ABSTRACT
Recently, the demands of biofuels have increased, because of their significant role in reducing various pollutants created by fossil fuels. Here, we have collected 25 samples containing various thermotolerant microorganisms from the nine natural fermented sources of Bangladesh, such as Boiled potato (Bp), Decomposed foods (Df), Municipal liquid waste (Mlw), Municipal solid waste (Msw), Sugarcane juice (Sc), Pantavat (Pv), Sugar molasses (Sm), Tari (Tari) and Watermelon juice (Wm) for bioethanol production. Among them, 18 isolates are capable of producing bioethanol. Cultural, morphological, physiological, biochemical and genetic analyses were carried out under various physiological conditions. Ethanol fermentation was checked by different carbon sources, temperatures and pH. All of the isolates could grow well in the medium containing Dextrose and Arabinose and only two strains Pv-1 and Bp-2 could ferment Xylose as a sole carbon source. At 42 °C, the highest ethanol concentration 6.58% (v/v) was obtained by a strain Wm-1 isolated from Watermelon juice. At 37 °C, maximal ethanol concentrations of 6.74% (v/v), 6.50% (v/v) and 6.22% (v/v) were obtained by the strains Bp-2, Wm-l and Pv-1, respectively. Among the various pH tested, the highest ethanol concentration 6.6% (v/v) was obtained at pH 4.5 by a strain named Tari-2. Finally, yeast 26S rDNA sequencing information identified the strains Sc-2 as Saccharomyces cerevisiae Pv-2, Tari-2 and Df-1 as Pichia kudriavzevii, Mlw-l and Bp-2 as Candida tropicalis, Pv-1 as Pichia guilliermondii and Df-2 as Candida rugosa.
Introduction
Ethanol is an important industrial chemical with emerging potential as a biofuel to replace vanishing fossil fuels [Citation1]. The production of pure ethanol apparently began in the twelfth to fourteenth century along with improvements in the art of distillation, permitting the condensation of vapours of liquids boiling at lower temperatures. During the middle ages, alcohol was not only used for the production or as a constituent of medical drugs, but also for the manufacture of painting pigments and other chemical industries, and it was only in the nineteenth century that this trade became an industry with enormous production, due to vast improvements of the distilling process which emerged with modern biotechnology [Citation2].
In general, yeast strains are able to grow and efficiently ferment ethanol at pH values of 3.5–6.0 and temperatures of 28—30 °C, with efficiency dropping rapidly at higher temperatures. However, many thermotolerant yeasts ferment ethanol at higher temperatures ranging from 37 to 50 °C [Citation1]. There are several potential benefits of thermotolerant yeast for using in the production of industrial alcohol: thermotolerant yeast exhibits rapid metabolic activity and a high fermentation rate with high product output. The solubility of oxygen and other gases in the fermentation broth decreases with increasing temperature. This phenomenon supports the establishment and long-term maintenance of anaerobic conditions, which is required for the optimum activity of yeast. The viscosity of the fermentation broth decreases with increasing temperature. Therefore, the energy required to maintain proper agitation of the growth media is reduced. The metabolic activity of microbes and frictional effects of agitation serves to generate large amounts of heat. Thus, additional energy to maintain the vessels at the desired temperature, as well as the cooling requirements after sterilization is reduced. The chances of contamination are also minimized [Citation2].
The thermotolerant organisms could promote high yield of bioethanol at high temperature [Citation3]. In this study, our main target is to identify some thermotolerant microbes from the natural fermented products of Bangladesh for bioethanol production. Samples were screened under various physiological conditions. We have found several yeast strains capable of fermenting sugars at high temperatures and producing bioethanol.
Materials and methods
Sample collection
Nine different fermented sample sources were used for sample collection [Citation4]. They were collected from various natural fermented sources and locations in Bangladesh, where temperatures fluctuate between 35 and 40 ºC during summer. Selected sample sources were: Tari (overnight natural fermented palm juice/sap collected from the xylem tissue of palm tree, Phoenyx sylvestries, at around 35–40 ºC), Pantavat (Pv, an overnight natural fermented rice in tap water at around 35–38 ºC), Boiled potato (Bp, Boiled potato was exposed in open air where various starch-containing garbage are available), Watermelon juice (Wm, from street vendors), Sugarcane juice (Sc, from street vendors), Sugar molasses (Sm, from sugar mill), Decomposed food materials (Df, from food markets), Municipal solid waste (Msw) and Municipal liquid waste (Mlw). All of the samples were inoculated into Yeast-Peptone-Dextrose (YPD) agar plates and incubated at 37 ºC for two days. Yeast-like colonies were isolated for further study.
Isolation of thermotolerant microorganisms
Thermotolerant microorganisms were isolated by an enrichment technique in a media containing Sugarcane juice (8% total sugars), 0.05% (NH4)2SO4 and 4% (v/v) ethanol and with pH 4.5. Medium pH was adjusted by 1 N HCl. After inoculation, cultures were incubated for three days in a rotary shaker at a predetermined temperature with shaking speed of 160 rpm. Enriched cultures were then streaked on YPD agar plates containing the same medium and incubated at 30, 37, 40, 42, 45 and 50 ºC. Purified yeast cultures were kept on YPD agar plates and slants and then stored at 4 and −20 ºC, respectively. Thermotolerant yeast strains were screened based on their growth performances at the above-mentioned temperatures in YPD agar plates.
Screening of thermotolerant microorganisms under various physiological conditions
Thermotolerance of the isolates was determined by growing them on temperatures ranging from 30 to 50 °C. Microscopic studies were used to identify strain morphologically, after growing them under various physiological conditions. Furthermore, sequencing of yeast 26S rDNA (D1/D2 region) was used for further identification/screening of microorganisms, according to the method developed by Kurtzman and Fell [Citation5].
Morphological and physiological characterizations of isolated yeasts were carried out in medium containing various carbon sources and at various temperatures and pH. Three different carbon sources, YPD/Yeast–Peptone–Xylose (YPX)/Yeast–Peptone–Arabinose (YPA) medium (10 g/L yeast extract and 20 g/L peptone) were prepared and supplemented with 20 g/L of Dextrose/Xylose/Arabinose, which were designated as YPD, YPX and YPA, respectively. At first, one loop-full yeast colony was inoculated from a fresh YPD plate into test tube containing 3 mL of YPD broth and incubated at 30 ˚C for 24 h in a shaking water bath (incubator). Then yeast cells were streaked separately on the YPD, YPX and YPA agar plates and incubated at 30 ˚C for 48 h.
The cell morphology of thermotolerant yeast and their appearance on solid medium (YPD agar plate) were examined, after incubating at 37 °C for two days. The appearance of the cultures (texture, colour and surface of colonies) was recorded. Cells from a young actively growing culture were inoculated into test tube containing 5 mL of YPD broth, incubated at 37 °C for four days. The culture was examined to determine the growth of thermotolerant yeast visually on the surface of YPD liquid medium.
In order to identify the optimum growth temperature and pH, YPD agar plates were streaked with thermotolerant yeasts from actively growing culture and incubated overnight at various temperatures (30, 37, 40, 42, 45, 48 and 50 ºC) and pH (4.5, 5.0, 5.5 and 6.0). YPD broth was prepared in several conical flasks and pH was adjusted by concentrated sulphuric acid (H2SO4) or 2 N NaOH, where necessary. For the measurement of growth curve, 25 μL (200-fold dilution) from a young actively growing culture was inoculated into test tubes containing 5 mL of YPD or YPX broth and then incubated at various temperatures (25–45 ˚C) for at least 96 h [Citation6]. During incubation, cell suspension was collected in every 4 h interval, the optical density was measured in a spectrophotometer (Specord UV/Visible Spectrophotometer, Analytic Jena, Germany) at 600 nm against the corresponding broth medium as blank.
Fluorescent microscopic observation
For microscopic imaging, cells from liquid culture were harvested by mild centrifugation (5000 rpm for 5 min). In brief, cells were washed, fixed and stained with 5 μg/mL DAPI (4′,6-diamidino-2-phenylindole dihydrochloride). The fixed cells (5 μL) were dropped into the well of a 10-well multi-test microscope slide (76 × 26 mm with 24 × 60 mm coverslip; Matsunami glass Ind., Ltd., Japan) and air dried at 27 °C. Immunofluorescent microscope was used for characterization of thermotolerant yeasts morphologically, specially shape, size and visualization of nucleoid area of cell [Citation7].
Selection of thermotolerant microorganisms for ethanol production
Bioethanol production was conducted independently under various medium temperatures, pH and carbon sources as described in the results section below. Inoculums were prepared by transferring one loop full of 24 h culture grown on a plate of YPD agar to an Erlenmeyer flask containing 50 mL of a sugar cane juice medium as described earlier. The inoculums were transferred at the rate of 0.5% to the screening medium, followed by incubation on a rotary shaker at various temperatures ranging from 30 to 42 ºC in 250 mL Erlenmeyer flasks containing 100 mL of a basal Sugarcane juice medium composed of Sugarcane juice supplemented with Glucose up to 18% total sugars and 0.05% (NH4)2SO4.
Ethanol fermentation at various temperatures and pH
One loop-full of 24 h fresh culture grown on a plate of YPD agar was transferred to a test tube containing 3 mL YPD broth and incubated overnight. A conical flask containing 25 mL of basal media was inoculated with 125 μL (200 fold dilution) inoculums and incubated at various temperatures ranging from 30 to 42 ºC for 72 h, or different pH (4, 4.5, 5.5 and 6.0, adjusted by 1 N HCl) and incubated overnight. Basal media was composed of sugar (Glucose) up to 18% total sugars, water and 0.05% (NH4)2SO4. The yeast count was determined by using Neubauer counting chamber (Japan). The standard concentration of the inoculums (108 cells/mL) was prepared by the appropriate dilution with sterile distilled water.
Estimation of bioethanol
Ethanol produced in the fermentation medium was estimated by potassium dichromate oxidation method [Citation8]. Potassium dichromate (33.882 g/L), ferrous ammonium sulphate (135.5 g/L) and diphenylamine (0.5 g/100 mL concentrated H2SO4) solutions were used as reagents. The fermented sample was centrifuged at 3000 rpm for 10 min. After centrifugation, the supernatant was collected and the pellet was discarded. Then the supernatant was diluted 10 times with distilled water and was shaken well by vortex mixer. Ten millilitre of the diluted sample was distilled against K2Cr2O7 (10 mL) containing concentrated H2SO4 (5–6 mL). Then distilled product was titrated against freshly prepared ferrous ammonium sulphate solution with diphenylamine as an indicator. Appearance of green colour indicated the end point of the titration. Burette reading (amount of ferrous ammonium sulphate) was recorded to calculate the amount (in percentage) of ethanol present in the sample.
Genomic DNA extraction and 26S rDNA sequencing
The growth medium and culture conditions were same as described earlier. In brief, genomic DNA extraction, purification, polymerase chain reaction (PCR) and DNA sequencing reaction were carried out as follows: cells were washed once with distilled water and re-suspended in 2 mL of distilled water. One millilitre of cell suspension was collected in the 1.5 mL micro-centrifuge tube. After centrifugation, excess water was removed, and the cells were stored in a freezer (−20 ºC) until use. Genomic DNA was separated and purified by using DNA extraction kit (Takara, Japan). The sequencing of the D1/D2 domain of the yeast 26S rDNA was carried out from PCR products of genomic DNA fragment that were extracted from yeast cells. The D1/D2 domain of the rDNA was amplified by PCR with forward primer NL-1 and NL-4 forward primer NL-1: 5’-GCATATCAATAAGCGGAGGAAAAG-3’. Reverse primer NL-4: 5’-GGTCCGTGTTTCAAGACGG-3’ [Citation9]. The PCR product was checked by Agarose gel electrophoresis, purified using the ABI Big Dye terminator Cyclo sequencing Kit Version 3.1 (Applied Biosystems, California, USA) with the external primer IL-1 and IL-4 [Citation5]. The sequences were determined with an ABI PRISON BIO genetic Analyzer (Applied Biosystems) according to the instructions of the manufacturer. The sequence compared pair-wise using the basic local alignment search tool (BLAST) homology search.
Results and discussion
Collection of thermotolerant microorganisms from the natural fermented sources of Bangladesh
Thermotolerant microorganisms were collected from nine different natural fermented sources of Bangladesh. Our collected sample sources were from traditional fermented products with a possibility to identify some rare microorganisms capable of producing bioethanol under given conditions. Those fermented samples have various amounts of carbohydrate, lipids, sugars and vitamins [Citation10,Citation11]. A total of 25 yeast-like colonies were isolated independently from the 25 samples in YPD agar plate. Among them, three were isolated from Tari (Tari-1, Tari-2 and Tari-3); two were isolated from Pantavat (Pv-1 and Pv-2); four from Sugarcane juice (Sc-1, Sc-2, Sc-3 and Sc-4); two were isolated from Watermelon juice (Wm-l and Wm-s); two from Boiled potato (Bp-1 and Bp-2); four were from Sugar molasses (Sm-1, Sm-2, Sm-3 and Sm-4); two were isolated from Decomposed food materials (Df-1 and Df-2); two from Municipal solid waste (Msw-1 and Msw-2); four were isolated from Municipal liquid waste (Mlw-1, Mlw-2, Mlw-3 and Mlw-4). Detailed descriptions of the samples are shown in
Table 1. Thermotolerant microorganisms collected from the various natural sources of Bangladesh.
Screening of thermotolerant microorganisms
To understand the thermotolerant nature, the isolates were grown on temperatures ranging from 30 to 50 °C on YPD agar plates. All of the 25 isolates were able to grow on 37 and 42 °C (). Most of the strains were also able to grow from 45–50 °C. Six isolates (Tari-1, Bp-1, Sm-1, Sm-2, Sm-3 and Sm-4) were able to produce colonies even at 50 °C. They were initially identified as fungus while the rest of the isolates were presumably yeasts. Among them, two strains (Tari-2 and Bp-2), collected from Tari and Boiled potato, were promising thermotolerant microorganisms. They can grow at high temperatures (45, 48 and 50 ºC). The colonies were rough, irregular, elevated and with pink or deep brown colour.
Thermotolerant yeast cell morphology and their appearance on YPD agar medium were examined as shown in . Cells from a young actively growing culture were checked for their abilities to ferment Dextrose. Out of 25 isolates, 18 isolates could produce gas (CO2) and ethanol ((d) for four representative isolates). shows growth of these 18 thermotolerant yeasts at 37, 42 and 45 ºC on YPD agar plates. From this figure, it was clear that many of these 18 ethanol-producing isolates showed good growth at higher temperature.
Figure 1. Screening of thermotolerant yeast collected from various natural fermented product of Bangladesh. Growth of thermotolerant yeast was observed on YPD agar plates and YPD broth. (a) Mother YPD plate having Pv-1, (b) four thermotolerant yeasts isolated from Wm-l, Tari-2, Sc-4 and Pv-1, (c) colony morphology of Wm-1. Colonies were large, round, cream coloured, smooth with elevated surfaces, and (d) the growth of yeast cells in YPD broth. The leftmost tube represents control (without sample). After incubation, the YPD broth became turbid and formed gas (CO2) represented by a foamy white layer on the top of the tubes.
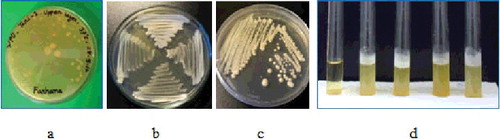
Figure 2. Growth of thermotolerant yeasts under various growth temperatures in YPD solid medium. Here, three panels of images – upper, middle and bottom – represent that yeast strains were grown at 37, 42 and 45 ºC, respectively, for two days. Various microorganisms were isolated from the natural fermented sources of Bangladesh like Tari (T), Boiled potato (Bp), Decomposed food (Df), Sugarcane juice (Sc), Watermelon juice (Wm), Pantavat (Pv), Municipal solid waste (Msw), Municipal liquid waste (Mlw), etc.
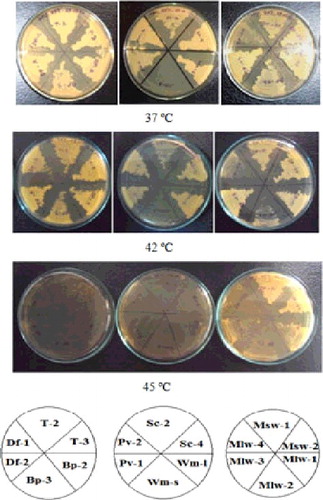
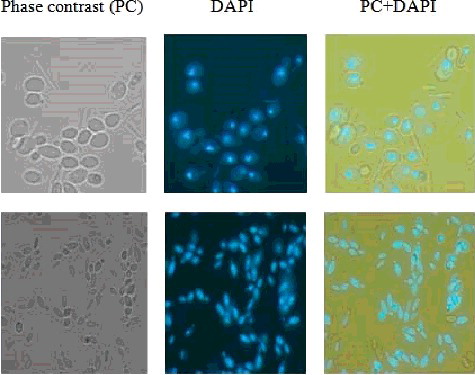
Isolated samples were grown in various physiological conditions of growth medium containing various carbon sources, different temperatures and pH. Among the various strains analysed here, immunofluorescent microscopic view of Pv-1 and Df-1 are shown in . This result concludes that our isolates having various yeast strains. Based on initial results, eight isolates were selected for further analysis. They are: Tari-2 from Tari; Sc-2 from Sugarcane juice; Pv-1 and Pv-2 from Pantavat; Wm-l from Watermelon juice; Bp-2 from Boiled potato; Df-1 and Df-2 from Decomposed food.
Figure 3. Fluorescent microscopic image of thermotolerant yeasts isolated from the natural sources of Bangladesh. Top and bottom three images represent strain Pv-1 and Df-1, respectively. Left, middle and right panel represent phase contrast, DAPI and combined images of PC + DAPI, respectively. Procedure for cell culture preparation and fixation for microscopic study is shown detail in the ‘Materials and methods’ section.
Physiological characterization of thermotolerant yeasts
Eight thermotolerant microbes were characterized under various carbon sources, pH and temperatures. When grown at 37 ºC, all strains could grow well in the medium supplemented by Glucose. However, when we added Xylose instead of Glucose, only strain Pv-1 isolated from Pantavat (overnight natural fermented rice with tape water at around 35–37 ºC) could grow well, as shown in Supplementary Figure 1. This result suggested that the strain isolated from Pantavat is one of the potential candidates for bioethanol production, which can utilize Xylose as a sole carbon source [Citation12]. Furthermore, the effect of medium temperature was monitored in temperatures ranging from 30 to 42 ºC. Three carbon sources (YPD, YPX and YPA) were applied in solid agar plates. Comparative study among the result of various temperatures sensitivity is shown in . This result again concludes that all yeast strains could grow well at 37–42 ºC in YPD solid medium. However, in YPX medium no growth was seen except Pv-1 and Bp-2 strain, as mentioned before.
Table 2. Effect of various temperatures and carbon sources on growth of thermotolerant yeasts isolated from the various fermented sources of Bangladesh.
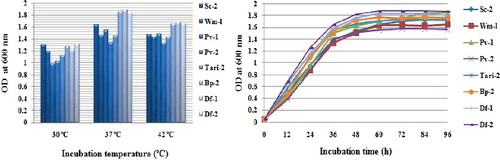
The growth of yeast cells was examined in YPD liquid medium under three different temperatures for three days (). The highest and lowest growth rates observed were at 37 and 30 ˚C, respectively, under the conditions tested. The highest and lowest growths were observed in Df-1 and Pv-2 strains, respectively at 37 ºC (, left panel). A typical growth curve of eight thermotolerant yeast cells is shown in (right panel). Among the eight yeast cells that were grown here, the highest and the lowest cell growths were observed from strain Df-2 and Pv-2, respectively. A little variation was seen in the case of Df-1 and Df-2 strains.
Figure 4. Effect of temperatures on yeast cell growth in YPD medium. Here, Y-axis represents OD (optical density) and X-axis represents three different temperatures. After 72 h incubation, growth was measured by spectrophotometer at 600 nm. The optimum growth rate was found at 37 ºC. Growth curve of various yeast samples isolated from the natural fermented sources of Bangladesh. Yeast samples were grown to YPD broth at 37 °C from 0 to 96 h. Samples were collected in various indicated time point and seize the growth and then their growth were measured by spectrophotometer (Specord UV/Visible Spectrophotometer, Analytic Jena, Germany) at 600 nm against the YPD broth as blank.
The optimum temperature, which gave the highest cell dry weight, was at 37 ºC. At temperature higher than 42 ºC, the growth of yeast was decreased. The concentrations of bioethanol at various temperatures are shown in . The maximum and the minimum bioethanol content were measured from the strain Bp-2 and Sc-2, respectively, at 37 and 30 ºC. Almost similar amount of bioethanol was estimated from the strains of Wm-l, Pv-1, Pv-2 and Df-2 in all tested temperatures. The result concludes that our isolated strains have wide ranges of bioethanol production abilities.
Figure 5. Effect of medium temperatures and pH on bioethanol production. Various cells were grown in a basal medium composed of total sugar (18%), H2O and 0.05% (NH4)2SO4 at various medium temperatures and pH for 72 h. The highest amount of bioethanol was produced by Wm-l and Bp-2 at 42 and 37 °C, respectively. The overall bioethanol production rate was good even at 42 °C. The highest amount of bioethanol was also produced at pH 4.5 by Tari-2 strain.
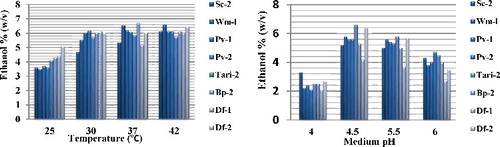
Comparative study was performed among the eight bioethanol producing strains under identical conditions, as shown in (right panel). Cell growth rate increased along with the pH increase from 4.0 to above. The pH of the medium had no different effect on the growth rate at pH 4.5–6.0, but beyond that, the growth rates were decreased. However, medium pH had an important impact on bioethanol production. Maximum and minimum bioethanol productions were observed in medium pH 4.5 and 4.0, respectively. Different samples have different rates of bioethanol production. Tari-2 (6.6% v/v) produced maximum bioethanol at pH 4.5 (). Among various physiological conditions tested, Tari-2 strain showed highest bioethanol productivity in presence of Xylose as a carbon source. Xylose is a five-carbon sugar capable of being converted to Glucose easily by an inexpensive enzymatic reaction. Several researchers have identified Xylose fermenting microorganisms under various conditions [Citation13]. This strain has a broad substrate spectrum, thermotolerance and high growth, as recently reported by Laluce et al. [Citation14].
In this study, ethanol fermentation was observed at temperature higher than optimum temperature, because yeast currently used for industrial fermentation is rapidly inactivated at 33–35 ºC [Citation15]. Significant cooling costs would be eliminated, especially during the summer or in tropical countries, with fermenting temperatures of 40 ºC and above. Only a few screening surveys have been carried out for the ability of yeasts to grow in a flask at or above 40 ºC [Citation16,Citation17]. Our studied samples were collected in summer where temperatures fluctuated from 35 to 40 ºC (). Therefore, we assumed to find out some natural thermotolerant microbes used for high temperature bioethanol production. Although temperature dependent variations of yeast cell growth were not consistent always (), maximum cell growth and bioethical concentration was found at 37 ºC with some exceptions (). Similar thermotolerant nature of yeast was also observed previously [Citation18], indicating that the thermotolerant mechanisms can be stable and it depends on cell physiology and genome stability under high temperature fermentation conditions.
26S rDNA sequence analysis
Finally, eight thermotolerant yeast strains Sc-2, Wm-1, Pv-1, Pv-2, Tari-2, Bp-2, Df-1 and Df-2 were identified using yeast 26S rDNA sequencing. Yeast D1/D2 region of each was partially sequenced, each representing about 500 base pairs. The results are shown in . Out of eight strains, four belonged to the genus Pichia and two were identified as Candida tropicalis. Single strains of Saccharomyces cerevisiae and Candida rugosa were also identified. S. cerevisiae (Sc-2) is well known for its biotechnological applications as well as availability of genetic tools for its modifications [Citation19]. C. tropicalis, although an occasional human pathogen, has also been used as industrial scale for the production of ethanol and long chain dicarboxylic acids [Citation20,Citation21]. C. rugosa is well known for its biotechnological application in the production of lipases [Citation22]. Pichia guilliermondii is a widely distributed natural strain for alcohol production and also isolated from clinical specimens [Citation23,Citation24]. Its ability to utilize Xylose has been reported earlier [Citation25]. Finally, Pichia kudriavzevii (Tari-2) is a non-pathogenic yeast found in decomposed fruit sources [Citation26]. Its ability to ferment Xylose and availability of its genome sequence make it a suitable candidate for bioethanol production.
Table 3. 26S rDNA sequencing information of thermotolerant microorganisms.
Conclusions
After screening of thermotolerant microorganisms, we concluded that the isolate Tari-2 (P. kudriavzevii) is a potential thermotolerant yeast strain that produced higher amount of bioethanol. Therefore, further research is required to establish suitable conditions and various parameters for optimum growth for production of bioethanol in laboratory, as well as in an industrial scale. Besides, molecular biology approaches can help finding some interesting isolates containing specific genes of interest. The genes of interest may be cloned and sequenced to enhance gross productions of desired products.
tbeq_a_1228477_sm9526.docx
Download MS Word (32.2 KB)Acknowledgment
We thank Sukanya Nitiyon for discussion and help for DNA sequencing. Authors also wish to thank Dr L. Noppon for the critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alfenore S , Molina-Jouve C , Guillouet SE , et al. Improving ethanol production and viability of Saccharomyces cerevisiae vitamin feeding strategy during fed-batch process. Appl Microbiol Biotechnol. 2002;60:67–72.
- Roehr M . The biotechnology of ethanol. Weinheim : Wiley-VCH Verlag GmbH & Co. KGaA; 2001.
- Murata M , Rodrussamec N , Suprayogi NY , et al. High-temperature ethanol fermentation with thermotolerant microbes. Proceedings of the 5th Bangladesh-Japan Joint International Conference; 2010 Dec 26–28; Dhaka, Bangladesh . p. 199–205.
- Talukder AA , Sujon SI , Hossain MM , et al. Production of bioethanol at high temperature from Tari. Adv Microbiol. 2015;5:325–335.
- Kurtzman CP , Fell JW . The yeasts, a taxonomic study. 4th ed. Amsterdam: Elsevier Publication; 1998.
- Sree NK , Sridhar M , Rao LV , et al. Ethanol production in solid substrate fermentation using thermotolerant yeast. Process Biochem. 1999;34:115–119.
- Talukder AA , Hiraga S , Ishihama A . Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells. 2000;5:613–626.
- Caputi A Jr , Ueda M , Brown T . Spectrophotometric determination of ethanol in wine. Am J Enol Viticult. 1968;19:160–165.
- O'Donnell K. Fusarium and it's near relatives. In: Reynolds DR , Taylor JW , editors. The fungal Holomarph: mitotic, meiotic and pleomorphic speciation in fungal systematics. Wallingford : CAB International; 1993. p. 225–233.
- Murata M , Nitiyon S , Lertwattanasakul N , et al. High-temperature fermentation technology for low-cost bioethanol. J Japan Inst Energy. 2015;94:1154–1162.
- Matsushita K , Azuma Y , Kosaka T , et al. Genomic analyses of thermotolerant microorganisms used for high-temperature fermentations. Biosci Biotech Biochem. 2016;80:655–658.
- Rodrussamec N , Lertwattanesakul N , Hirata K , et al. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus . Appl Microbiol Biotechnol. 2011;90:1573–1586.
- Yuangsaard N , Yongmanitchai W , Yamada M , et al. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie Van Leeuwenhoek. 2013;103:577–588.
- Laluce C , Bertolini MC , Hernandes J , et al. Screening survey for yeasts that ferment sucrose at relatively high temperature. Ann Microbiol. 1987;37:151–159.
- Anderson PJ , McNeil K , Watson K . High-efficiency carbohydrate fermentation to ethanol at the temperature above 40 °C by Kluyveromyces ma rxianus var. Marxianus isolated from sugar mills. Appl Environ Microbiol. 1986;51:1314–1320.
- Hacking AJ , Taylor IWF , Hanas CM . Selection of yeast able to produce ethanol from glucose at 40 ˚C. Appl Microbiol Biotechnol. 1984;19:361–363.
- Abdel-Banat BM , Hoshida H , Ano A . et al. High-temperature fermentation: how can process for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl Microbiol Biotechnol. 2010;85:861–867.
- Li H , Wu M , Xu L , et al. Evaluation of industrial Saccharomyces cerevisiae strains as the chassis cell for second-generation bioethanol production. Microbiol Biotechnol. 2015;8:266–274.
- Jeffries TW . Conversion of xylose to ethanol under aerobic conditions by Candida tropicalis . Biotechnol Lett. 1981;3:213–218.
- Picataggio S , Rohrer T , Deanda K , et al. Metabolic engineering of Candida tropicalis for the production of long-chain dicarboxylic acids. Biotechnology. 1992;10:894–898.
- Benjamin S , Pandey A . Candida rugosa lipases: molecular biology and versatility in biotechnology. Yeast. 1998;14:1069–1087.
- Leathers TD , Dien BS . Xylitol production from corn fibre hydrolysates by a two-stage fermentation process. Process Biochem. 2000;35:765–776.
- San Milian RM , Wu LC , Salkin IF , et al. Clinical isolates of Candida guilliermondii include Candida fermentati . Int J Syst Bacteriol. 1997;47:385–393.
- Zou YZ , Qi K , Chen X , et al. Favorable effect of very low initial K(L)a value on xylitol production from xylose by a self-isolated strain of Pichia guilliermondii . J Biosci Bioeng. 2010;109:149–152.
- Meroth CB , Hammes WP , Hertel C . Identification and population dynamics of yeasts in sourdough fermentation processes by PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2003;69:7453–7461.
- Chan GF , Gan HM , Ling HL , et al. Genome sequence of Pichia Kudriavzevii M12, a potential producer of bioethanol and phytase. Eukarot Cell. 2012;11:1300–1301.
