?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study explored the nutritional qualities of black mash bean flour impacted by fermentation, gelatinization and their combination. Protease activity, in vitro protein digestibility (IVPD), and protein solubility were evaluated for both fermented and unfermented black mash bean flour samples. Interestingly, when compared to fermented samples and gelatinized mash bean flour (GMBF), raw mash bean flour (RMBF) showed the highest protein solubility. Due to enzymatic activity and protein hydrolysis, fermentation with Lactobacillus E14 and Saccharomyces cerevisiae MK-157 enhanced protein solubility. Prolonged fermentation timeframes were positively correlated with protease activity and IVPD, suggesting the degradation of complex proteins. The highest levels of IVPD (90.12%) and protease activity (5546.78 U/g) were detected in GMBF fermented by S. cerevisiae MK-157, indicating the impact of fermentation on protein breakdown. The Lactobacillus E14-fermented RMBF exhibited 5521.45 U/g of protease activity and 87.54% IVPD. The mineral content of the fermented samples was substantially greater than that of the unfermented GMBF. The increased calcium, magnesium, potassium, sodium, iron, zinc, and copper levels for the GMBF fermented by S. cerevisiae MK-157 indicated that fermentation facilitated the release of minerals from chelated compounds. Microscopic examination revealed higher protein quantities in the fermented flour as well as hydrolyzed and broken starch granules embedded in the protein matrix. Large mash bean proteins were shown to be proteolyzed during fermentation into smaller peptides by SDS PAGE. Storage proteins were extensively broken down during fermentation with S. cerevisiae MK-157, whereas Lactobacillus E14 displayed a more focused pattern of hydrolysis. The nutritional profile of black mash bean flour was improved by fermentation with Lactobacillus E14 and S. cerevisiae MK-157.
Introduction
Legumes represent a crucial yet cost-effective reservoir of proteins, dietary fiber, essential vitamins, minerals, and phytonutrients. Regular legume consumption correlates with several health benefits, notably the reduction in susceptibility to cancer, type-II diabetes, and cardiovascular diseases[Citation1]Interestingly, throughout the previous three decades, the average amount of legumes consumed by one individual has not changed, even despite an increase in worldwide population measurements.[Citation1] Therefore, additional study on legumes is needed to provide creative substitutes for food ingredients that can increase their intake while also satisfying the increased need for nutrient-dense food options. Commonly termed as black gram, black bean, or black mash bean (Vigna mungo) is a member of the Leguminoseae family. It is known for containing high levels of protein, mineral, fiber, and phytochemical contents with number of medicinal benefits. In particular, its components have been scientifically associated with cholesterol lowering, antioxidant, anti-carcinogenic, and anti-diabetic properties.[Citation1]
Despite the health advantages associated with black gram lentils, the digestion process might compromise nutrient bioavailability. This limitation arises from the presence of anti-nutritional components (i.e., phytic acid, saponin, protease inhibitors and raffinose) which inhibits digestive enzymes.[Citation2] Specifically, within these ant-nutritional factors, phytic acid in black mash beans obstructs the accessibility of vitamins, vital minerals (such as calcium, magnesium, iron, and zinc), and proteins due to its strong chelating properties which causes nutrient deficiencies by interfering with the absorption mechanism of small intestine.[Citation2] To mitigate these anti-nutritional elements in legumes, prior treatments are necessary such as heat treatment (boiling, steaming and roasting), sprouting/germination, and fermentation. Notably, boiling induces full or partial starch gelatinization, enhancing the adaptability of legume flours in the production of baked products, pasta, and snack items.[Citation3]
Thermal processing of legume seeds, before milling, is a pivotal step when aiming to utilize them as efficacious functional food components. Exposing the whole seeds to moist heat leads to the gelatinization of starch, a process facilitated by water molecules permeating the seed coat.[Citation4] Simultaneously, certain sections of the middle lamellae within the cell walls disassociate due to the degradation of pectin.[Citation4] Such modifications notably enhance the digestibility of primary constituents like proteins and starch in legume flours (Ali et al., 2023).[Citation5] Additionally, fermenting legumes enhances their texture, shelf life, organoleptic properties, nutrient digestibility, and augments bioactive compounds while markedly diminishing anti-nutritional components.[Citation6] Moreover, pretreating lentils, beans, and peas via the enzymatic conversion of anti-nutritional compounds by fermentation is a reliable method. Fermentation of legumes flour is known to enhance the bioavailability of proteins, minerals, antioxidants, vitamins and bioactive compounds.[Citation2] Fermented food products are enjoyed globally representing the consumption ratio of 60% and 25% by developing countries and Europe, respectively.[Citation6] The beneficial effects of solid-state fermentation (SSF) overcomes importance of liquid-state fermentation because of accelerated procedures, economic viability, environmental sustainability, and desirable environment for microbial growth.[Citation7] Moreover, the moisture level and its distribution within the solid substrate is essential and critical factor for the desirable microbial growth. Numerous researchers had demonstrated the remarkable impact of SSF of peas, beans and lentils.[Citation8–10]
More than one microbial culture can be used in the SSF. Additionally, according to [Citation11] lactic acid bacteria (LAB) are commonly referred as probiotics. The LAB such as Pediococcus acidilactici, P. pentosaceus, Lactobacillus plantarum and, Lactobacillus sakei[Citation12] have been used to ferment legumes and have shown increased antioxidant levels with the corresponding decrease in antinutrients.[Citation13] Furthermore, legumes fermentation with yeast cells has also been reported by researchers. Several Saccharomyces cerevisiae microbial strains are employed in the production of fermented foods i.e., cereals, dairy products, and beverages.[Citation14] The boulardii strain of S. cerevisiae is one of the commercial probiotics that is well-known for favorable nutritional qualities.[Citation14] Moreover, [Citation8] and [Citation15] have respectively evaluated the suitability of specific strains of S. cerevisiae, such as LM and KY794742, for fermenting dehydrated cocoa beans.
In food preparation, only endosperm fraction of black mash bean have been utilized which accounts for the generation of 25% bio-waste of the beans in terms of their byproducts i.e., shells.[Citation5] In this investigation, intact black mash beans, inclusive of their shells, were subjected to treatment, elevating the commercial relevance of these typically discarded fractions. Notably, the external husks of legumes, despite their rich composition of bioactive substances and antioxidants, are predominantly classified as organic waste and remain unexploited in human diets or food products.[Citation15] Therefore, the aim of this research was to utilize whole seeds of black mash beans to evaluate the combined effect of gelatinization and fermentation on macronutrients and micronutrients. To date, the scholarly database indicates that neither raw nor pre-gelatinized black mash bean flour has been investigated for solid-state fermentation via Lactobacillus sp. and S. cerevisiae for the determination of SDS-Page pattern, protein digestibility, mineral concentration, and structural profile. The findings from this study could potentiate the sustainable handling of legume by-products and may foster the idea for advancements in pilot-scale legume processing.
Materials and methods
Materials
The black mash beans were purchased from the local market in Karachi, Pakistan. The chemicals and microbiological cultures used were of the analytical reagent grade (Oxoid, USA; Sigma-Aldrich, Germany; Dae-Jung, South Korea).
Preparation of raw and gelatinized black mash bean flour
The whole black mash beans were converted into fine flour using a laboratory miller (Model 3100 from Perten Instruments). The obtained milled bean flour was sieved through a 60 µm mesh to achieve consistent particle size. The seeds underwent a gelatinization process where they were boiled in purified water at 100°C for 15 min. After boiling, the seeds were air-dried at 60°C for 8 h. The post-gelatinization dried seeds were then milled and sifted similarly to the initial raw black mash bean flour procedure. Additionally, to ensure sterility, the resulting flour samples were autoclaved at 121°C and 15 psi for 20 min.
Microorganism and preparation of inoculum
In our previous studies, S. cerevisiae MK-157, and Lactobacillus sp. E14 demonstrated enhanced enzyme production, antioxidant activities, and anti-nutrient inhibition in fermented mash bean flour.[Citation4] All the above mentioned strains were obtained from the microbial collection at the University of Karachi, Pakistan. To reactivate the strains of S. cerevisiae MK-157 and Lactobacillus sp. E14, they were cultured in Sabouraud Dextrose Broth (SDB), Oxoid, USA and De Man, Rogosa, & Sharpe agar (MRS), respectively. The culture was then incubated at 30°C for 72 h. Following incubation, the culture underwent centrifugation at 6,000 × g for 15 min. The resulting supernatant was removed, and the sediment was rinsed three times using saline. Following this, a mineral salt medium (MSM) was used to suspend again the cleaned pellet.[Citation4] After vortex mixing (5 min), the optical density was measured at 600 nm and adjusted to achieve 0.5 OD.
Solid-state fermentation of black mash bean flour
The optimal conditions for solid-state fermentation, including inoculum size, incubation temperature, and incubation time, were determined in a previous study conducted by our research team.[Citation4] Both the raw and gelatinized mash bean flour (RMBF and GMBF) 5 g each were fermented in petri plates. The RMBF was fermented for 80 ho at 36°C with a 3.76 mL Lactobacillus E14 inoculum. In contrast, 2.58 mL of Lactobacillus E14 inoculum was used to ferment GMBF for 25 h at 24°C. S. cerevisiae MK-157 strain utilized for fermenting RMBF and GMBF at inoculum levels of 4.92 mL for 80 h at 25°C and 3.88 mL for 44 h at 37°C, respectively. The moisture content of the fermenting medium was maintained to 80% by using MSM. Then after fermentation samples were sterilized followed by drying for 24 h at 60°C. For further utilization, fermented flour was ground into a fine powder, passed through a 60 µm sieve, and stored at 4°C. A control sample was also processed under identical conditions but without the introduction of any culture medium.
Protein extraction
Protein isolates from both fermented and control flour samples were prepared according to the method outlined by Ladjal-Ettoumi et al..[Citation16] Briefly, 30 g of flour was mixed with distilled water in a ratio of 1:10 (w/v). The pH of the mixture was adjusted to 8.00 using 1 M NaOH and stirred at 500 rpm for 45 min at 20°C. The suspension was then centrifuged at 4°C for 20 minutes at 4500 g. The pellet obtained was re-suspended in distilled water (pH 8.0, 1:5 (w/v)), vortexed for 45 min, and finally centrifuged (4500 × g, 20 min, 4°C). The supernatants from the two centrifugations were consolidated and maintained at pH 4.5 utilizing 0.1 M HCl to precipitate the protein. The resulting precipitate was washed with distilled water (4°C) twisted, and freeze-dried.
SDS polyacrylamide gel electrophoresis
The SDS-PAGE analysis was conducted based on the method described by He Fanglian[Citation17] with minor modifications. Resolving and stacking gels were prepared with 10% and 5% protogel, respectively. The resolving gel was composed of 1.5 M Tris-base with 20% SDS (pH 8.8), while a solution of 0.5 M Tris-base with 20% SDS at pH 6.8 was used for the stacking gel. The stacking gel was poured over the resolving gel, and a comb was used to create the wells, which polymerized for 45 min. Once the gel was fully polymerized, the comb was removed, and the wells were rinsed with deionized water. The gel tank was filled up to 1 L of running buffer (Tris-base; 18 g, SDS; 6 g, and Glycine; 86.4 g). Each protein sample (25 µL) was loaded onto the gel. The gel tank was sealed, and a continuous current (90 V) was applied. Protein standard served as a reference, and staining was performed using Coomassie Brilliant Blue.
Protein solubility
Protein extract samples were prepared according to the method of Li & Wang.[Citation18] Flour samples were sifted through a 0.2 mm sieve and subsequently extracted through ultra-sonication by utilizing a buffer (boric acids and sodium hydroxide) for 1 h at 25°C. The concentrates underwent centrifugation (7500 × g, 4°C) for 15 min. The soluble protein levels in the supernatants were assessed using Biorad Quickstart® Kit (Bradford reagent 5,000,203, Bio-Rad) by following the manufacturer instructions. The standard curve was prepared by using bovine serum albumin (BSA). Bradford reagent (300 µL) was mixed with sample (12.5 µL) and incubated at 37°C for 45 min. Distilled water was added in blank instead of sample. Absorbance was recorded at 595 nm.
Estimation of protease activity
A flour sample (1 g) was subjected to extraction in 25 mL of PBS (0.1 mol/L, pH 7.5) for a duration of 1 h at 25°C, with intermittent shaking. Subsequently, the mixture underwent centrifugation for 15 min at 8000 × g and 4°C. The resulting supernatant, obtained after centrifugation, was utilized for the assessment of protease activity following the protocol outlined by.[Citation18] The quantification of protease activity was expressed in units (U), where one unit represents the quantity of enzyme necessary to librate 1 µg of tyrosine/min under the specific experimental states applied. A standard curve was constructed employing tyrosine concentrations ranging from 0 to 50 mg/L. Finally, the protease activity was indica as units per gram (U/g) of the dry sample.
In-vitro protein digestibility
The protein digestibility of samples was studied using an In-vitro method presented by Almeida et al.[Citation19] The nitrogen levels present in the enzymatic digested samples and undigested samples were determined with the help of the Kjeldahl process. The In-vitro protein digestibility was analyzed as follows:
Mineral analysis and bio-availability
Mineral contents of fermented flour samples were assessed using atomic absorption spectrophotometry.[Citation20] Briefly, 2 g of each sample was incinerated for 7 h at 550°C in a Nabertherm furnace (Nabertherm B150, Lilienthal, Germany). The resulting samples were dissolved with 10 mL of 10% HNO3 (w/v) in a 25 mL volumetric flask. Following digestion, the sample was diluted to a final volume of 25 mL with distilled water, and an atomic absorption spectrophotometer (AAnalyst 200 PerkinElmer CT 06484–4794 USA) was used for analysis. The operational conditions were as follows: Air to acetylene ratio of 13.50:2, Nebulizer uptake rate of 5 L/min. Detection limits were established for Mg (0.02 ppm), K (0.06 ppm), Fe (0.03 ppm), Cu (0.03 ppm), Mn (0.03 ppm), and Zn (0.03 ppm). Calibration was performed by of utilizing standard solutions form each element with concentrations ranging from 10 to 50 ppm. Utilizing a replicated gastrointestinal model, the in vitro bioavailability of calcium, iron, and zinc was examined.[Citation21] Following the recommendations of Sadh et al.. (2017),[Citation22] the formulations of inorganic and organic solvents, saliva, gastric juice, duodenal fluids, and bile contents were mimicked. Using the following formula, the in vitro bioavailability of minerals was determined:
Bright field microscopy
Light microscopy of fermented RBMF, fermented GBMF and their control samples were performed with a light microscope (TE2000, Nikon, Japan) equipped with a digital camera (DS-5 M, Japan) according to the method of Torres et al.[Citation23] Flour samples (0.2 g) were slurred with distilled water in Eppendorf microfuge tubes (2.0 mL). The tubes were capped and gently agitated. Then, the thin layer of uniform thickness of slurry was immobilized on a slide. After drying, slides were separately stained for 1 min with 0.1% fast green and 0.1% iodine. Then the prepared slides were washed with distilled water, air dried and covered with a cover slip. The images were captured at 50 objective lens and analyzed by NIS-Elements software.
Scanning electron microscopy
The micrographs of flour samples were assessed by utilizing a scanning electron microscope (JEOL, Analysis system, Model # JSM-6380, Japan).[Citation24] The samples were affixed to the sample holder and coated with a thin layer of gold (2 min, 2 mbar) using a sputter-coating process. Scanning electron microscopy (SEM) studies were conducted at an applied potential of 10 kV, and all micrographs were captured at a magnification of 3500 X.
Statistical analysis
Version 17.0 of the Statistical Package for the Social Sciences (SPSS) software was used to apply the Analysis of Variance (ANOVA) to the observed values of the activities (Inc., Chicago, USA). To identify significant differences (p ≤ .05), Duncan’s multiple range test was employed. All analyses were conducted in triplicate to ensure statistical robustness.
Results and discussion
Protein solubility of fermented black mash bean flour
The concentrations of soluble protein of fermented and unfermented flour samples are presented in . The RMBF demonstrated highest protein solubility (2313.51 µg/mL) compared to GMBF and fermented flour samples. The reduced protein solubility observed in fermented samples compared to raw legume flour might be credited due to numerous interrelated determinants associated with the fermentation process. During fermentation, the action of microorganisms induces protein denaturation, leading to alterations in protein structure. The formation of complex molecules and shifts in pH during fermentation may contribute to proteins adopting less soluble configurations.[Citation25] Furthermore, structural alterations in proteins, including alterations in their composition of amino acids or the generation of cross-links, may decrease their water solubility substantially.[Citation25] These combined effects demonstrated the complex influence of fermentation on the characteristics of proteins. However, RMBF Lactobacillus E14 and RMBF S. cerevisiae MK-157, GMBF Lactobacillus E14 and GMBF S. cerevisiae MK-157 demonstrated higher levels of protein solubility compared to unfermented GMBF. It was likely that the denaturation and leaching of soluble protein in hot water during the gelatinization process was the cause of GMBF lower protein solubility value (513.52 µg/mL). Generally, heating is responsible for aggregation of the unfolded protein molecules, which results in loss of solubility. Moreover, thermal denaturation involves an initial stepwise dissociation of subunits and a subsequent re-association of only partially unfolded molecules with the formation of either soluble or insoluble complexes.[Citation25] Additionally, S. cerevisiae fermentation of gelatinized flour demonstrated higher protein solubility, or 758.50 µg/mL, which can be related to a number of characteristics associated with S. cerevisiae activities. In addition proteins may be broken down into smaller peptides and amino acids by the enzymatic activity of S. cerevisiae, especially proteases, which might cause the noticeable rise in concentrations of proteins.[Citation26] Similarly, RMBF fermented by Lactobacillus E14 depicted increased protein solubility due to the greater duration of the fermentation process (80 h) which facilitated more hydrolysis of protein into lower molecular weight proteins and peptides, and degradation of starch molecules which reduced the their interaction with the proteins.[Citation18] Therefore, the solvating effect of protein improved subsequent to fermentation of RMBF and GMBF with Lactobacillus E14 and S. cerevisiae MK-157, respectively. Moreover, less incubation time (44 h) for GMBF-Lactobacillus E14 resulted lower value of protein solubility compared to other fermented flour samples. Similar observations were reported by Li and Wang[Citation18] for chickpea flour fermented by Bacillus subtilis lwo.
Table 1. Effect of gelatinization and fermentation of black mash bean flour fermented via Saccharomyces cerevisiae MK-157 and Lactobacillus E14 on protein solubility, in vitro protein digestibility and proteases activity.
Protease activity and in vitro digestibility of fermented mash bean flour
The values of In-vitro protein digestibility (IVPD) and protease activity of fermented and unfermented flour samples are depicted in . The observations revealed that the increased protease activity of fermented sample correspondingly resulted in the enhanced level of IVPD. Highest proteases activity (5546.78 U/g and 5521.45 U/g) and IVPD (90.12% and 87.54%) was observed for GMBF fermented by S. cerevisiae MK-157 followed by RMBF fermented by Lactobacillus E14, respectively. In addition the protease activity increased remarkably with highest increase in fermentation time (80 h). In line with earlier findings, the protease generated during lactic acid bacteria and S. cerevisiae fermentation was found to facilitate the breakdown of complex proteins into simpler, soluble proteins.[Citation27] This observation aligns with the observed alterations in soluble proteins of fermented RMBF and fermented GMBF throughout the fermentation process and was also confirmed form the results of SDS PAGE (). The peptides and free amino acids generated through protease activity have the potential to enhance the functional properties of mash bean.[Citation28] The IVPD is a crucial nutritional assessment parameter for dietary protein. The IVPD of raw and gelatinized flour fermented with S. cerevisiae MK-157 and Lactobacillus E14 showed positive correlation with the observed values of protease activity. Besides, enhanced digestibility of fermented samples was presumably occurred because of the increased protein dissolvability. The observations from depicted that the increment in protein digestibility was directly proportional to protein solubility. During in vitro digestion, the soluble protein of mash bean flour was further hydrolyzed by digestive enzymes. Simultaneously digestive enzymes degraded the residual macromolecular protein into shorter peptides and free amino acids which ultimately resulted in increased values of IVPD of fermented legume flour.[Citation29] The RMBF demonstrated negligible protease activity and IVPD (68.11%). In contrast GMBF showed greater values of protease activity (1010.87 U/g) and IVPD (76.56%). The anti-nutritional factors of RMBF were responsible for binding proteins which exert the inhibitory role on proteases and reduced IVPD.[Citation27] Gelatinizing mash beans activates endogenous proteases, and the heat treatment involved in the gelatinization process directly reduces anti-nutrients.[Citation2] This dual effect enhanced the protein digestibility of GMBF fermented by S. cerevisiae MK-157 (90.12%). Furthermore, within the context of fermentation for microbial enzyme production, the degradation of cell-wall constituents and concurrent reduction of anti-nutritional factors occur. This process results in the liberation of proteins from polyphenolic complexes, thereby enhancing their accessibility to proteolytic enzymes.[Citation27] Therefore, the combined effect of the endogenous proteases triggered during gelatinization and microbial peptidases and proteases led to the increased values of protein solubility, protease activity and IVPD of fermented GMBF.[Citation2] However, GMBF fermented with Lactobacillus E14 showed lower value of IVPD (81.67%) compared to other fermented samples. This could be possible due to less incubation time compared to other samples which resulted in lower production of bacterial digestive enzymes.[Citation11] Previous study showed the similar observations of increased solubility and digestibility of raw and gelatinized legume flour proteins fermented through lactic acid bacteria.[Citation2]
Mineral composition and bioavailability of fermented flour
Several essential functions crucial for maintaining strong bones structure and facilitating nerve impulses are carried out by minerals, contributing to a healthy and prolonged lifespan. Various macro minerals, including calcium (Ca), magnesium (Mg), potassium (K), and sodium (Na), as well as micro minerals like iron (Fe), zinc and copper (Cu), were examined in the extracts of samples and reported in . Fermentation increased significantly (p < .05) the mineral content of flour samples. In addition, compared to unfermented GMBF the Ca, Mg, K, Na, Fe, Zn and Cu of GMBF fermented by S. cerevisiae MK-157 were increased by 27.97%, 5.54%, 2.40%,2.40%, 224.5%, 95.83%,5.61%, and 40%, respectively. RMBF with Lactobacillus E14 depicted the 2nd highest increase in the values of minerals content among the fermented flour samples. The lowest increase was observed for GMBF with Lactobacillus E14. The mineral content of all the fermented flour samples was found to be higher than unfermented GMBF. Similarly increased contents of macro and microminerals were reported by Jan et al. (2022)[Citation30] in finger millet fermented by yeast. The increased mineral concentrations observed in fermented flour samples were attributed to the action of microbial enzymes, which facilitated the release of minerals from chelated complex compounds i.e. phytic acid.[Citation4] Another reason of declined values of mineral content of unfermented GMBF was because of the discharging of minerals into water during gelatinization.[Citation2] Although, the unfermented RMBF demonstrated greater values of mineral contents then fermented samples. The lower micronutrient content of fermented flour compared to unfermented RMBF could be attributed to processes such as leaching and lixiviation of minerals, influenced by microbial activity during fermentation. Enzymatic breakdown of complex compounds and the utilization of micronutrients by microorganisms contribute to these changes. Additionally, the duration of the fermentation process plays a crucial role in determining the extent of these alterations in micronutrient content.[Citation31] The diminished value of Zn was also documented by Mbaeyi-Nwoha and Obetta[Citation32] for fermented pigeon pea flour, in contrast to unfermented flour. The mineral substances in food doesn’t address the aggregate sum of minerals accessible and consumed by the human body, because only a particular amount is bioavailable. Mineral bioavailability is characterized as the extent to of the ingested mineral is retained and used in different physiological activities of the body.[Citation33] The In vitro bioavailability of Ca, Fe, and Zn of all the fermented flour samples was significantly (p ≤ .05) higher than unfermented flour (). The highest increase in mineral concentration was observed for flour samples fermented for 80 h regardless of the kind of microbial culture. The bioavailability of minerals plays a crucial role in their efficient extraction from the food system. Moreover, bioavalibility is highly impacted by the target mineral content in food sources as well as the rate at which gastrointestinal cells digest and absorb them.[Citation31] The declined bioavailability of Ca, Fe, and Zn of RMBF and GMBF was might be due to the antinutrients i.e., phytates and polyphenols which binds with the minerals and ultimately reduced their absorption.[Citation34] Furthermore, fermentation diminished the linkage of these antinutrients with minerals and resulted in their increased digestibility. In addition, fermentation process leads to the production of organ acids which are responsible for the breakdown of coordination complex between antinutries and nutrients.[Citation35] These observation of increased mineral bioaccessibility of fermented GMBF and fermented RMBF can be linked with the increased phytases activity of raw and gelatinized mash bean flour fermented by S. cerevisiae MK-157 and Lactobacillus E14.[Citation4] Similarly Ray and Didier[Citation36] found that lactic acid fermentation contributed to decreased levels of tannin which potentially resulted in increased iron absorption.
Table 2. Effect of gelatinization and fermentation of black mash bean flour fermented via Saccharomyces cerevisiae MK-157 and Lactobacillus E14 on mineral composition and in vitro mineral bioavailability.
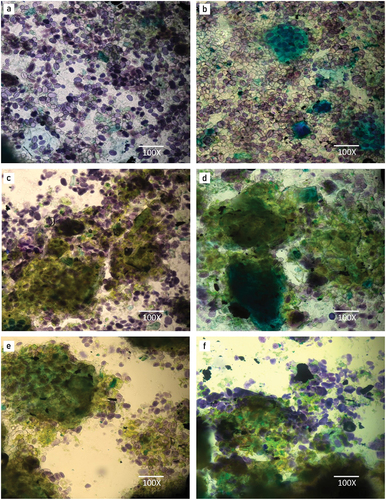
Bright field microscopy of fermented black mash bean flour
The bright field microstructures of control (RMBF and GMBF), RMBF-Lactobacillus E14, GMBF-Lactobacillus E14, raw and gelatinized flour fermented by S. cerevisiae MK-157 are presented in . Blue and light green colors of legume flour cells are indicated by the presence of starch and protein contents respectively. Starch granules appeared uniformly distributed in RMBF and GMBF. In addition, the milling process resulted in noticeable disruption of certain protein matrix fragments in both raw and gelatinized flour samples, as depicted in Fig. 4a,b. Micrograph of GMBF showed partly gelatinized starch granules in . He gelatinization process, known to modify both amylose and amylopectin, led to the increased proportion of available starch relative to total starch.[Citation37] Remarkably, the integrity of the starch granules was maintained regardless of the high-temperature gelatinization treatment (). According to [Citation38] this preservation was made possible by the encapsulation of proteins and starch granules within seed shells, which somewhat prevented cross-linkages with other molecules. Furthermore, the phases of pre- and post-treatment gelatinization resulted in a notable destabilization and eventual elimination of protein body residues (). Large protein aggregates surrounded by starch granules are visible for fermented flour samples (). In addition the fermentation of mash bean flour depicted higher green areas and less blue areas, indicating more protein content and lower starch molecules (). This fact is explicable, taking into account that fermentation increases the production, liberation, and hydrolysis of protein into their simpler units of amino acids.[Citation10] Therefore, more interaction of amino acids/protein bodies with the staining dye occurred in fermented flour samples. The starch granules appeared more dispersed within the protein phase (). Contrary, to the unfermented flour samples the continuous phase i.e., starch granules clumped together and became more concentrated into large areas, and protein fragments (discontinuous phase) are smaller and fewer in numbers (). The fermentation of starch granules of RMBF and GMBF was observed to be a very heterogeneous process (). The granules were selectively degraded by microorganisms, with a few intact granules observed even after 80 h of fermentation. Similar observations were reported by Sozer et al.[Citation39] when faba bean flour was fermented by Lactobacillus plantarum.
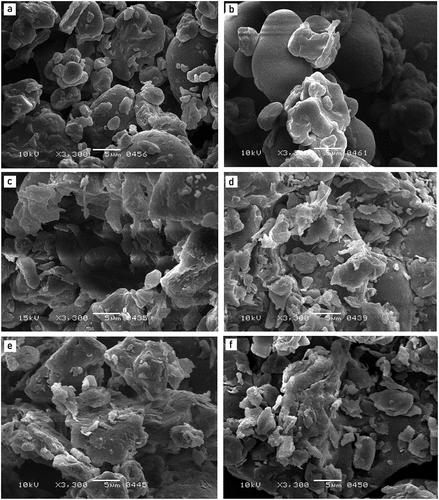
Scanning electron microscopy of fermented flour
Scanning electron micrographs show the microstructure of samples of raw, fermented, and gelatinized flour (). Starch granules and globular protein particles were evenly dispersed in raw flour; the milling process appeared to have disturbed the protein matrix (). Due to the alteration of amylose and amylopectin during the gelatinization process, partly gelatinized starch granules were visible in the micrograph of GMBF ().[Citation40] Proteins and starch were enclosed in seed shells, which significantly limited cross-linkages with other molecules, but this also conserved the integrity of the starch granules throughout the heat treatment.[Citation38] The microstructure of both RMBF and GMBF was dramatically changed by solid-state fermentation (). The structural integrity of starch granules and protein bodies in fermented samples was compromised, leading to the formation of amorphous flake-like structures. These characteristics were likely ascribed due to the pre-gelatinization blends of proteins and starch, followed by fermentation, bringing about irregular patterns of more small chains of amino acids and dextrins. Amylolytic and proteolytic compounds produced by S. cerevisiae and Lactobacillus during fermentation were responsible for the breakdown of the components of starch and protein into their smallest units.[Citation41] The fermentation time of more than 24 h, i.e., 80 h for RMBF fermented by Lactobacillus sp. E14 and S. cerevisiae MK-157, and 44 h for GMBF fermented by S. cerevisiae MK-157 caused the most extreme internal disintegration of molecules of starch and protein (). Consistent with the findings of this research, past studies have revealed that the fermentation of beans by Lactobacillus plantarum initiated the internal disintegration of starch particles and modified the protein network.[Citation41,Citation42]
SDS PAGE of protein extract of fermented black mash bean flour
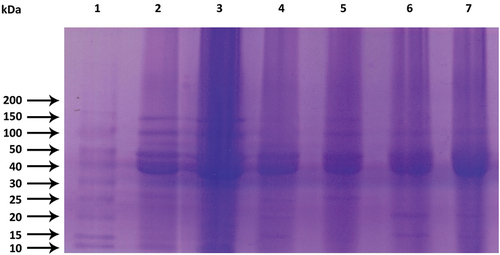
The molecular weight of protein subunits and alterations in mash bean protein induced by fermentation and gelatinization were evaluated using SDS-PAGE. Figure 4 illustrates that RMBF displayed discrete protein bands ranging from 10 to 150 kDa (Lane 3), while GMBF exhibited intense bands at 30 to 150 kDa. Gelatinization led to the loss of soluble protein which is evident from the faint bands at 10 to 25 kDa (Lane 2). RMBF fermented by Lactobacillus E14 and S. cerevisiae MK-157 depicted predominant proteins with molecular weights between 30 kDa and 50 kDa, followed by proteins between 15 kDa and 20 kDa (Lane 6 and 7). Microbial protease activity in fermented samples hydrolyzed higher molecular weight proteins into smaller fragments.[Citation43] Furthermore, SDS-PAGE indicated protein hydrolysis during fermentation, suggesting the presence of short-chain peptides in fermented flour. The intensity of protein bands in RMBF samples was higher than in corresponding fermented samples due to proteolysis during fermentation, releasing peptides with lower molecular masses than native proteins. Fermentation also led to significant increases in free amino acid contents not detectable by SDS-PAGE.[Citation44] GMBF fermented by Lactobacillus E14 showed minor changes in the electrophoretic pattern due to a shorter incubation time compared to other fermented samples (Lane 5), aligning with observations reported by Liu et al.[Citation45] for Lactobacillus fermentation of chickpeas. Additionally, fermentation of hydrolyzates did not alter the molecular weight distribution, possibly because Lactobacillus E14 was unable to metabolize large polypeptides.[Citation46] However, proteins with lower molecular weights below 25 kDa disappeared, indicating the increased degree of hydrolysis caused by the metabolic process of bacteria.[Citation44] The protein pattern in mash bean fermentation indicated reduced concentrations of all protein subunits with progressing fermentation, yet major subunits of molecular weights 30 to 50 kDa remained intact even after 80 h. This preservation might be attributed to the increased availability of concentrated vicilin globulin protein (50 kDa, 32 kDa) with intense bands (30 to 50 kDa), as visualized in . These observations suggested that vicilin degradation was not a rapid process even after 80 h of fermentation.[Citation18] In contrast, extensive degradation and breakdown of mash bean storage proteins: legumin (65 kDa), and convicilin (72 kDa) – occurred in fermented RMBF and GMBF fermented by S. cerevisiae MK-157 (Lane 4) (Ma et al., 2018).[Citation40] In alignment with earlier research, our results demonstrated that the fermentation process led to the proteolysis of higher molecular weight mash bean proteins into comparatively smaller molecular weight proteins or peptides.[Citation45,Citation46]
Figure 3. Electrophoretic analysis of extracted protein samples: Lane 1: Marker, Lane 2: Gelatinized mash bean flour (GMBF), Lane 3: Raw mash bean flour (RMBF), Lane 4: GMBF fermented by S. cerevisae MK-157, Lane 5: GMBF fermented by Lactobacillus sp. E14, Lane 6: RMBF fermented by S. cerevisae MK-157, Lane 7: RMBF fermented by Lactobacillus sp. E14.
Conclusion
In the present research, gelatinization and the combination of bacterial cultures namely, Saccharomyces cerevisiae MK-157 and Lactobacillus E14 were used to improve the nutritional value of black mash bean flour. Through meticulous assessment, it was revealed that the process substantially improved the nutritional profile of the beans. Notably, fermentation, facilitated by strains such as S. cerevisiae and Lactobacillus spp., induced protein denaturation and subsequent enzymatic breakdown, leading to increased solubility and digestibility of proteins. Additionally, gelatinization and fermentation enhanced mineral bioavailability by releasing minerals from chelated complexes and reducing anti-nutritional factors.The black mash bean flour which received bioprocessing showed potential as a value-added component for the production of functional food items. Its bio-functional qualities make it an appealing option for use in gluten-free formulations as well as the nutritional enhancement of extruded and baked goods. Studies on humans might be incorporated into further studies to obtain more profound understanding of the physiological impacts of fermented black mash bean flour consumption on mineral bioavailability and protein digestion.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia, for funding this work through the project number [RSPD2024R589].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wani, I. A.; Sogi, D. S.; Gill, B. S. Physicochemical and Functional Properties of Flours from Three Black Gram (Phaseolus Mungo L.) Cultivars. Int. J. Food Sci. & Tech. 2013, 48(4), 771–777. DOI: 10.1111/ijfs.12025
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C. G. Nutritional and Functional Effects of the Lactic Acid Bacteria Fermentation on Gelatinized Legume Flours. Int. J. Food Microbiol. 2020, 316, 108426. DOI: 10.1016/j.ijfoodmicro.2019.108426
- Gómez, M.; Martínez, M. M. Changing Flour Functionality Through Physical Treatments for the Production of Gluten-Free Baking Goods. J. Cereal Sci. 2016, 67, 68–74. DOI: 10.1016/j.jcs.2015.07.009
- Ali, S. A.; Saeed, S. M. G.; Ejaz, U.; Baloch, M. N.; Sohail, M. A Novel Approach to Improve the Nutritional Value of Black Gram (Vigna Mungo L.) by the Combined Effect of Pre-Gelatinization and Fermentation by Lactobacillus sp. E14 and Saccharomyces cerevisiae MK-157: Impact on Morphological, Thermal, and Chemical Structural Properties. LWT. 2022, 172, 114216.
- Ali, S. A.; Saeed, S. M. G.; Sohail, M.; Elkhadragy, M. F.; Yehia, H. M.; Giuffrè, A. M. Functionalization of Pre-Gelatinized Urad Bean Fermented by Saccharomyces cerevisiae MK-157 As a Fat Replacer and Its Impact on Physico-Chemical, Micromorphology, Nutritional and Sensory Characteristics of Biscuits. Arabian J. Chem. 2023, 16(9), 105029. DOI: 10.1016/j.arabjc.2023.105029
- Senanayake, D.; Torley, P. J.; Chandrapala, J.; Terefe, N. S. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation. 2023, 9(7), 635. DOI: 10.3390/fermentation9070635
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T., et al. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods. 2020, 10(1), 69.
- Kouamé, C.; Loiseau, G.; Grabulos, J.; Boulanger, R.; Mestres, C. Development of a Model for the Alcoholic Fermentation of Cocoa Beans by a Saccharomyces cerevisiae Strain. Int. J. Food Microbiol. 2021, 337, 108917. DOI: 10.1016/j.ijfoodmicro.2020.108917
- Manfredini, P. G.; Cavanhi, V. A. F.; Costa, J. A. V.; Colla, L. M. Bioactive Peptides and Proteases: Characteristics, Applications and the Simultaneous Production in Solid-State Fermentation. Biocatal. Biotransform. 2021, 39(5), 360–377. DOI: 10.1080/10242422.2020.1849151
- Alrosan, M.; Tan, T.-C.; Mat Easa, A.; Gammoh, S.; Alu’datt, M. H.; Stankovic, M. Effects of Fermentation on the Quality, Structure, and Nonnutritive Contents of Lentil (Lens Culinaris) Proteins. J. Food Qual. 2021, 2021, 1–7. DOI: 10.1155/2021/5556450
- Kumitch, H. M.; Stone, A.; Nosworthy, M. G.; Nickerson, M. T.; House, J. D.; Korber, D. R.; Tanaka, T. Effect of Fermentation Time on the Nutritional Properties of Pea Protein‐Enriched Flour Fermented by Aspergillus Oryzae and Aspergillus Niger. Cereal. Chem. 2020, 97(1), 104–113. DOI: 10.1002/cche.10234
- Wu, H.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Mung Bean (Vigna Radiata) as Probiotic Food Through Fermentation with Lactobacillus Plantarum B1-6. LWT Food Sci. Technol. 2015, 63(1), 445–451. DOI: 10.1016/j.lwt.2015.03.011
- Licandro, H.; Ho, P. H.; Nguyen, T. K. C.; Petchkongkaew, A.; Van Nguyen, H.; Chu-Ky, S.; Nguyen, T. V. A.; Lorn, D.; Waché, Y. How Fermentation by Lactic Acid Bacteria Can Address Safety Issues in Legumes Food Products? Food Cont. 2020, 110, 106957. DOI: 10.1016/j.foodcont.2019.106957
- Lazo‐Vélez, M.; Serna‐Saldívar, S.; Rosales‐Medina, M.; Tinoco‐Alvear, M.; Briones‐García, M. Application of Saccharomyces cerevisiae Var. Boulardii in Food Processing: A Review. J. Appl. Microbiol. 2018, 125(4), 943–951. DOI: 10.1111/jam.14037
- Girish, T.; Pratape, V.; Rao, U. P. Nutrient Distribution, Phenolic Acid Composition, Antioxidant and Alpha-Glucosidase Inhibitory Potentials of Black Gram (Vigna Mungo L.) and Its Milled By-Products. Food Res. Int. 2012, 46(1), 370–377. DOI: 10.1016/j.foodres.2011.12.026
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11(1), 43–51. DOI: 10.1007/s11483-015-9411-6
- He, F. Laemmli-sds-page. Bio-protocol. 2011, 1(11), e80–e80. DOI: 10.21769/BioProtoc.80
- Li, W.; Wang, T. Effect of Solid-State Fermentation with Bacillus subtilis Lwo on the Proteolysis and the Antioxidative Properties of Chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. DOI: 10.1016/j.ijfoodmicro.2020.108988
- Almeida, C. C.; Monteiro, M. L. G.; da Costa-Lima, B. R. C.; Alvares, T. S.; Conte-Junior, C. A. In vitro Digestibility of Commercial Whey Protein Supplements. LWT Food Sci. Technol. 2015, 61(1), 7–11. DOI: 10.1016/j.lwt.2014.11.038
- AOAC. Official Methods of Analysis of AOAC International, 16th ed; The Association: Gaithersburg, MD, 2012.
- Dhull, S. B.; Punia, S.; Kumar, R.; Kumar, M.; Nain, K. B.; Jangra, K.; Chudamani, C. Solid State Fermentation of Fenugreek (Trigonella Foenum-Graecum): Implications on Bioactive Compounds, Mineral Content and in vitro Bioavailability. J. Food Sci. Technol. 2021, 58(5), 1927–1936. DOI: 10.1007/s13197-020-04704-y
- Sadh, P. K.; Chawla, P.; Bhandari, L.; Kaushik, R.; Duhan, J. S. In vitro Assessment of Bio-Augmented Minerals from Peanut Oil Cakes Fermented by Aspergillus Oryzae Through Caco-2 Cells. J. Food Sci. Technol. 2017, 54(11), 3640–3649. DOI: 10.1007/s13197-017-2825-z
- Torres, M. D.; Moreira, R.; Chenlo, F.; Morel, M. H.; Barron, C. Physicochemical and Structural Properties of Starch Isolated from Fresh and Dried Chestnuts and Chestnut Flour. Food Technol. Biotechnol. 2014, 52(1), 135–139.
- Saeed, S. M. G.; Ali, S. A.; Ali, R.; Sayeed, S. A.; Mobin, L.; Ahmed, R. Exploring the Potential of Black Gram (Vigna Mungo) Flour as a Fat Replacer in Biscuits with Improved Physicochemical, Microstructure, Phytochemicals, Nutritional and Sensory Attributes. SN Appl. Sci. 2020, 2(12), 1–17. DOI: 10.1007/s42452-020-03797-6
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation. 2022, 8(6), 244. DOI: 10.3390/fermentation8060244
- Sirisena, S.; Chan, S.; Roberts, N.; Dal Maso, S.; Gras, S. L.; Martin, G. J. Influence of Yeast Growth Conditions and Proteolytic Enzymes on the Amino Acid Profiles of Yeast Hydrolysates: Implications for Taste and Nutrition. Food Chem. 2024, 437, 137906. DOI: 10.1016/j.foodchem.2023.137906
- Liu, Y.; Danial, M.; Liu, L.; Sadiq, F. A.; Wei, X.; Zhang, G. Effects of Co-Fermentation of Lactiplantibacillus plantarum and Saccharomyces cerevisiae on Digestive and Quality Properties of Steamed Bread. Foods. 2023, 12(18), 3333. DOI: 10.3390/foods12183333
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung Bean Proteins and Peptides: Nutritional, Functional and Bioactive Properties. Food Nutr. Res. 2018, 62. DOI: 10.29219/fnr.v62.1290
- Çabuk, B.; Nosworthy, M. G.; Stone, A. K.; Korber, D. R.; Tanaka, T.; House, J. D.; Nickerson, M. T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56(2), 257. DOI: 10.17113/ftb.56.02.18.5450
- Jan, S.; Kumar, K.; Yadav, A. N.; Ahmed, N.; Thakur, P.; Chauhan, D.; Hyder Rizvi, Q. U. E.; Dhaliwal, H. S. Effect of Diverse Fermentation Treatments on Nutritional Composition, Bioactive Components, and Anti-Nutritional Factors of Finger Millet (Eleusine Coracana L.). J. App. Biol. Biotech. 2022, 10(1), 46–52. DOI: 10.7324/JABB.2022.10s107
- Kumari, M.; Platel, K. Impact of Soaking, Germination, Fermentation, and Thermal Processing on the Bioaccessibility of Trace Minerals from Food Grains. J. Food Process. Preserv. 2020, 44(10), e14752. DOI: 10.1111/jfpp.14752
- Mbaeyi Nwaoha, I.; Obetta, F. Production and Evaluation of Nutrient-Dense Complementary Food from Millet (Pennisetum Glaucum), Pigeon Pea (Cajanus Cajan) and Seedless Breadfruit (Artocarpus Altillis) Leaf Powder Blends. Afr. J. Food Sci. 2016, 10(9), 143–156. DOI: 10.5897/AJFS2015.1393
- Dhull, S. B.; Punia, S.; Kidwai, M. K.; Kaur, M.; Chawla, P.; Purewal, S. S.; Sangwan, M.; Palthania, S. Solid-State Fermentation of Lentil (Lens Culinaris L.) with Aspergillus Awamori: Effect on Phenolic Compounds, Mineral Content, and Their Bioavailability. Legume Sci. 2020, 2(3), e37. DOI: 10.1002/leg3.37
- Kiewlicz, J.; Rybicka, I. Minerals and Their Bioavailability in Relation to Dietary Fiber, Phytates and Tannins from Gluten and Gluten-Free Flakes. Food Chem. Feb 1, 2020, 305, 125452. DOI: 10.1016/j.foodchem.2019.125452
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M. E.; Grauwet, T. Barriers Impairing Mineral Bioaccessibility and Bioavailability in Plant-Based Foods and the Perspectives for Food Processing. Crit. Rev. Food Sci. Nutr. 2020, 60(5), 826–843. DOI: 10.1080/10408398.2018.1552243
- Ray, R. C.; Didier, M. Microorganisms and Fermentation of Traditional Foods; CRC Press, 2014. DOI: 10.1201/b17307
- Zhang, H.; Wang, R.; Chen, Z.; Zhong, Q. Enzymatically Modified Starch with Low Digestibility Produced from Amylopectin by Sequential Amylosucrase and Pullulanase Treatments. Food Hydrocolloids. 2019, 95, 195–202. DOI: 10.1016/j.foodhyd.2019.04.036
- Ma, Z.; Boye, J. I.; Hu, X. Nutritional Quality and Techno-Functional Changes in Raw, Germinated and Fermented Yellow Field Pea (Pisum Sativum L.) Upon Pasteurization. LWT. 2018, 92, 147–154. DOI: 10.1016/j.lwt.2018.02.018
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C. G.; Flander, L.; Poutanen, K. Lactic Acid Fermentation as a Pre-Treatment Process for Faba Bean Flour and Its Effect on Textural, Structural and Nutritional Properties of Protein-Enriched Gluten-Free Faba Bean Breads. Foods. 2019, 8(10), 431. DOI: 10.3390/foods8100431
- Awolu, O. O.; Ojewumi, M. E.; Isa, J.; Ojo, D. O.; Olofin, H. I.; Jegede, S. O.; Yildiz, F. Comparative Analyses of Functional, Pasting and Morphological Characteristics of Native and Modified Tigernut Starches with Their Blends. Cogent. Food Agric. 2017, 3(1), 1306934. DOI: 10.1080/23311932.2017.1306934
- Yakubu, C. M.; Sharma, R.; Sharma, S. Fermentation of Locust Bean (Parkia Biglobosa): Modulation in the Anti‐Nutrient Composition, Bioactive Profile, in vitro Nutrient Digestibility, Functional and Morphological Characteristics. Int. J. Food Sci. & Tech. 2022, 57(2), 753–762. DOI: 10.1111/ijfs.15288
- Toor, B. S.; Kaur, A.; Kaur, J. Fermentation of Legumes with Rhizopus Oligosporus: Effect on Physicochemical, Functional and Microstructural Properties. Int. J. Food Sci. & Tech. 2022, 57(3), 1763–1772. DOI: 10.1111/ijfs.15552
- Christensen, L. F.; García-Béjar, B.; Bang-Berthelsen, C. H.; Hansen, E. B. Extracellular Microbial Proteases with Specificity for Plant Proteins in Food Fermentation. Int. J. Food Microbiol. 2022, 381, 109889. DOI: 10.1016/j.ijfoodmicro.2022.109889
- Rizzello, C. G.; Verni, M.; Koivula, H.; Montemurro, M.; Seppa, L.; Kemell, M., et al. Influence of Fermented Faba Bean Flour on the Nutritional, Technological and Sensory Quality of Fortified Pasta. Food Funct. 2017, 8(2), 860–871.
- Liu, Y.; Zhu, S.; Li, Y.; Sun, F.; Huang, D.; Chen, X. Alternations in the Multilevel Structures of Chickpea Protein During Fermentation and Their Relationship with Digestibility. Food Res. Int. 2023, 165, 112453. DOI: 10.1016/j.foodres.2022.112453
- García Arteaga, V.; Demand, V.; Kern, K.; Strube, A.; Szardenings, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic Hydrolysis and Fermentation of Pea Protein Isolate and Its Effects on Antigenic Proteins, Functional Properties, and Sensory Profile. Foods. 2022, 11(1), 118. DOI: 10.3390/foods11010118