 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Plant growth-promoting rhizobacteria (PGPR) enhance plant growth under the influence of multigenic processes, including nitrate () and ammonium (
) uptake genes, which could potentially explain the improvement in plant nutrition and plant growth promotion. Studies on the effects of PGPR inoculation on regulation of
and
plant uptake genes and nutrient accumulation using soil or soil-like substrates are limited. Here, we tested the hypothesis that the application of PGPR Bacillus mixtures increases overall plant growth, nutrient uptake and the transcript levels of nitrate and ammonium uptake genes in Arabidopsis thaliana. All three PGPR mixtures tested in this study significantly increased plant shoot fresh weight, root fresh, chlorophyll content, nutrient uptake and plant diameter. The transcript levels of five nitrate and four ammonium uptake genes were significantly higher in PGPR-treated plants compared to untreated plants. These results demonstrate that plant growth promotion and enhanced nutrient uptake by select PGPR mixtures.
Introduction
Microbial associations with roots growing in soil are complex and can enhance the capacity of plants to acquire nutrients from soil through a number of mechanisms. Nitrogen (N) availability represents a limiting factor in crop production. Low N use efficiency promotes excessive N chemical fertilization, which increases production costs (Kaiser et al. Citation2002) and can negatively impact the environment. In this respect, plant growth-promoting rhizobacteria (PGPR) are of particular importance and have been widely studied as biofertilizers to enhance plant growth and yield. The biofertilizer effects of specific PGPR result from several mechanisms including production of volatile compounds (Zhang et al. Citation2007), increased mineral and nitrogen availability in the soil (Lin et al. Citation1983), and production of plant growth regulators (indole-3-acetic acid [IAA], gibberellins, and cytokinins) which directly increase plant growth by increasing root surface area and number of root hairs (Bottini et al. Citation2004; Khalid et al. Citation2004; Liu et al. Citation2017; Ryu et al. Citation2005b; Tsavkelova et al. Citation2006).
Application of many PGPR inoculants have resulted in enhanced nutrient uptake in various crops, including cotton and pea (Egamberdiyeva and Hoflich Citation2004), tomato (Kirankumar et al. Citation2008), maize (Biari et al. Citation2008; Lin et al. Citation2018) and peanut (Dey et al. Citation2004). Some PGPR-mediated increases in nutrient uptake are related to increases in root surface area and root morphology; as a result there is an indirect enhancement of nutrient uptake by the plant (Bákonyi et al. Citation2009). Increased root development leads to increased root surface area that could improve plant nutrient uptake which can be a key factor for plant growth promotion. In addition, PGPR also can have more direct effects on the root’s nutrient transport systems. For example, Bertrand et al. (Citation2000b) showed that a strain of Achromobacter spp. enhanced the rate of nitrate () uptake of Brassica napus roots.
A first step in improving N use efficiency of plants could be to increase the primary acquisition process occurring at the root/soil interface. In A. thaliana, and
transporters are located mainly at the root plasma membrane and, therefore, are positioned to uptake N from the soil (Forde and Clarkson Citation1999a). Uptake mechanisms of N in roots are specific and rely on high-affinity and low-affinity transport systems (Touraine et al. Citation2001). The high – affinity transport systems (HATS) transport ions when the concentration in the medium is low, in the range of 10 µM. The low-affinity transport systems (LATS) operate only when this substrate is present in relatively high concentrations in the medium, in the range of mM (Mort-Gaudry Citation2001).
Nitrate transporters in A. thaliana have been classified as NRT1 transporters (also known as AtNRT1 in Arabidopsis), which include 53 members, and NRT2 transporters (also known as AtNRT2 in Arabidopsis), which include 7 members (Orsel et al. Citation2002). The relative contribution of these transporters to nitrate uptake depends on the developmental stage of the root and the N status of the plant (Wang et al. Citation2012). In Arabidopsis, the involvement in nitrate uptake has been extensively studied for two members of the AtNRT1 gene family (AtNRT1.1 and AtNRT1.2) and two members of the AtNRT2 gene family (AtNRT2.1 and AtNRT2.2) (Ho et al. Citation2009; Krouk et al. Citation2010b). Both of these gene families are expressed mainly in root tissues (Forde and Clarkson Citation1999a). Expression of NRT1.1, NRT1.2, NRT2.1, NRT2.2, and/or NRT2.4 is regulated at the transcriptional level by nitrate, nitrite, ammonium, glutamine, N starvation, light, sucrose, diurnal rhythm, and/or pH (Krouk et al. Citation2010a). A strong correlation between the transcript abundance of these nitrate transporter genes and nitrate uptake by the plant suggests that transcriptional regulation plays a key role in modulating nitrate uptake activities (Krouk et al. Citation2010b; Wang et al. Citation2012).
The uptake of ammonium () has been less intensively studied than uptake of
. Many plant species that normally use
also have an efficient system for absorbing
, identified as AMT (AtAMT in Arabidopsis), which is constitutively expressed at high
levels (Yuan et al. Citation2007b). There are uncertainties regarding the exact chemical species transported by AMT, which can be in the form of either hydrophobic NH3 or charged ammonium. The AMT1 family of high-affinity
transporters contains five members, of which AtAMT1.1, AtAMT1.2, and AtAMT1.3 have been studied in detail (Glass et al. Citation2002). The location in which the transporter genes are expressed in the plant seems to be dependent on the plant species and type of transporter. AtAMT1.2, AtAMT1.3 and AtAMT1.5 genes are expressed in roots, while only AtATM1.1 is expressed in root and leaf tissue (Forde and Clarkson Citation1999a; Glass et al. Citation2002; Khademi et al. Citation2004). Furthermore, the AtAMT transporters are localized in different types of root tissue. AtAMT1.1, AtAMT1.3, and AtAMT1.5 are localized in the plasma membrane of rhizodermis cells, while AtAMT1.2 is in the plasma membrane of endodermal and cortical cells (Ludewlg et al. Citation2007). These locations indicate a spatial arrangement of AMT1-type transporters that assures ammonium uptake for efficient radial transport across the root tissue via both symplastic and apoplastic routes (Yuan et al. Citation2007b).
Among all PGPR, species in the genus Bacillus have been the most often commercialized as biofertilizers and biological control agents in agriculture. An important characteristic of those bacteria is their ability to form thermo-stable and chemically-resistant endospores that allow them to survive for a long period of time. The underlying signaling mechanisms triggered in plants by this specific bacterial genus have not yet been fully identified. Currently, there is little information about the potential effect of Bacillus PGPR on and
plant uptake genes. The higher expression of
and
gene transporters are might be related to higher N-uptake and enhanced plant growth. Furthermore, few of the published studies have been performed in soil or soil-like substrate, which limits the information available to in vitro environments (López-Bucio et al. Citation2007; Ryu et al. Citation2005b; Saia et al. Citation2015) The main objective of the present study is to determine the effects of selected Bacillus PGPR mixtures on plant development and regulation of
and
uptake genes on Arabidopsis thaliana roots. In order to examine these effects, a soil-like system (in vivo), instead of an in vitro system, was used to grow A. thaliana plants. This method allowed conditions that mimic the relationship between PGPR and roots under agricultural conditions.
Materials and methods
Bacterial strains and microbial source preparation
Three different PGPR Bacillus mixtures (i.e. 3 PGPR treatments) were tested. Mixture one contained strains: INR-7 (Bacillus pumilus), IN937a (Bacillus subtilis), T4 (Bacillus safensis) and SE56 (Lysinibacillus xylanilyticus). Mixture two contained strains: INR-7 (B. pumilus), SE56 (L. xylanilyticus), SE76 (B. safensis), and FZB42 (Bacillus amyloliquefaciens). Mixture three contained strains: INR-7 (B. pumilus), SE56 (L. xylanilyticus), SE52 (B. safensis), and E681 (Paenibacillus peoriae). All of the bacterial strains were obtained from the PGPR culture collection at the Department of Entomology and Plant Pathology, Auburn University (Auburn, AL, USA). These strains have plant growth-promoting effects (Enebak et al. Citation1998; Jetiyanon et al. Citation2003; Kokalis-Burelle et al. Citation2003; Sato et al. Citation2000) and were previously selected for their potential to increase nitrogen uptake in cabbage and pepper (Calvo Citation2013). The bacterial mixtures were prepared by mixing equal volumes of spore suspensions of each strain to a final concentration of log 5 per ml.
Plant source, growth conditions, treatments, and experimental design
Seeds of wild-type A. thaliana (ecotype Columbia 0, seeds originally provided by ABRC, Ohio State University, OH, USA) were maintained at 4°C for at least 2 days before sowing. Seeds were then surface-sterilized by immersion in 70% ethanol (v/v) for 5 min, washed subsequently four times with sterile distilled water, immersed in 1 mL solution that contained 0.5 ml 0.1% Triton X-100 + 0.5 ml of calcium hypochlorite for 3 min, and subsequently washed four times with sterile distilled water. Approximately 100 seeds were sown in 4 separate plastic trays (22 cm (L) × 14 cm (W) × 5 cm (H)) containing 70 g of sieved Sunshine Professional Growing Mix # 8 (Sun Gro Horticulture Canada Ltd. http://www.sungro.com/professional-products?brandID=1&productID=83). The PGPR mixtures and a control with no PGPR (untreated) were compared for a total of four treatments. Before sowing seeds 175 ml of bacterial spore suspensions was applied to each tray. Enough additional water was applied to each tray to reach the water holding capacity (WHC) of the sunshine mix, and trays were covered with plastic wrap and placed into a growth chamber (Percival Scientific E-41L2) with a photoperiod of 16 h of light, day/night temperatures of 23°C/22°C, and an average of 100 µmol m−2 s−1 light intensity at the rosettes level. At 15 days after sowing (Vadassery et al. Citation2012), uniform seedlings were selected and transplanted to individual pots containing 70 g of sunshine mix plus 200 ml water (amount of water to reach WHC).
The experimental design was a complete randomized design (CRD) with 4 treatments (PGPR-mixture1, PGPR-mixture2, PGPR-mixture3, and untreated control) and 10 replicates per treatment. Pots were placed in a growth chamber with the same conditions described above. Three days after transplanting, a second bacteria inoculation of 3 ml containing log 7 spores per ml bacteria was applied to the base of the plant. Plants were watered every other day by weighing each pot and adding enough water to reach 270 g. Six-week old plants were used for the following experiments.
Evaluation of plants and nutrient uptake analysis
Plant growth was measured at 8, 12, 14, 16, and 19 days after transplanting (DAT), using digital photography and image processing via ImageJ (ImageJ 1.47v; http://rsbweb.nih.gov/ij/webcite) software to determine rossette areas. At 21 DAT, plants were harvested and evaluated. From the 10 replicates per treatment, five were used to evaluate plant diameter, chlorophyll content (SPAD 502 meter), number of leaves, fresh shoot weight (FSW), root fresh weight (RFW), root dry weight (RDW), and maximum root length. For nutrient content, shoot samples were freeze-dried and then total N determined using a Micro-Dumas combustion system (Borda and Hayward Citation1967), while P and K were determined using Inductively Coupled Atomic Emission Spectroscopy (ICP-AES) (Houk et al. Citation1980). Freeze dry weights were recorded and nutrient uptake was calculated using this freeze dry weights.
Total RNA isolation and cDNA synthesis
Plants from the above experimental setup were carefully uprooted 21 DAT. Roots were gently washed, immediately frozen in liquid nitrogen, and were stored at −80°C. A dome-shaped metal spatula was used to grind frozen samples to a fine powder in an Eppendorf tube containing liquid nitrogen. Total RNA was extracted from 50 mg root, and each sample was obtained using TRIzol® Plus Purelink RNA Mini Kit (Ambion® Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDrop™ Spectrophotometer ND-2000 (Thermo Scientific, Wilmington, USA). The absense of contaminant DNA in the RNA samples was verified by PCR, in which specific primers of a known gene and PCR master mix (Promega, Madison, WI, USA) were used for PCR reactions. PCR reactions were performed according to the manufacturer’s instruction under the following conditions: 95°C for 3 min, 40 cycles (95°C for 30 s, 57°C for30 s, and 72°C for 1 min), and 72°C for 5 min. PCR products were loaded onto 1% agarose gel, and the integrity of RNA was assessed by gel electrophoresis and ethidiumbromide staining. In all samples tested in this work, no fragments of genomic DNA were identified. First strand cDNA was synthesized from 500 ng RNA using a Goscrpit™ Reverse Ttranscription System Kit (Promega USA) according to the manufacturer’s instructions. The experiments repeated at least five times using five individual root system and at least five techincal replicates were tested with in each individual root system.
Real-time PCR
The transcript levels of genes involved in the uptake and transport of nitrate and ammonium in A. thaliana were measured by quantitative RT-PCR (). PCR was carried out on an ABI 7500 Real Time PCR System (Life Technologies, Carlsbad, CA, USA) with a 96 well rotor. The amplification reactions were performed with 25 µl of mixture consisting of 12.5 µl of PerfeCTA® SYBR® Green Fastmix® LowROX qPCR Master Mix (Quanta Biosciences, Inc, Gaithersburg, MD, USA), 0.5 µl of cDNA, and 100 nM primers (Integrated DNA Technologies, Coralville, IA, USA). The reactions were incubated at 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 57°C for 34 s, and a final cycle of 72°C for 34 s. PCR conditions were determined by comparing threshold values in a dilution series of the RT product, followed by non-template control for each primer pair. Four housekeeping genes: Cytoplasmic Glyceraldehyde-3-Phosphate Dehydrogenase (GAPC2), Actin (ACT1), Ubiquitin-specific protease 6 (UBP6), and 18S ribosomal RNA (18S rRNA) were used to normalize the results of the real-time PCR. The most stable housekeping gene was selected using the Normfinder software (Andersen et al. Citation2004). The most stable housekeeping gene was 18S (AT2G03810) and used to quantify the relative transcrpit levels of target genes (i.e. AtNRT1, AtNRT2 and AtAMT gene families). The sequencing of amplicons confirmed that single products corresponding to the contigs that were used for primer design. All genes were similar to known nucleotide sequences using BLAST with a score value higher than 100 and identity ranging from 90% to 97%.
Table 1. Summary of most important genes analyzed and involved in nitrate and ammonium uptake and transport as expressed in root tissue of A. thaliana.
Statistical analyses
Data on plant parameter and gene expresion response were analyzed by analysis of variance (ANOVA), using a general linear model (GLM). All statistical analyses were performed using SAS software version 9.2 (SAS Institute Citation2010) with a significance level of α = 0.05 set a priori. The least significant difference (LSD) test was used to identify significant differences of plant paramters between treatments, and Tukey’s test was used to analyze gene expression results.
Results
Effects of PGPR on growth and nutrient uptake of A. thaliana
Treatment with each of the three PGPR mixtures significantly increased the growth of A. thaliana at 21 DAT ( and ). On average, PGPR treatment increased fresh and dry shoot weight, root fresh weight, and dry root weight by 81%, 21%, 52%, and 100%, respectively. PGPR treatment also significantly increased SPAD meter measurements (). Application of PGPR mixtures enhanced plant growth throughout the growth cycle of A. thaliana (). Differences in the rosette area were observed as early as the 8th day after transplanting (8 DAT). Inoculated plants were bigger and had larger and greater numbers of leaves compared to the control plants. Rosette areas were significantly increased by the PGPR mixtures in all five stages evaluated (). The growth promotion elicited by the PGPR mixtures was maintained constantly in all stages until the last harvest at 21 DAT, when differences between the control and the PGPR treatments were greater than previous days. PGPR also increased the number of leaves and the total leaf area ().
Figure 1. Effects of PGPR mixtures on rosette areas during long-term growth of A. thaliana plants. (A) Representative photographs of A. thaliana rosettes of plants exposed to the different treatments (control, PGPR mixture 1, PGPR mixture 2, and PGPR mixture 3) at 8, 12, 14, 16, and 19 days after transplanting (DAT), bars correspond to 1 cm. (B) Graphic representation of rosette average of plants subjected to the different treatments at 8, 12, 14, 16, and 19 DAT. Media and SE were calculated with 10 plants per treatment. Asterisks indicate significant differences among the control treatment and the three other PGPR treatments at each time (One or two-way ANOVA, p < 0.05).
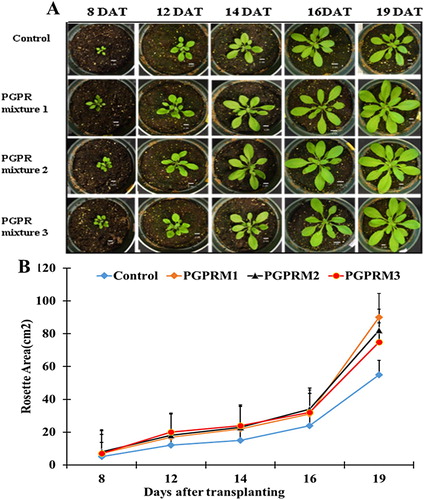
Table 2. Effect of PGPR mixtures on plant parameters: diameter, chlorophyll content (SPAD), fresh shoot weight (FSW), dry shoot weight (DSW), number of leaves, and total leaf area evaluated at 21 DAT.
Table 3. Effect of PGPR mixtures on root plant parameters: fresh root weight (FRW), dry root weight (DRW), and maximum root length at 21 DAT.
There was a significant increased on the percentage of total nitrogen by mixtures 1 and 2. PGPR showed a non-significant increased on total P and K (%) compare to the control. PGPR mixtures do not showed a significant effect on C/N ratio (). In the other hand uptake of N, P, and K by A. thaliana was significantly increased by the three PGPR mixtures. Nitrogen uptake was enhanced more by PGPR than was P and K ().
Figure 2. Effect of PGPR mixtures on A.thaliana shoot nutrient uptake. Total A. thaliana shoot uptake of nitrogen (N), phosphorus(P), and potassium (K) evaluated 21 DAT. Data are means ± SE. Columns within the same nutrient labeled with the same letter are not significantly different based on LSD test (α = 0.05).
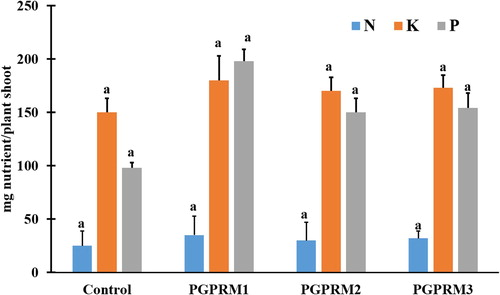
Table 4. Effect of PGPR mixtures on total nitrogen %, total Phosphorous % (calculated as PO4%), potassium % and Carbon to Nitrogen ratio (C/N) at 21 DAT.
PGPR mixtures regulates nitrate and ammonium uptake and transport genes
The expression of genes that regulate nitrate uptake and transport were generally induced by treatment of A. thaliana with PGPR mixtures. The transcript levels of nitrate uptake genes were significantly higher when plants were treated with PGPR mixtures. However, the expression level of AtNRT2.2 gene in PGPR-treated plants was not significantly different from untreated plants (). The expression of AtNRT2.1 and AtNRT1.1genes were higher in PGPR-treated plants. The transcript levels of AtNRT2.1 gene were significantly increased by 62, 45, and 29 fold in PGPR mixture 1, 2, and 3 treated plants respectively. Likewise, the transcript levels of AtNRT1.1 gene were increased by 39, 16, and 17 fold in A. thaliana plants treated with PGPR mixture 1, 2, and 3, respectively.
Figure 3. Quantitative real time PCR of selected nitrate uptake (AtNRT1.1, AtNRT2.1, AtNRT1.2, AtNRT2.2, and AtNRT2.3) and nitrate transport (AtNRT1.5) genes expressed in root tissue of A. thaliana after inoculation with three PGPR mixtures. Quantitative RT-PCR determinations of relative levels of gene expression in complete plants at 21 DAT. Data are means ± SE. Columns labeled with the same letter are not significantly different based on Tukey’s test (α = 0.05).
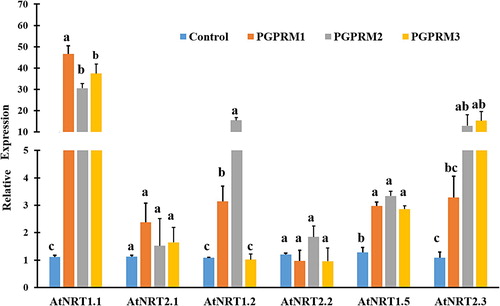
The expression level of the gene that regulates low-affinity bidirectional transport of nitrate from outer root tissue to xylem AtNRT1.5 was increased significantly in plants treated by all three PGPR mixtures. The overall gene expression pattern varied among the three PGPR mixtures. For instance, AtNRT1.1 gene was more highly expressed in plants treated with PGPR mixture 1 than in plants treated with mixtures 2 and 3. AtNRT1.2 gene was more highly expressed in plants treated with mixture 2 than in plants treated with mixtures 1 and 3. Furthermore, the expression level of AtNRT1.2 gene in plants treated with mixture 3 was not significantly different from that found in plants in other treatments or the control ().
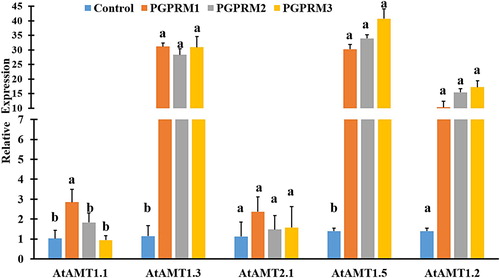
The transcript levels of ammonium uptake genes in all PGPR mixture-treated plants were significantly higher, except in AtAMT2.1 gene. AtAMT1.1 genes showed significantly enhance expression only in PGPR-M1 treated plants (). The transcript level of AtAM1.5 gene significantly increased by 35 and 42 fold in plants treated with PGPR-mixture 2 and 3, respectively. Plants treated with PGPR-mixture 2 and 3 showed very similar patterns of gene expression in all ammonium uptake genes (). When comparing nitrate and ammonium genes, PGPR mixtures were able to induce higher level expressions of nitrate uptake genes than ammonium uptake genes.
Figure 4. Quantitative real time PCR of selected ammonium uptake (AtAMT1.1, AtAMT1.2, AtAMT1.3, AtAMT1.5, and AtAMT2.1) genes expressed in root tissue of A. thaliana after inoculation with three PGPR mixtures. Quantitative RT-PCR determinations of relative levels of gene expression in complete plants at 21 DAT. Data are means ± SE. Columns labeled with the same letter are not significantly different based on Tukey’s test (α = 0.05).
Discussion
It was demonstrated that Bacillus PGPR mixtures do enhanced plant growth and nutrient uptake in A. thaliana. Similar results of plant growth promotion (total leaf area, plant weight, chlorophyll content, and root length) during early and late stages of A. thaliana development were previously reported using other PGPR species such as Burkholderia phytofirmans (Poupin et al. Citation2013), Phyllobacterium brassicacearum STM196 (Mantelin et al. Citation2006), and B. subtilis GB03 (Liu et al. Citation2017). Furthermore Ryu et al. (Citation2005b) reported that two of the strains tested in the present study (T4 and INR937a) increased foliar fresh weight and total leaf area of A. thaliana plants. The increased on nutrient uptake could be related the growth promotion activity. Jang et al. (Citation2018) hypothesized that the enhanced plant growth observed in all stages of PGPR-inoculated plants could partially be explained by improved availability and acquisition of nutrients. It was observed that PGPR treatment also significantly increased SPAD meter measurements, which are highly correlated to chlorophyll concentration (Liu et al. Citation2017).
Due to none of the Bacillus spp. strains tested in this experiment were positive for nitrogen fixation, it has been hypothesized that the mechanisms used by PGPR Bacillus species in order to increase nitrogen uptake and in general other nutrient uptakes could be related to hormone production, solubilization of soil nutrients and enhancement of root growth or root surface (Calvo Citation2013). However, lately other mechanisms has been proposed for other PGPR bacteria. Mantelin and Touraine reported that Phylobacterium spp. enhanced the nutrient uptake of A. thaliana. They found that this could be due to an enhanced in the synthesis of end products from the first intermediates of the N assimilatory pathway. Furthermore they also showed that inoculated plants alleviated the N-dependent regulation of root development which could indirectly affect nutrient uptake. In a separate study it has been proposed that microbial inoculation could impact soil mineralization process therefore affecting the direct availability of nutrient in soil solution which will potentially could be absorb by the plant (Brimecombe et al. Citation1999). Nevertheless it is important to take into account that the increased on nutrient uptake by PGPR inoculants could be due to a combination of mechanisms more than a result of a single one. Even though we found that several nitrate and ammonium uptake genes were upregulated by PGPR mixtures the increased on nutrient uptake may be not only due to this upregulation effect at the end it is very probable that different mechanisms are involve and the final effect is the results of a complex system. For instance, recent study by Gaudinier et al. (Citation2018) demonstrated that the transcriptional regulatory network and twenty-one transcription factors regulates the architecture of root and shoot systems in response to changes in nitrogen availability. PGPR increases nitrogen availability in the rhizosphere of tomato plants (Fan et al. Citation2017).
Results in this study have demonstrated that different PGPR strain mixtures can differentially affect genes involved in nitrate transport into the plant. (Forde and Clarkson Citation1999b) reported that these tested nitrate transport genes were highly expressed when A. thaliana plants were grown under optimal conditions. The strong correlation between the transcript abundance of these specific nitrate transporter genes and nitrate uptake activities suggests that transcriptional regulation plays a key role in modulating nitrate uptake activities (Krouk et al. Citation2010b; Wang et al. Citation2012; Gaudinier et al. Citation2018). In the present study, two of the genes reported by Forde and Clarkson (Citation1999b), AtNRT1.1 and AtNRT2.1, were highly expressed in plants treated with PGPR mixtures, indicating that the selected PGPR strains exerted a significant effect on two of the most important genes involved in nitrate uptake in A. thaliana roots.
Ammonium uptake transporters are all high-affinity transporters. Unlike nitrate uptake genes, they are expressed in different parts of the root tissue. Ammonium can enter the symplastic route for radial transport toward the root stele via regulation of AMT1.1, AMT1.3, and AMT1.5, which are localized at the plasma membrane of rhizodermis cells, including root hairs. Ammonium can also bypass outer root cells via the apoplastic transport route and subsequently enter the root symplast by AMT1.2-mediated transport across the plasma membrane of endodermal (in the root hair zone) and cortical (in more basal root zones) cells (Yuan et al. Citation2007a). In the current study, AMT1.1, AMT1.2, AMT1.3, and AMT1.5 genes showed significantly high transcript levels in A. thaliana plants treated with three different PGPR mixtures, which demonstrates that PGPR affect apoplastic and symplastic ammonium transport in different root tissues including root hair, endodermal, and cortical cells. It is important to note that the one ammonium uptake gene in which PGPR mixtures failed to show any effect (AtAMT2.1) is distantly related to the AtAMT1 family (Sohlenkamp et al. Citation2000). It has not been characterized as well as the AtAMT1 family and has a lower activity in root cortex and meristematic root tissue. It may express in leaves and other A. thaliana organelles. Because AtAMT2.1 is expressed in various tissues, it is likely that this transporter plays diverse roles in the plant (Sohlenkamp et al. Citation2000).
Only a few previous studies have focused on the impact of PGPR on the regulation of nutrient uptake in plants. Inoculation of canola with Achromobacter sp.strain U80417 resulted in an increase of NO3− and K+ net influx rates per root surface area unit (Bertrand et al. Citation2000a).
Mantelin et al. (Citation2006) reported that there was no effect on expression of nitrate and ammonium genes (AtNRT1.1, AtNRT1.2, AtNRT2.1, AtNRT2.2, AtNRT2.3, AtAMT1.1, AtAMT1.2, AtAMT1.3, AtAMT1.5, and AtAMT2.1) in A. thaliana roots inoculated with P. brassicacearum strain STM196. However, the transcript levels of nitrate transporter genes AtNRT2.5 and AtNRT2.6 in A.thaliana shoot increased by 20 and 25 fold, respectively, in P.brassicacearum inoculated plants. Notably, the genus Phyllobacteriumis a gram-negative bacterial genus, unlike Bacillus which is a gram-positive genus. Hence, the potential effect on specific nitrate or ammonium uptake genes may vary among genera of PGPR.
Effects of Bacillus spp. PGPR on nitrate and ammonium uptake genes have not been reported previously. Previous in vitro microarray studies by Lakshmanan et al. (Citation2013) showed that inoculation with B. subtilis FB17 resulted in upregulation of membrane transport genes in roots. They also reported upregulation levels as high as 104 fold in unknown protein genes when bacteria were applied in vitro to A. thaliana plants. These results support the idea that PGPR significantly affect plant gene expression and confirm that PGPR have an effect that is not limited to the production of metabolites that promote plant growth.
With only a few exceptions (Ryu et al. Citation2005a), most studies involving A. thaliana and PGPR until now were performed under in vitro conditions. In vitro studies using A. thaliana are important for genetic research; however, when performing in vitro PGPR experiments with A. thaliana, the interaction between PGPR and microorganisms in the plant rhizosphere is not taken into account, which could affect the gene expression results. Through the release of root exudates in the rhizosphere, plants can affect bacterial colonization and also bacterial gene expression, especially genes encoding plant-beneficial traits. Moreover, PGPR also interact with bacteria naturally found in soil outside the rhizosphere. Thus it is important to consider the complexity of the interaction of PGPR with their rhizosphere environment (Vacheron et al. Citation2013). For this reason, there may be a difference in results from PGPR applications in plants grown in vitro and plants grown in a soil-like substrate (in vivo). In support of this hypothesis, Ryu et al. (Citation2005a) observed that the majority of PGPR strains that were more effective at promoting A. thaliana growth in vitro were only moderately effective in vivo.
Here we have reported for the first time in vivo effects of PGPR mixtures that are related to more than one bacterial species in the regulation of plant growth and expression of genes that are involved in nitrate and ammonium uptake and transport. When PGPR mixtures are applied as inoculants their effect on plant growth is the result of the contribution of all active individual cells from each bacterial species (Vacheron et al. Citation2013). Furthermore, a synergistic effect between bacterial species in the same mixtures could be occurring, meaning that the performance level might be higher than if only one type of strain was involved (Bashan Citation1998). The results of this study also show that PGPR mixtures positively affect plant growth, plant development, and nutrient uptake. Plants inoculated with each of the three PGPR mixtures showed increases in plant parameters such as plant diameter, fresh shoot and root weights, dry weights, number of leaves, chlorophyll content, and N, P, K uptake. These increases in plant growth parameters by PGPR mixtures were associated with the expression of several genes involved in nitrate and ammonium uptake and transport in roots of A. thaliana. Moreover, to farther understand the role of PGPR in nitrogen uptake root architecture will be studied in follow up future studies. Overall, these findings contribute to a better understanding of plants and beneficial bacteria interactions and provide novel information about the effects of PGPR on plant development and nitrate uptake regulations.
Acknowledgements
We thank Mr John Mcinroy for assisting with PGPR preparations and Dr Michael Miller for assisting with qPCR analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Pamela Calvo
Dr. Pamela Calvo is working at the global seed treatment and product development manager at BASF chemical company. Her research interest focuses on PGPR research: biocontrol, nutrient uptake, nutrient management, greenhouse gases from agriculture, soil fertility, soil microbiology, rhizosphere ecology.
Simon Zebelo
Dr. Simon Zebelo is an Assistant Professor of Entomology at the University of Maryland Eastern Shore. Dr. Zebelo’s research interests include both the fundamental and applied aspects of insect behavior, chemical ecology, and integrated pest management.
David McNear
David McNear is an Associate Professor at the University of Kentucky. His research interest focuses on exploring the biogeochemical processes occurring at the soil-water-plant interface (a.k.a. the Rhizosphere) and how these processes influence the mobility and bioavailability of trace elements in natural systems.
Joseph Kloepper
Dr. Joseph Kloepper is a Professor at Auburn University. His research focus is on the use of plant growth-promoting rhizobacteria (PGPR) for promoting plant growth, plant health, and nutrient uptake.
Henry Fadamiro
Dr. Henry Fadamir is a Professor of entomology at Auburn University. Dr. Fadamiro’s research focuses on arthropod behavior, neurobiology, chemical and molecular ecology and integrated pest management.
References
- Abel S, Theologis A. 1996. Early genes and auxin action. Plant Physiol. 111:9–17. doi: 10.1104/pp.111.1.9
- Andersen CL, Jensen JL, Orntoft TF. 2004. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496
- Bákonyi GÉ, Lévai L, Veres S, Tóth B, Marozsán M. 2009. Comparison of effects of different biofertilisers on early development of cucumber and wheat seedlings. Ratarstvo. 44:491–495.
- Bashan Y. 1998. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv. 16:729–770. doi: 10.1016/S0734-9750(98)00003-2
- Bertrand H, Plassard C, Pinochet X, Touraine B, Normand P, Cleyet-Marel JC. 2000a. Stimulation of the ionic transport system in Brassica napus by a plant growth-promoting rhizobacterium (Achromobacter sp.). Can J Microbiol. 46:229–236. doi: 10.1139/w99-137
- Bertrand H, Plassard C, Pinochet X, Touraine B, Normand P, Cleyet-Marel JC. 2000b. Stimulation of the ionic transport system in Brassica napus by a plant growth-promoting rhizobacterium (Achromobacter sp.). Can J Microbiol. 46:229–236. doi: 10.1139/w99-137
- Bi Y-M, Wang R-L, Zhu T, Rothstein SJ. 2007. Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genomics. 8:281. doi: 10.1186/1471-2164-8-281
- Biari N, Gholami A, Rahmani A. 2008. Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth promoting rhizobacteria in arid region of lran. J Biol Sci. 8:1015–1020. doi: 10.3923/jbs.2008.1015.1020
- Borda P, Hayward LD. 1967. Nitrogen analysis of nitrate esters by micro-dumas combustion. Anal Chem. 39:548–549. doi: 10.1021/ac60248a029
- Bottini R, Cassan F, Piccoli P. 2004. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biot. 65:497–503. doi: 10.1007/s00253-004-1696-1
- Brimecombe MJ, De Leij FA, Lynch JM. 1999. Effect of introduced pseudomonas fluorescens strains on soil nematode and protozoan populations in the rhizosphere of wheat and pea. Microb Ecol. 38:387–397. doi: 10.1007/s002489901004
- Calvo P. 2013. Effect of microbial inoculation on Nitrogen plant uptake and Nitrogen losses from soil and plant-soil systems Auburn University.
- Dey R, Pal KK, Bhatt DM, Chauhan SM. 2004. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res. 159:371–394. doi: 10.1016/j.micres.2004.08.004
- Egamberdiyeva D, Hoflich G. 2004. Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J Arid Environ. 56:293–301. doi: 10.1016/S0140-1963(03)00050-8
- Enebak SA, Wei G, Kloepper JW. 1998. Effects of plant growth-promoting rhizobacteria on loblolly and slash pine seedlings. Forest Sci. 44:139–144.
- Fan X, Zhang S, Mo X, Li Y, Fu Y, Liu Z. 2017. Effects of plant growth-promoting rhizobacteria and N source on plant growth and N and P uptake by tomato grown on calcareous soils. Pedosphere. 27(6):1027–1036. doi: 10.1016/S1002-0160(17)60379-5
- Forde BG, Clarkson DT. 1999a. Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Adv Bot Res. 30:1–90. doi: 10.1016/S0065-2296(08)60226-8
- Forde BG, Clarkson DT. 1999b. Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Adv Bot Res. 30:1–90. doi: 10.1016/S0065-2296(08)60226-8
- Gaudinier A, Rodriguez-Medina J, Zhang L, Olson A, Liseron-Monfils C, Bågman A-M, Brady SM. 2018. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature. 563(7730):259–264. doi: 10.1038/s41586-018-0656-3
- Glass ADM, Britto DT, Kaiser BN, Kinghorn JR, Kronzucker HJ, Kumar A, Okamoto M, Rawat S, Siddiqi MY, Unkles SE, et al. 2002. The regulation of nitrate and ammonium transport systems in plants. J Exp Bot. 53:855–864. doi: 10.1093/jexbot/53.370.855
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants. Cell. 138:1184–1194. doi: 10.1016/j.cell.2009.07.004
- Houk R, Fassel V, Flesh G, Svec H, Gray A, Taylor C. 1980. Inductively copled argon plasma as an ion source for mass spectrometric determination of trace elements. Annal Chem. 52:2283–2289. doi: 10.1021/ac50064a012
- Huang NC, Liu KH, Lo HJ, Tsay YF. 1999. Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell. 11:1381–1392. doi: 10.1105/tpc.11.8.1381
- Jang JH, Kim S-H, Khaine I, Kwak MJ, Lee HK, Lee TY, Lee WY, Woo SY. 2018. Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica. 56:1188–1203. doi: 10.1007/s11099-018-0801-0
- Jetiyanon K, Fowler WD, Kloepper JW. 2003. Broad-spectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis. 87:1390–1394. doi: 10.1094/PDIS.2003.87.11.1390
- Kaiser BN, Rawat SR, Siddiqi MY, Masle J, Glass ADM. 2002. Functional analysis of an Arabidopsis T-DNA “knockout” of the high-affinity NH4+ transporter AtAMT1;1. Plant Physiol. 130:1263–1275. doi: 10.1104/pp.102.010843
- Khademi S, O’Connell J, Remis J, Robles-Colmenares Y, Miericke LJW, Stroud RM. 2004. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.3.5 angstrom. Science. 305:1587–1594. doi: 10.1126/science.1101952
- Khalid A, Arshad M, Zahir ZA. 2004. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol. 96:473–480. doi: 10.1046/j.1365-2672.2003.02161.x
- Kirankumar R, Jagadeesh KS, Krishnara PU, Patil MS. 2008. Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of Tobacco Mosaic Virus pathogen. Karnataka J Agr Sci. 21:309–311.
- Kokalis-Burelle N, Vavrina CS, Reddy VS, Kloepper JW. 2003. Amendment of muskmelon and watermelon transplant media with plant growth-promoting rhizobacteria: effects on seedling quality, disease, and nematode resistance. Horttechnology. 13:476–482. doi: 10.21273/HORTTECH.13.3.0476
- Krouk G, Crawford NM, Coruzzi GM, Tsay YF. 2010a. Nitrate signaling: adaptation to fluctuating environments. Curr Opin Plant Biol. 13:266–273. doi: 10.1016/j.pbi.2009.12.003
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. 2010b. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 18:927–937. doi: 10.1016/j.devcel.2010.05.008
- Lakshmanan V, Castaneda R, Rudrappa T, Bais HP. 2013. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta. 238:657–668. doi: 10.1007/s00425-013-1920-2
- Lin X, Kaul S, Rounsley S, Shea TP, Benito M-I, Town CD, Fujii CY, Mason T, Bowman CL, Barnstead M, et al. 1999. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature. 402:761–768. doi: 10.1038/45471
- Lin W, Okon Y, Hardy RWF. 1983. Enhanced mineral uptake by Zea Mays and Sorghum Bicolor roots inoculated with azospirillum-brasilense. Appl Environ Microb. 45:1775–1779.
- Lin Y, Watts DB, Kloepper JW, Torbert HA. 2018. Influence of plant growth-promoting rhizobacteria on corn growth under different fertility sources. Commun Soil Sci Plant Anal. 49:1239–1255. doi: 10.1080/00103624.2018.1457155
- Liu K, McInroy JA, Hu C-H, Kloepper JW. 2017. Mixtures of plant-growth-promoting rhizobacteria enhance biological control of multiple plant diseases and plant-growth promotion in the presence of pathogens. Plant Dis. 102:67–72. doi: 10.1094/PDIS-04-17-0478-RE
- López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E. 2007. Bacillus megaterium rhizobacteria promote growth and Alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact. 20:207–217. doi: 10.1094/MPMI-20-2-0207
- Ludewlg U, Neuhduser B, Dynowski M. 2007. Molecular mechanisms of ammonium transport and accumulation in plants. Febs Lett. 581:2301–2308. doi: 10.1016/j.febslet.2007.03.034
- Mantelin S, Desbrosses G, Larcher M, Tranbarger TJ, Cleyet-Marel JC, Touraine B. 2006. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta. 223:591–603. doi: 10.1007/s00425-005-0106-y
- Mayer M, Dynowski M, Ludewig U. 2006. Ammonium ion transport by the AMT/Rh homologue LeAMT1; 1. Biochemical J. 396:431–437. doi: 10.1042/BJ20060051
- Mort-Gaudry JF. 2001. Nitrogen assimilation by plants. New Hampshire: Science Publishers.
- Orsel M, Filleur S, Fraisier V, Daniel-Vedele F. 2002. Nitrate transport in plants: which gene and which control? J Exp Bot. 53:825–833. doi: 10.1093/jexbot/53.370.825
- Poupin MJ, Timmermann T, Vega A, Zuniga A, Gonzalez B. 2013. Effects of the plant growth-promoting bacterium burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. Plos One. 8. doi: 10.1371/journal.pone.0069435
- Ryu CM, Hu CH, Locy RD, Kloepper JW. 2005b. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil. 268:285–292. doi: 10.1007/s11104-004-0301-9
- Ryu C-M, Hu C-H, Locy RD, Kloepper JW. 2005a. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil. 268:285–292. doi: 10.1007/s11104-004-0301-9
- Saia S, Rappa V, Ruisi P, Abenavoli MR, Sunseri F, Giambalvo D, Martinelli F. 2015. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci. 6:815. doi: 10.3389/fpls.2015.00815
- Salanoubat M, Lemcke K, Rieger M, Ansorge W, Unseld M, Fartmann B, Valle G, Blöcker H, Perez-Alonso M, Obermaier B, et al. 2000. Sequence and analysis of chromosome 3 of the plant Arabidopsis thaliana. Nature. 408:820–823. doi: 10.1038/35048706
- SAS Institute Inc. 2010. Base SAS® 9.3 procedures guide. Cary, NC: SAS Institute Inc.
- Sato S, Nakamura Y, Kaneko T, Katoh T, Asamizu E, Kotani H, Tabata S. 2000. Structural analysis of Arabidopsis thaliana chromosome 5. X. sequence features of the regions of 3,076,755 bp covered by sixty P1 and TAC clones. DNA Res. 7:31–63. doi: 10.1093/dnares/7.1.31
- Sohlenkamp C, Shelden M, Howitt S, Udvardi M. 2000. Characterization of Arabidopsis AtAMT2, a novel ammonium transporter in plants. FEBS Lett. 467:273–278. doi: 10.1016/S0014-5793(00)01153-4
- Touraine B, Daniel-Vedele F, Forde B. 2001. Nitrate uptake and its regulation. In: Lea P, Morot-Gaudry J-F, editors. Plant nutrition. Berlin: Springer-Verlag; p. 1–36.
- Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI. 2006. Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Micro. 42:117–126. doi: 10.1134/S0003683806020013
- Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moenne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dye F, Prigent-Combaret C. 2013. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 4:356. doi: 10.3389/fpls.2013.00356
- Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithoefer A. 2012. CML42-mediated calcium signaling coordinates responses to spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 159:1159–1175. doi: 10.1104/pp.112.198150
- Wang YY, Hsu PK, Tsay YF. 2012. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 17:458–467. doi: 10.1016/j.tplants.2012.04.006
- Yuan L, Loque D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wiren N. 2007a. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell. 19:2636–2652. doi: 10.1105/tpc.107.052134
- Yuan LX, Loque D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wiren N. 2007b. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell. 19:2636–2652. doi: 10.1105/tpc.107.052134
- Zhang HM, Jennings A, Barlow PW, Forde BG. 1999. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA. 96:6529–6534. doi: 10.1073/pnas.96.11.6529
- Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, et al. 2007. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 226:839–851. doi: 10.1007/s00425-007-0530-2
