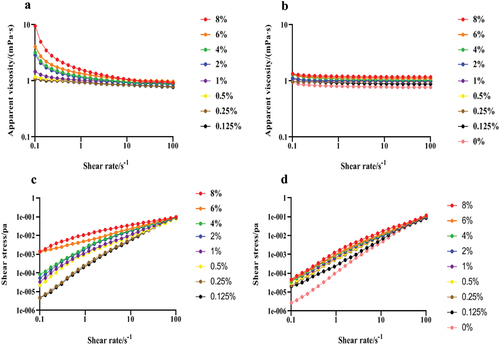
Table 2. Power Law model parameters for the LNT and AS mixture at different concentrations.
The apparent viscosity of AS increased with the increase of shear rate, indicating it is a shear thinning pseudoplastic fluid. After AS was mixed with LNT, at a concentration of 0.125%−8% had the decreased apparent viscosity with the increase of shear rate, showing a typical non-Newtonian flow behavior and a shear thinning pseudoplastic fluid. Moreover, at the same shear rate, the apparent viscosity of elevated with the rise of LNT concentration, but was lower than that of the LNT solution ().
The shear stress increased with the increase of shear rate, and the intercept of the shear rate-shear stress curve was less than one, indicating that both LNT solution and the mixture exhibit shear thinning behavior and are non-Newtonian fluids. Moreover, at the same shear rate, the shear stress of LNT solution in the range of 0.125%−8% raised with the increased LNT concentration. After adding AS, the shear stress of the mixed solution was lower than that of the LNT solution ().
Dynamic rheology
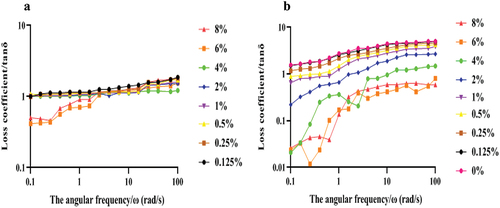
With the increase of angular frequency, the tan δ of LNT solution gradually increased (). When the concentration of LNT solution was 0.125%−4%, the tan δ was always greater than one, indicating that the liquid was in a flowing state. However, the tan δ of 6% and 8% LNT solution was less than one under low-frequency scan, while it was greater than one under high-frequency scan. The tan δ of was significantly higher than that of LNT solution.
Particle size distribution
The particle size distribution of LNT solution was mainly 10–100 nm, which increased with the increase of LNT concentration (). However, most of the particle sizes of the LNT and AS mixture were evenly distributed between 100 and 1000 nm, except for a small amount between 10 and 100 nm. The average particle sizes of LNT and the mixture of LNT and AS gradually decreased with the increase of LNT concentration increased (). The addition of AS significantly increased the average particle size of LNT and AS mixture (p < .05), which was positively correlated with the LNT dose.
Figure 3. Particle size distribution of the LNT and AS mixture at different concentrations. (a) LNT; (b) LNT + AS (1:1/v:v).
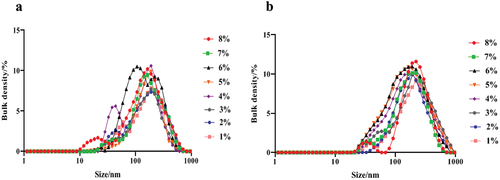
Table 3. Average particle size of the LNT and AS mixture with different concentration.
Surface tension
The surface tension of AS was 80.64 mN/m, which was higher than that of LNT solution (). The surface tension of LNT solution decreased with the increase of LNT concentration. However, the mixture with LNT concentration higher than 2% gradually elevated the surface tension. Moreover, at the same concentration of LNT, the surface tension of the mixed solution was significantly higher than that of LNT solution.
Table 4. Surface tension of the LNT and AS mixture at different concentrations.
Tensile rheology
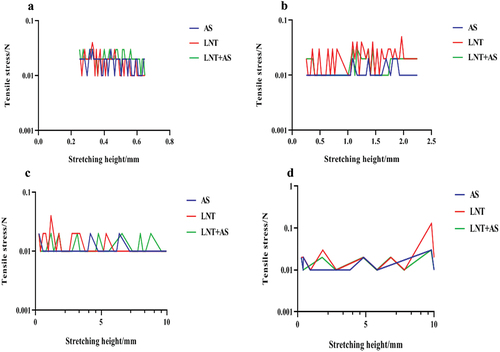
The fracture time of each solution becomes shorter with the acceleration of stretching speed (). At the same stretching rate, the breaking time of AS solution was the shortest, while the breaking time of LNT solution was the longest, which was significantly higher than that of AS solution and the mixture of LNT and AS. As shown in , the fracture height increased significantly with the increase of the tensile speed. When the maximum tensile speed was 5 mm/s, the fracture height of LNT solution and the mixture of LNT and AS solution were significantly higher than that of AS solution.
Table 5. Comparison of solution fracture time at different tensile speeds.
Table 6. Comparison of solution fracture height at different tensile speeds.
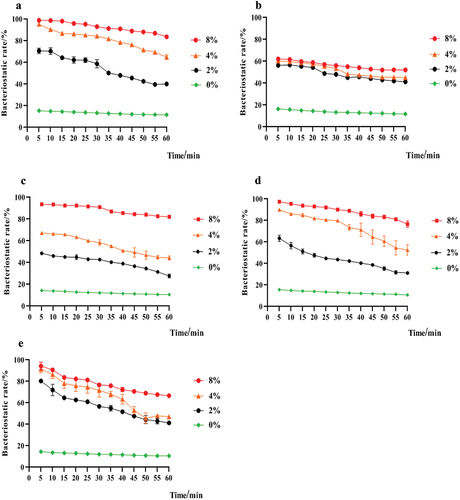
As shown in , different concentrations of LNT and AS mixture could inhibit S. mutans, S. salivalis, A. viscosus, S. sanguinis, and C. albicans. The antibacterial rate of the mixed solution to S. mutans increased with the increase of LNT concentration in mixture (R2 = 0.932–0.9926). The mixture containing 8% LNT had the strongest antimicrobial activity, with the best effect on S. mutans. With the increase of time, the mixture containing 8% LNT had stable inhibitory effects on S. mutans, with the inhibition rate of 99%−83%.
Antibacterial rate on oral caries-causing bacteria
As shown in , different concentrations of LNT and AS mixture could inhibit S. mutans, S. salivarius, A. viscosus, S. sanguinis, and C. albicans. The antibacterial rate of the mixed solution to S. mutans increased with the increase of LNT concentration in mixture (R2 = 0.932–0.9926). The mixture containing 8% LNT had the strongest antimicrobial activity, with the best effect on S. mutans. With the increase of time, the mixture containing 8% LNT had stable inhibitory effects on S. mutans, with the inhibition rate of 99%−83%.
Cell morphology and structure
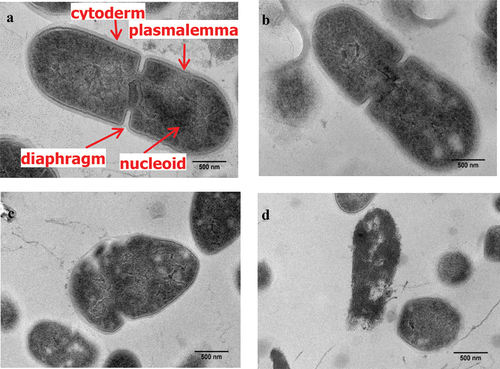
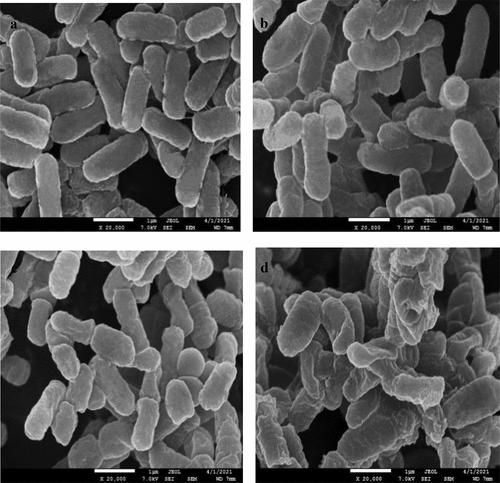
S. mutans in the control group had an intact membrane and a smooth surface. In contrast, treatment with the 2%, 4%, and 8% mixture of LNT and AS caused damage to the morphology and structure of S. mutans in a dose-dependent manner, including rough surface, atrophy, and indentation (), with the most severe damage in 8% LNT group. After treatment with the mixture containing 4% LNT, S. mutans had blurred cell boundaries and distorted morphology. After treatment with the 8% LNT and AS mixture, many bacteria were significantly distorted and deformed, the cell walls were seriously damaged, and some cells were hollow and sunken ().
Figure 6. Effects of the mixture of LNT and AS on cell morphology of S. mutans. (a) 0%; (b) 2%; (c) 4%; (d) 8%.
As shown in , the cell wall and cell membrane of the bacteria in the control group were intact with a high central electron density, evenly distributed structure, clearly visible membrane, and closely combined cell wall and plasma membrane. After treatment with the mixture containing 2% LNT, the pseudo nuclei showed distortion with low-density blank areas (). When the concentration continuously increased to 4% and 8%, the bacteria showed irregular morphology, asymmetric poles, seriously blurred cell walls and plasma membranes, uneven distribution of materials in cells, and some high-density particles in adjacent cell walls ().
Effects of the LNT and as mixture on cell membrane integrity
Intracellular biomacromolecule leakage, electrical conductivity, and β-galactosidase
After treatment with 2%, 4%, and 8% LNT and AS mixture for 15 min, the absorbance of nucleic acid and the mass concentration of soluble protein in S. mutans were significantly increased (p < .05) (). With the continuous increase of time, the leakage of nucleic acid in S. mutans tended to be flat. After treatment for 120 min, the nucleic acid absorbance in the three LNT groups was significantly increased by 0.906 ± 0.046, 1.656 ± 0.047, and 1.819 ± 0.063 (p < .05) (), respectively, and the soluble protein concentration was remarkably increased by 0.005 ± 0.002 g/L, 0.102 ± 0.003 g/L, and 0.13 ± 0.009 g/L (p < .05) ().
Figure 8. Effects of the mixture of LNT and AS on intracellular biomacromolecules in S. mutans. Error bars show standard deviation (n = 3). *, ** and *** indicated respectively p < .05, p < .01 and p < .001 statistically significant difference compared with blank group. a. nucleic acid leakage; b. soluble protein concentration; c. electrical conductivity; d. β -galactosidase activity.
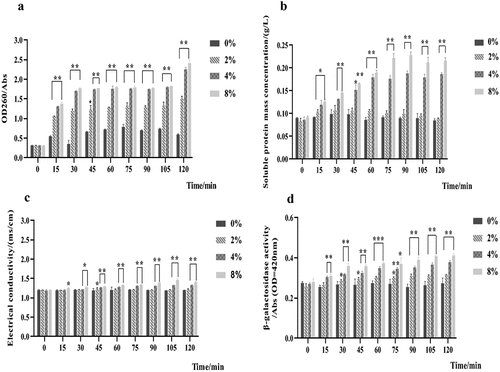
The mixture of LNT and AS enhanced the conductivity and β-galactosidase activity of S. mutans in a dose- and time-dependent manner (). Compared with the control group, the conductivity of S. mutans was significantly increased in the 8% LNT mixture group at 15 min, and in the 4% and 8% LNT mixture at 30–120 min (p < .05) (). Within 15–120 min, the activities of β-galactosidase in the three LNT groups were significantly higher than those of the control group ().
Fluorescence intensity
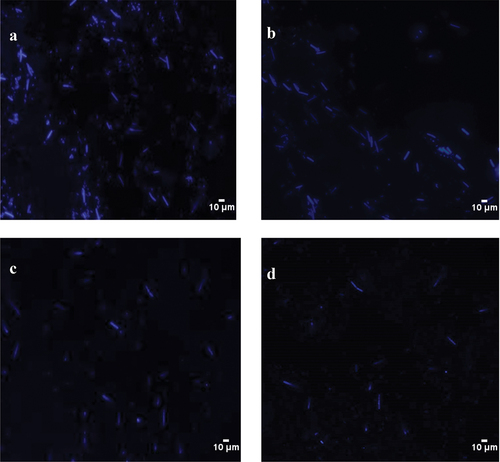
The fluorescence intensity in the three treatment groups decreased in a dose-dependent manner, indicating that the mixture elevates the permeability of the cell membrane in S. mutans and results in the loss of intracellular nucleic acid and DNA content ().
AKP activity and energy metabolism (ATP) of S. mutans
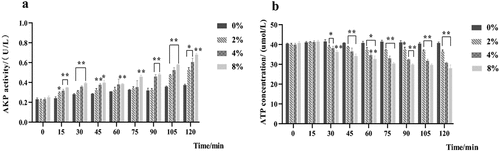
The AKP activities of S. mutans in the three mixture groups were significantly higher than those of the control group from 15 to 120 min (p < .05) (). After treatment for 120 min, the AKP activity in each treatment group (2%, 4%, 8%) was increased by 32.52%, 37.93%, and 44.97% (p < .05, p < .01). However, after treatment from 30 min to 150 min, the ATP concentration in the three LNT groups was significantly reduced ().
Gene expression in the glycolytic pathway
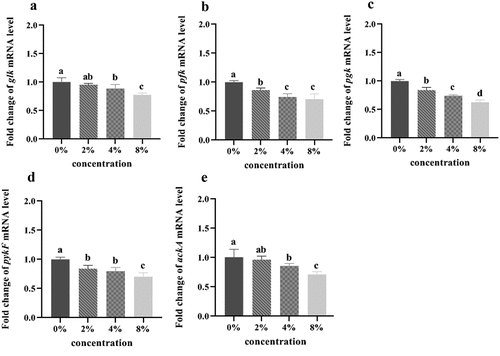
Compared with the control group, the expression levels of glk, ackA, pfk, pykF, and pgk in the three mixture groups were decreased in a dose-dependent manner (p < .05) (). When the mixture concentration was 2%, the expression levels of pfk, pgk, and pykF were significantly decreased (p < .05). When the mixture concentration was increased to 4% and 8%, the expression levels of these genes were significantly decreased by 11.24%, 29.31%, 25.89%, 20.17%, and 14.70%, and by 22.30%, 29.80%, 37.07%, 29.80%, and 29.03%, respectively (p < .05).
Acid production
In the concentration range of 0%−8%, the acid production of S. mutans in the three LNT groups was inhibited with the increased LNT content in the mixture (). The mixture containing 4% and 8% LNT could significantly inhibit the acid production of S. mutans (p < .05). However, the mixture containing 2% LNT produced more acid than the control group.
Table 7. Effects of the LNT and AS mixture on acid production of S. mutans.
Discussion
Polysaccharides have different rheological properties due to the interactions between polymer chains. In this study, the apparent viscosity of LNT solution gradually decreased with the increase of shear rate, which was supported by a previous study.[Citation17] With the increase of shear rate, the apparent viscosity of the mixed solution was significantly decreased, showing typical now-Newtonian flow and shear thinning pseudoplastic fluid, which was proved by the increased shear stress with the increase of shear rate and the intercept of shear rate-shear stress curve of less than one. The phenomenon may be caused by that the LNT molecules in the mixture gradually interact with the components of AS to change the molecular structure of LNT and increase the hydrogen bonding force of LNT molecules. Similar result was obtained in the study by Shengyu Fan et al.,[Citation18] which showed that the shear stress of the low methoxyl pectin (LMP)/sodium caseinate (CAS) complex after adding salt ions was significantly higher than that of the control group, possibly related to the stability of the complex after salt addition; the Na+ interacted with the complex and reduced the binding rate of the protein to the polysaccharide, changed the viscosity of the complex to some extent. Moreover, at the same shear rate, the apparent viscosity of the mixture is continuously elevated with the increase of LNT concentration. This is consistent with a previous study, which indicated that at the same shear rate, the higher the polysaccharide concentration from floral mushrooms cultivated in Huangshan Mountain, the higher the viscosity value.[Citation19]
The increase of apparent viscosity can effectively raise the swallowing time of pharyngeal transport to better coordinate the oral cavity and pharynx. In addition, the apparent viscosity of the mixed solution was lower than that of the LNT solution at the same shear rate, which was consistent with the change of shear stress. The decrease in the apparent viscosity and shear stress of the mixed solution may be a result of the dilution of the mixture caused by the addition of AS (99% water and 1% mucus). The hydrogen bonds and interaction between LNT molecules and water or metal ions disturb the interaction between LNT molecules and AS, resulting in a reduction in apparent viscosity and shear stress.[Citation20]
Loss tangent tan δ = G″/G’ (δ, loss angle) is the ratio of viscosity modulus to elastic modulus, which can directly reflect the viscoelastic characteristics of fluid. Tan δ > 1indicates that the system’s viscosity is higher than the elasticity, indicating the viscosity of the liquid. While tan δ < 1indicates that the system’s elasticity is greater than the viscosity, showing solid gel characteristics. Tan δ in the 0.125%−4% LNT solution was greater than one, showing that the viscosity of the liquid is in flowing state. However, tan δ in the 6% and 8% LNT solution was less than one under low-frequency scan showing weak gel state, while it was greater than one under high-frequency scan showing fluid state. The changes of tan δ in LNT solution may be because the increase of angular frequency changes the 6% and 8% LNT solution system, which unties the links between polysaccharide molecules and makes the viscous components gradually dominant, showing the nature of the fluid. However, tan δ in the mixture was significantly higher than that of LNT solution, indicating that the interaction between LNT and AS can increase the viscoelastic characteristics, which makes the compound easier to flow.
Polysaccharides can change the rheological properties of solutions because they tend to form gel aggregation between macromolecules.[Citation21] Therefore, the particle size distribution of LNT solution and the mixture of LNT and AS can better understand the changes in apparent viscosity caused by the molecular aggregation in polysaccharide solution. In this study, the particle size distribution of LNT solution was mainly 10–100 nm, which may be due to the glycoprotein molecules formed by the combination of polysaccharide molecules and a small amount of proteins. In addition, the particle size distribution between 100 and 1000 nm increased with the raise of LNT concentration, which may be due to aggregation between polysaccharide molecules forms relatively larger polymers. This result is consistent with the elevation of viscosity and shear stress of LNT solution. Chenyu Zhao et al.[Citation22] determined the average particle size of nanoemulsions from glycated soy protein isolate, which showed that the emulsion particle size was smaller, it had higher electrostatic repulsion, and the emulsion was more stable. Therefore, the uniform particle size distribution of the solution indicates that the solution system is relatively stable.
Compared with LNT solution, the average particle size of the mixed solution increased and the particle size distribution was more uniform. Most particle sizes of the mixture were evenly distributed between 100 and 1000 nm, which indicates that LNT has good stability in AS, and the interaction between ions in AS and polysaccharides can promote the uniform distribution of LNT in solution. The average particle size of the LNT was smaller than that of the mixture, which might cause the larger particle size of the mixture due to the entanglement of the polysaccharide molecules with the metal ions in the AS.
Surface tension is necessary for measuring tensile rheology and can reflect the interaction between macromolecules. In this study, the surface tension of LNT solution was lower than that of water (72.4 mN/m), which may be because that the hydrophilicity of LNT molecules in water reduces the strength of hydrogen bond and the surface tension of aqueous solution.[Citation23] The surface tension of AS (80.64 mN/m) was higher than that of water, which may be because that the interaction force among metals in AS is more potent than that of hydrogen bond. However, the LNT gradually reduced the force and surface tension between metal ions. When the LNT concentration of the mixture was higher than 2%, the increase of LNT molecules gradually elevated the surface tension. In addition, at the same concentration of LNT, the surface tension of the mixed solution was significantly higher than that of the LNT solution.
Understanding the tensile rheology of LNT in AS adds a new dimension for exploring the oral processing mechanisms. At low stretching speed, the deformation that can occur is large, and the stretching liquid column can maintain a fracture for a long time. With the increase of the stretching speed, the deformation became smaller, and the fracture time of the stretched liquid column gradually shortened. Due to the increased spacing and viscosity of macromolecules in the mixture, the viscoelasticity of the liquid will hinder the stretching and fracture, and the molecular weight decreased relative to LNT, which indicates that adding LNT to AS can better improve the tensile ductility of AS.[Citation24] This is proved by a previous study.[Citation6]
At different tensile velocities, the maximum tensile stress could be achieved due to the relatively high molecular weight of LNT, which was more challenging to break during the stretching process. The tensile stress of the mixture was better than that of AS solution with smaller intermolecular mobility and enhanced chain entanglement, as proved by the shear viscosity of the LNT and AS mixture. Moreover, the addition of LNT can improve the tensile viscosity, tensile strength, and ductility of the AS solution.
Patients with xerostomia have poor oral self-cleaning ability and high caries rates. S. mutans is considered the most cariogenic bacteria. Therefore, inhibition of S. mutans is a crucial target for caries prevention.[Citation25] In this study, the mixture had inhibitory effects on S. mutans, and the bacteriostatic rate was positively correlated between the LNT concentration of the mixture (R2 = 0.932–0.9926). The mixture containing 8% LNT had the highest bacteriostatic rate of 99% ± 0.85. With the increase of time, it had a lasting inhibitory effect on S. mutans, with an inhibition rate of 99%−83%. A study by He Li[Citation26] found that the inhibitory effects of the peony total polysaccharide on the growth of S. mutans variation mainly appear in bacteria during the logarithmic growth period and increase with increasing concentration, which is consistent with our study of the LNT and AS mixture S. mutans variation. Cell membranes are essential for maintaining cell morphology and life activity and it can protect bacteria from harmful compounds to maintain the stability of the intracellular environment.[Citation27] In this study, the mixture significantly changed the membrane morphology and structures, membrane permeability, and cell wall integrity of S. mutans. To further study the effects of the mixed solution on membrane permeability, S. mutans were stained with DAPI. The mixture significantly reduced the fluorescence intensity of the three treatment groups in a dose-dependent manner, which indicates that the mixture increases the permeability of the cell membrane in S. mutans, resulting in the loss of intracellular nucleic acid and a significant decrease of DNA content.
The damage to cell membranes will induce the leakage of macromolecular substances, including nucleic acid and protein, into the cell membrane and cytoplasm, resulting in an increase of cell membrane permeability and conductivity.[Citation28] In addition, β-galactosidase is a hydrolase located on the cell membrane, and its leakage can evaluate the permeability and destruction of cell membrane.[Citation29] In this study, the leakage of nucleic acids, soluble protein, and β-galactosidase in the three LNT groups was significantly increased compared with the control group, indicating that the mixture can inhibit the growth of S. mutans by causing the leakage of biomacromolecules, thus resulting in the damage and the increased permeability of cell membrane of S. mutans in a dose-dependent manner. Diana Royan et al.[Citation30] found that the phenol component of xanthorrhizol can cause protein coagulation, lysis of bacterial cell membranes, unequal cell membrane permeability, leaked of intracellular material, and cell death in S. mutans. Moreover, the mixture enhanced the conductivity of S. mutans in a dose- and time-dependent manner, similar to a previous study.[Citation31] AKP is a phosphatase involved in cellular phosphorus metabolism and signal transduction. Damage to the cell wall will lead to AKP leakage and increased AKP activity in the culture medium[Citation32] In this study, the mixture significantly increased the AKP activity and led to AKP leakage into the culture medium, which was proved by the destruction of the cell wall, as supported by a previous study.[Citation33]
There are many ion channels on the cell membrane of bacteria, which are essential for the exchange of materials and energy inside and outside the cell. Change in cell membrane permeability can cause the leakage of ATP, which plays a vital role in cell material transport and energy conversion.[Citation34] In this study, after treatment from 30 min to 150 min, the ATP concentration in the three LNT groups was significantly reduced. The reduced ATP concentration may be caused by that on the one hand, the electrolyte loss in the bacteria decreases the proton dynamic of cells, which disturbs the utilization of reducing hydrogen and inhibits the synthesis of ATP; on the other hand, the reduction of bacteria number and apoptosis results in a decrease in the rate of ATP synthesis and an increase in the rate of ATP hydrolysis. Bajpai Viwek K et al.[Citation35] studied the effects of cudrania tricuspidata fruit essential oil on membrane permeability, and they found that the reduction in intracellular ATP may be due to a decreased rate of intracellular ATP synthesis and an increased rate of intracellular ATP hydrolysis, which is consistent with our findings.
Glycolysis pathway is a critical cycle involved in intracellular energy metabolism and substance synthesis. There are multiple key enzymes in the glycolysis process, five of which produce or consume ATP, including glk, pfk, pgk, pykF, and ackA. Inhibition of these enzyme activities will interfere with the various metabolism of cells.[Citation36] In this study, the lower expression of glk can hinder the oxidative decomposition of sugars in glycolysis and reduce ATP content, which further blocks the formation of glucose 6-phosphate process of glk catalyzing glucose, which is consistent with a previous study.[Citation37] In addition, the mixture could directly inhibit the expression of the restriction enzymes of pfk, pgk, and pykf in late glycolysis, with the most significant inhibitory effects on pgk gene. Therefore, it can be speculates that the mixture can reduce the levels of relevant regulatory enzymes during the glycolytic reaction of S. mutans, weaken the biological activity of S. mutans, and inhibit the role of glycolytic metabolism until the end of the whole glycolytic pathway.[Citation38] In pyruvate metabolism, the ackA mRNA expression was significantly different from that in the control group, and acetyl-CoA produced by pyruvate formate-lyase pathway is used to produce acetate and ATP, indicating that S. mutans treated with the mixture prefers to use lactate fermentation to produce ATP, which further affects ATP synthesis. Similar results are obtained in a previous study.[Citation39]
Acid production in bacterial metabolism is the direct cause of dental caries. Change of ∆pH value in the liquid medium represents the accumulation of bacterial acid metabolites. The better the growth conditions of bacteria, the smaller the pH value, and the more acid metabolites.[Citation40] In this study, the acid production of S. mutans was decreased and inhibited with the increase of the LNT content (4%, 8%) in the mixture. The reduced ∆pH can adversely affect proton dynamics, resulting in a significant decrease in ATP concentration, which can depolarize the cell membrane.[Citation41]
Conclusion
In conclusion, the results indicate that LNT plays a dominant role in the oral processing rheology of food to better simulate the rheological properties of xerostomia patients in oral processing. The mixture of LNT and AS plays a vital role in the antibacterial mechanism against S. mutans by inhibiting the growth of S. mutans and causing the leakage of biological macromolecules and the destruction of intracellular homeostasis, therefore resulting in the deterioration and increased permeability of cell membrane, inhibition of ATP synthesis in glycolysis and the increase of acid production of S. mutans in a dose-dependent manner. It can provide an excellent theoretical basis for studying LNT as a functional food raw material. To meet the clinical needs, further studies on the side effects of LNT, cytotoxicity, and taste are needed to adapt the population with oral diseases.
Authorship contribution statement
Wei Han: Methodology, Data curation, Writing-orginal draft; Xingru Lu: Investigation, Methodology, Data curation; Honghu Ai: Data curation, Writing – review & editing, Resources; Rui Wu: Formal analysis, Methodology, Data curation; Shanshan Wu: Formal analysis, Investigation, Methodology; Yanfeng Cheng: Conceptualization, Validation, Writing-orginal draft; Shaojun Yun: Visualization, Methodology, Formal analysis, Writing-review & editing; Mingchang Chang: Software, Formal analysis, Investigation, Writing-review & editing; Feier Cheng: Validation, Investigation, Writing-orginal draft; Cuiping Feng: Funding acquisition, Conceptualization, Writing-review & editing. Jinling Cao: Funding acquisition, Project administration, Supervision, Data curation, Formal analysis, Writing-review & editing. All authors approved the final version of this manuscript.
Acknowledgments
This project was supported by the Shanxi Natural Science Foundation Project (202103021224126); the earmarked fund for Modern Agro-industry Technology Research System (2022HX027); Special Scientific Research Project of Agricultural Valley Construction in Shanxi Province (SXNGJSKYZX201903).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lavergne, M. D. D.; Young, A. K.; Engmann, J.; Christoph, H., Food Oral Processing—An Industry Perspective. Front Nutr. 2021, 8, 634410. DOI:10.3389/fnut.2021.634410.
- Witter, D. J.; Haan, A. F. D.; Käyser, A. F.; van Rossum, G. M. A 6-Year Follow-Up Study of Oral Function in Shortened Dental Arches. Part I: Occlusal Stability. J. Oral Rehabil. 2010, 21(2), 113–125. DOI: 10.1111/j.1365-2842.1994.tb01131.x.
- Rossetti, D.; Yakubov, G. E.; Stokes, J. R.; Williamson, A. M.; Fuller, G. G. Interaction of Human Whole saliva and Astringent Dietary compounds investigated by Interfacial Shear Rheology. Food Hydrocoll. 2008, 22(6), 1068–1078. DOI: 10.1016/j.foodhyd.2007.05.014.
- Panda, S.; Chen, J.; Benjamin, O. Development of Model Mouth for Food Oral Processing Studies: Present Challenges and Scopes. Innovative Food Sci. Emerging Technol. 2020, 66(1), 102524. DOI: 10.1016/j.ifset.2020.102524.
- Shi, J. J.; Zhang, J. G.; Sun, Y. H.; Xu, Q. X.; Li, L.; Prasad, C.; Wei, Z. J. The Rheological Properties of Polysaccharides Sequentially Extracted from Peony Seed Dreg. Int. J. Biol. Macromol. 2016, 91, 760–767. DOI: 10.1016/j.ijbiomac.2016.06.038.
- Yuan, B.; Ritzoulis, C.; Chen, J. Extensional and Shear Rheology of Okra Polysaccharides in the Presence of Artificial Saliva. NPJ Sci. Food. 2018, 2, 20. DOI: 10.1038/s41538-018-0029-1.
- He, L. I.; Qin, M. A.; Zhao, J. Study on Optimization of Extraction and Effects of Polysaccharides on Streptococcus Mutans from Paeonia Sinjiangensis K. Y. Pan. Guangdong Trace Elem. Sci. 2013, 20(4), 10–17. DOI: 10.16755/j.cnki.issn.1006-446x.2013.04.008.
- Zheng, Z.; Zhang, Y.; Liu, Y.; Wang, J.; Cui, Z.; Pan, X.; Liu, Y.; Tang, W.; Wang, K. Metabolic Degradation of Lentinan in Liver Mediated by CYP450 Enzymes and Epoxide Hydrolase. Carbohydr. Polym. 2021, 253(9), 117–255. DOI: 10.1016/j.carbpol.2020.117255.
- Ziaja-Sotys, M.; Radzki, W.; Nowak, J.; Topolska, J.; Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Kuczumow, A.; Bogucka-Kocka, A. Processed Fruiting Bodies of Lentinus Edodes as a Source of Biologically Active Polysaccharides. Appl. Sci. 2020, 10(2), 470. DOI: 10.3390/app10020470.
- Nagashima, Y.; Yoshino, S.; Yamamoto, S.; Maeda, N.; Azumi, T.; Komoike, Y.; Okuno, K.; Iwasa, T.; Tsurutani, J.; Nakagawa, K. Lentinula Edodes Mycelia Extract Plus Adjuvant Chemotherapy for Breast Cancer Patients: Results of a Randomized Study on Host Quality of Life and Immune Function Improvement. Mol. Clin. Oncol. 2017, 7(3), 359–366. DOI: 10.3892/mco.2017.1346.
- Yu, Z. H.; Yin, L. H.; Qian, Y.; Yan, L. Effect of Lentinus Edodes Polysaccharide on Oxidative Stress, Immunity Activity and Oral Ulceration of Rats Stimulated by Phenol. Carbohydr. Polym. 2009, 75(1), 115–118. DOI: 10.1016/j.carbpol.2008.07.002.
- Ciric, L.; Tymon, A.; Zaura, E.; Lingström, P.; Stauder, M.; Papetti, A.; Signoretto, C.; Pratten, J.; Wilson, M.; Spratt, D. In vitro Assessment of Shiitake Mushroom (Lentinula Edodes) Extract for Its Antigingivitis Activity. Biomed Res. Int. 2011, 2011(1), 1–7. Article 507908. DOI: 10.1155/2011/507908.
- Meng, J. L.; Feng, C. P.; Chang, M. C.; Cheng, H. Y. Study on the Antibacterial Effect of Shiitake Polysaccharide. J. Shanxi Agri. Univ. (Nat. Sci. Edition). 2012, 32(3), 261–264. DOI: 10.13842/j.cnki.issn1671-8151.2012.03.004.
- Ma, C. Y.; He, N.; Zhao, Y. Y.; Xia, D. D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189(4), 1291–1303. DOI: 10.1007/s12010-019-03067-1.
- He, N.; Wang, P. Q.; Wang, P. Y.; Ma, C. Y.; Kang, W. Y. Antibacterial Mechanism of Chelerythrine Isolated from Root of Toddalia Asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18(1), 261–269. DOI: 10.1186/s12906-018-2317-3.
- Li, P. F.; Sun, N.; Zeng, J. C.; Zeng, Y. R.; Fan, Y. G.; Feng, W. J.; Li, J. Differential Expression of MiR-672-5p and MiR-146a-5p in Osteoblasts in Rats After Steroid Intervention. Gene. 2016, 591(1), 69–73. DOI: 10.1016/j.gene.2016.06.045.
- Hao, Z. Q.; Chen, Z. J.; Chang, M. C.; Meng, J. L.; Liu, J. Y.; Feng, C. P. Rheological Properties and Gel Characteristics of Polysaccharides from Fruit-Bodies of Sparassis Crispa. Int. J. Food Prop. 2018, 21(1), 2283–2295. DOI: 10.1080/10942912.2018.1510838.
- Salehi, F.; Kashaninejad, M.; Behshad, V. Effect of Sugars and Salts on Rheological Properties of Balangu Seed (Lallemantia Royleana) Gum. Int. J. Biol. Macromol. 2014, 67(3), 16–21. DOI: 10.1016/j.ijbiomac.2014.03.001.
- Xu, J. L.; Zhang, J. C.; Liu, Y.; Sun, H. J.; Wang, J. H. Rheological Properties of a Polysaccharide from Floral Mushrooms Cultivated in Huangshan Mountain. Carbohydr. Polym. 2016, 139, 43–49. DOI: 10.1016/j.carbpol.2015.12.011.
- Ni, L. F.; Yu, J.; Wang, X. P.; Wang, J.; Cao, X. X.; Cao, S. L.; Ma, X. J. Studies on the Effects of Sodium Hydroxide on Hydrogen Bonding of Water and Ionic Liquid/H2O Systems by ATR-IR Analyses. Spectrosc. Spectral Anal. 2021, 41(10), 3106–3110. DOI: 10.3964/j.issn.1000-0593(2021)10-3106-05.
- Chen, J. S. Food Oral Processing: Some Important Underpinning Principles of Eating and Sensory Perception. Food Struct. 2014, 1(2), 91–105. DOI: 10.1016/j.foostr.2014.03.001.
- Zhao, C. Y.; Bu, G. H.; Chen, F. S.; Yu, Z. Preparation and Stability of Nanoemulsions from Glycated Soy Protein Isolate. J. Henan Univ. Technol: Nat. Sci. Edition. 2021, 42(4), 22–29. DOI: 10.16433/j.1673-2383.2021.04.004.
- Ren, N. N.; Gong, Y. H.; Lu, Y. Z.; Meng, H.; Li, C. X. Surface Tension Measurements for Seven Imidazolium-Based Dialkylphosphate Ionic Liquids and Their Binary Mixtures with Water (Methanol or Ethanol) at 298.15 K and 1 Atm. J. Chem & Eng Data. 2014, 59(2), 189–196. DOI: 10.1021/je400004j.
- Ren, D. D. Preparation and Performance Studies of Multifunctional Polyacrylamide Composite Hydrogels. Zhengzhou Univ. 2021. DOI: 10.27466/d.cnki.gzzdu.2021.005148.
- Ccahuana-Vásquez, R. A.; Cury, J. A. S.Mutans Biofilm Model to Evaluate Antimicrobial Substances and Enamel Demineralization. Braz. Oral. Res. 2010, 24(2), 135–141. DOI: 10.1590/S1806-83242010000200002.
- He, L. In Vitro Study of the Effect of Peony Total Polysaccharides on Bacterial Virulence Factors; Xinjiang Medical University: 2013. DOI: 10.7666/d.D600574.
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71(1), 519–538. DOI: 10.1146/annurev-micro-102215-095630.
- Zhou, J. C.; Zhou, G. Y.; Liu, J. A.; He, Y. H.; Dong, W. T. Antifungal Mechanisms of the Ethyl Acetate Extracts from Phytolacca americana and Ageratum Conyzoides Against Colletotrichum Gloeosporioides of Dalbergia Odorifera. Plant Prot. 2017, 43(4), 46–50+60. DOI: 10.3969/j.issn.0529-1542.2017.04.008.
- Wang, Z. C.; Yang, Q. Q.; Wang, X. Q.; Li, R. F.; Qiao, H. Z.; Ma, P. A.; Su, Q.; Zhang, H. R. Antibacterial Activity of Xanthan-Oligosaccharide Against Staphylococcus Aureus via Targeting Biofilm and Cell Membrane. Int. J. Biol. Macromol. 2020, 153(6), 539–544. DOI: 10.1016/j.ijbiomac.2020.03.044.
- Diana, R.; Joenoes, H.; Djais, A. A. The Effect of Curcuma Xanthorrhiza Ethanol Extract on the Viability of Streptococcus Mutans and Aggregatibacter Actinomycetemcomitans (Dental Biofilm Research: In vitro Study). Asian J. Pharm. Clin. Res. 2017, 10(17), 30. DOI: 10.22159/ajpcr.2017.v10s5.23087.
- Zhang, Z. H.; Dong, Y.; Tian, Y.; Ji, Y. R.; Yang, Q. L.; Shi, J. Antibiostatic Mechanism of Industrial Cannabis Leaves Against Staphylococcus Aureus. J. Food Biotechnol. 2021, 40(5), 95–103. DOI: 10.3969/j.issn.1673-1689.2021.05.012.
- Ji, J. Y.; Yang, J.; Zhang, B. W.; Wang, S. R.; Zhang, G.-C.; Lin, L.-N. Sodium Pheophorbide a Controls Cherry Tomato Gray Mold (Botrytis cinerea) by Destroying Fungal Cell Structure and Enhancing Disease Resistance-Related Enzyme Activities in Fruit. Pestic. Biochem. Physiol. 2020, 166, 104581. DOI: 10.1016/j.pestbp.2020.104581.
- Choi, B. K.; Kim, K. Y.; Yoo, Y. J.; Oh, S. H.; Choi, J. H.; Kim, K. Y. In vitro Antimicrobial Activity of a Chitooligosaccharide Mixture Against Actinobacillus Actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents. 2001, 18(6), 553–557. DOI: 10.1016/S0924-8579(01)00434-4.
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene Against Listeria Monocytogenes. Mol. 2020, 25(1), 33. DOI: 10.3390/molecules25010033.
- Bajpai, V. K.; Sharma, A.; Baek, K. H. Antibacterial Mode of Action of Cudrania Tricuspidata Fruit Essential Oil, Affecting Membrane Permeability and Surface Characteristics of Food-Borne Pathogens. Food Control. 2013, 32(2), 582–590. DOI: 10.1016/j.foodcont.2013.01.032.
- Zou, L.; Hu, Y.; Chen, X.; The Antimicrobial Mechanism of Black Pepper Petroleum Ether Extracts on Escherichia coli and Staphylococcus Aureus. Food Science and Technology. 2018, 43(6), 245–249. CNKI:SUN:SSPJ.0.2018-06-049.
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral Exerts Its Antifungal Activity Against Penicillium Digitatum by Affecting the Mitochondrial Morphology and Function. Food Chem. 2015, 178(jul.1), 76–81. DOI: 10.1016/j.foodchem.2015.01.077.
- Kong, J. H. Enzymatic Preparation of Theaflavins and Its Health-Promoting Activity on Oral Microbiota. Zhejiang Gongshang Univ. 2022. DOI: 10.27462/d.cnki.ghzhc.2022.000007.
- Park, S. N.; Lim, Y. K.; Choi, M. H.; Cho, E.; Bang, I. S.; Kim, J. M.; Ahn, S. J.; Kook, J. K. Antimicrobial Mechanism of Oleanolic and Ursolic Acids on Streptococcus Mutans UA159. Current Microbiol: An Int. J. 2017, 75(1), 11–19. DOI: 10.1007/s00284-017-1344-5.
- Zhao, G. J. Effect of Different Concentrations of Sucrose and Xylitol on the Interaction Between S. Sanguina and S. Mutans [master’s thesis]; Zhengzhou University: China, 2015.
- Ultee, A.; Bennik, M. H. J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol is Essential for Action Against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68(4), 1561–1568. DOI: 10.1128/AEM.68.4.1561-1568.2002.