ABSTRACT
This study investigates the impact of priming on the resistance of tomato plants to the potato aphid Macrosiphum euphorbiae, focusing on the role of glandular trichomes. Glandular trichomes are specific hairs that provide protection to tomato plants against herbivorous insects. The experimental priming conducted in this study revealed that prior infestation by Spodoptera littoralis caterpillars increased the plant's resistance against M. euphorbiae, pointing at the jasmonic acid (JA) signaling pathway in regulating this plant-aphid interaction. Glandular trichomes type IV were effective against aphids regardless of the previous infestation. Using JA-deficient tomato (spr2), we observed that M. euphorbiae multiplication increased, while the number of aphids on salicylic -deficient NahG plants was lower than in the wildtype Moneymaker. These findings emphasize the crucial role of the JA signaling pathway in tomato plant resistance to aphids and the importance of glandular trichomes to enhance plant defences against pests.
Introduction
Plants often suffer from attack by multiple herbivorous insects, either simultaneously or sequentially that may reduce the survival and fitness. To cope with the diversity of insect herbivory, plants have evolved different defense mechanisms to perceive and respond appropriately to specific attackers (Erb and Reymond Citation2019). While some defences are only induced in response to an herbivore attack other defence mechanisms are expressed constitutively (War et al. Citation2012; Fürstenberg-Hägg et al. Citation2013). Inducible defenses are activated when the surveillance system of the plant recognizes elicitors contained in insect oral secretions or signals released by injured cells which are followed by rapid activation of sophisticated plant signaling pathways (Nguyen et al. Citation2016). Moreover, induced defenses are not only triggered in the insect-attacked leaves but can also occur systemically i.e. in distal areas from the wounded parts facilitating rapid and effective responses to incoming herbivore attacks (Conrath et al. Citation2015; Erb and Reymond Citation2019). Plants expressing induced defences often exhibit a faster and stronger activation of specific defense responses after they have been previously challenged by an insect herbivore or a pathogen. This capacity for augmented defense expression is called priming, which is an adaptive strategy that improves the defensive capacity of plants. Induced plant defenses can have an impact on other herbivores that feed on the same plant simultaneously or subsequently (Agrawal Citation2014; Stam et al. Citation2014). Phytohormones, such as Jasmonic acid (JA) and salicylic acid (SA), play a crucial role in the activation of these induced plant defenses (Pieterse et al. Citation2012; Steinbrenner et al. Citation2020). The activation of these pathways by herbivores is often herbivore-specific, and their induction can be partly predicted by the feeding mode of the herbivore.
To fine-tune defense mechanisms in plants, the SA- and JA-responsive signaling pathways are interdependent and act through a complex network of regulatory interactions. One of the best characterized interactions in defense-related signaling is the crosstalk between the JA and SA response pathways. The crosstalk hypothesis predicts interference between plant signaling pathways when initial and subsequent attackers induce defenses associated with different pathways (Pieterse et al. Citation2012; Eisenring et al. Citation2018; Erb and Reymond Citation2019). Nonetheless, synergistic interactions have been described as well. For instance, a meta-analysis on plant-mediated effects of initial attackers on the performance of subsequent attackers indicated that JA-inducing herbivores resulted in a reduction in the performance of both JA- and SA- inducing herbivores (Moreira et al. Citation2018).
It is generally assumed that leaf-chewing herbivores, such as caterpillars, lead to an induction of defensive pathways mainly regulated by JA, whereas SA signaling primarily participates in defense against piercing-sucking herbivores that feed on the phloem, such as aphids and whiteflies (Morkunas et al. Citation2011; Züst and Agrawal Citation2016; Steinbrenner et al. Citation2020). However, the impact of SA defense pathway on resistance to aphids remains equivocal (van Emden and Harrington Citation2007; Javed et al. Citation2023). For instance, Moran and Thompson (Citation2001) reported that mutations blocking the SA signaling pathway and SA-deficient Arabidopsis plants do not compromise resistance to aphids, while other authors have showed that SA accumulation and signaling has an important role in the defense against aphids (Mewis et al. Citation2005; Feng et al. Citation2021; Javed et al. Citation2023) or the effect is aphid- species dependent (Gao et al. Citation2023) or depends of the host plant identity. For instance, SA is vital for countering Macrosiphum euphorbiae in tomato (Bhattarai et al. Citation2007), while in Arabidopsis, SA activation promotes whitefly infestation (Zhang and Li Citation2019). Interestingly, previous damage by leaf-chewing herbivores inducing the JA pathway, or the exogenous application of JA (or its precursors), enhances plant resistance to aphids (Ellis et al. Citation2002; Zhu-Salzman et al. Citation2004; Cooper and Goggin Citation2005; Li et al. Citation2023). Moreover, the role of JA signaling on aphid population growth has been put forward experimentally by various authors by working with plant mutants (Mewis et al. Citation2005; Kuśnierczyk et al. Citation2011) or by demonstrating the inhibition of JA-responsive gene expression (Schwartzberg and Tumlinson Citation2014). Nonetheless, SA-induced resistance may also provide defense against phloem-feeding insects by activating the JA signaling pathway (Alba et al. Citation2015).
Resistance to herbivores also depends on structural and chemical plant defenses. These defenses include physical barriers such as trichomes, thorns, spines and/or toxic chemicals (Schuurink and Tissier Citation2020; Kortbeek et al. Citation2021). Among these resistance traits, glandular trichomes have been studied in detail in tomato and are an important insect resistance trait (Simmons and Gurr Citation2005; McDowell et al. Citation2011; Glas et al. Citation2012). For example, cultivated tomato (S. lycopersicum) and its wild relatives contain four types of glandular trichomes named type I, IV, VI and VII. Type IV trichomes are particularly relevant as they have been shown to be responsible for the partial resistance against numerous herbivores among other, whiteflies (Rodríguez-López et al. Citation2020, Citation2011), spider mites (Alba et al. Citation2009), moths (Juvik et al. Citation1994; da Silva et al. Citation2016) and aphids (Goffreda et al. Citation1990; Blanco-Sánchez et al. Citation2021). The main metabolites produced on glandular trichomes type IV are acylsugars (McDowell et al. Citation2011) i.e. viscous polyesters that include one or several acyl chains on sucrose or glucose backbones. Acylsugars are related with movement impairment and also with toxicity on insects and consequently, with a reduced access to leaf epidermis for feeding or oviposition (Simmons and Gurr Citation2005; Planelló et al. Citation2022). Insect resistance mediated by glandular trichomes in tomato can also be induced on newly formed leaves by insect feeding and application of methyl jasmonate (Tian et al. Citation2012; Escobar-Bravo et al. Citation2016). Recently, we reported the detrimental effect of glandular trichomes type IV on the aphid Macrosiphum euphorbiae (Blanco-Sánchez et al. Citation2021; Planelló et al. Citation2022) Haga clic o pulse aquí para escribir texto. In previous experiments, we found that type IV glandular trichomes affect host selection and aphid proliferation with aphids avoiding and reducing rate of multiplication on the genotype with these glandular trichomes (Ferrero et al. Citation2020; Blanco-Sánchez et al. Citation2021). In addition, the exposure to type IV glandular trichomes activated the detoxication and the ecdysone pathways underlying important metabolic trade-offs in aphids exposed to glandular trichomes (Planelló et al. Citation2022).
To investigate the signaling pathway governing the interactions between M. euphorbiae and tomato, we conducted two experiments. First, we performed a priming experiment in which we briefly exposed two different tomato genotypes, ‘Moneymaker’ (MM) and its near-isogenic line with enhanced acylsucrose production (ABL 10-4), to either M. euphorbiae or S. littoralis caterpillars. The underlying hypothesis was that if JA is a key hormone in the interaction of M. euphorbiae with the plant, we would expect better aphid multiplication on genotypes with impaired JA pathway. The rationale for using these two near-isogenic genetic lines, one with enhanced acylsucrose production, was to disentangle the effects of a known resistance trait (type IV trichomes) from resistance mediated by hormonal pathways. We expected the aphid's performance to be better in the treatment where the initial feeding pulse would not trigger the JA pathway, resulting in higher aphid multiplication. Second, we used three tomato genotypes with varying abilities to operate the JA pathway to further test this hypothesis. We hypothesized that if JA is a key hormone, then the aphid's performance would be better on genotypes with an impaired JA pathway.
Material and methods
Plant materials and growth conditions
Five tomato (S. lycopersicum) genotypes were used in this study: (1) the tomato cultivar MM; (2) its near-isogenic line ABL 10–4 with type-IV glandular trichomes and enhanced production of acylsucroses; (3) the decreased SA transgenic line NahG in the genetic background of MM (Oldroyd and Staskawicz Citation1998; Brading et al. Citation2000); (4) the mutant line spr2, impaired for the synthesis of JA and the expression of the JA-responsive proteinase inhibitor II (PIN-II) gene, a marker of induced resistance to insects; (5) and its corresponding wild-type background S. lycopersicum cv CastleMart (CM) (Li et al. Citation2003).
For all experiments, seeds were sterilized with an aqueous solution of 50% of household bleach for 30 min, rinsed two times with distilled water and sown in seedbeds with autoclaved soil mixture (45% peat, 45% coconut fiber, and 10% perlite) after germination on filter paper. Seedlings were grown in an insect-proof greenhouse at 25 ± 2 °C, 50–70% relative humidity and a 16/8 h light/dark photoperiod. Two weeks after germination, seedlings were transplanted into plastic pots (18 cm diameter, 15 cm depth) filled with the same sterile soil mixture. Plants were watered daily and fertilized weekly with water-soluble NPK (SO3) [1.98-3, 41-20, (4.46)] fertilizer mixture (Fertiluq®) and water-soluble N (Ca–Mg) [4.32 (6.51-4.02)] fertilizer mixture (Fertiluq®) with micronutrients.
Aphid and caterpillar populations
A clonal population of M. euphorbiae was obtained from an individual gravid female coming from an infested tomato field in Aranjuez (Spain). Aphids were reared on the susceptible tomato ‘MM inside insect-proof cages (W46.5 × D46.5 × H46.5 cm) in a climatic chamber at 22-25°C, 65% of relative humidity (HR) and a 16/8 h light/dark photoperiod. Plants were watered twice a week. Fertilizer was applied as described above.
Spodoptera littoralis pupae were reared in plastic boxes (W12 × D18 × H6 cm) with mesh on the top and lined with filter paper in a climatic chamber at 25 ± 2°C during day and 18 ± 2°C at night, 70% relative humidity, and with a 16 h photoperiod. Fresh laid eggs were hand-picked and transferred into new boxes. Freshly hatched larvae were reared in artificial diet until the larvae developed into second instars (Ferrero et al. Citation2020).
Priming experiment
In another assay, we assessed to what extent a short pulse of herbivory could prime tomato plants (i.e. induce plant defenses) and in consequence, affect aphid performance in a second aphid attack. With this purpose, we compared aphid multiplication on ‘MM and on the near-isogenic line (ABL 10-4) with type-IV glandular trichomes and enhanced production of acylsucroses that either had been previously challenged by the aphid M. euphorbiae; had been challenged by caterpillars of S. littoralis and (3) control plants that had not received any previous herbivory treatment. Experimental plants (with ten repetitions per treatment) were placed in a greenhouse with conditions as explained in the mutant assays. All plants from all treatments were caged individually with tul-mesh (i.e. confined within a cage of W30 × D30 × H70 cm). For the treatment with caterpillars, two second-instar larvae caterpillars were transferred to the experimental plants. Each caterpillar was weighted before placement on the plants to ensure that caterpillars with comparable sizes were used across experimental plants. For the pulse with aphids, plants were infested with three adult apterous females. After five days of feeding, aphids and caterpillars were manually removed from the treated plants and a week later three apterous female adults of M. euphorbiae were placed on all experimental plants of the three treatments (i.e. plants previously treated with aphid, caterpillars or control plants) and were allowed to reproduce for 20 days.
To confirm the induction of JA-dependent defenses we evaluated by means of RT-qPCR the gene expression of the proteinase inhibitor II (PIN- II), a marker gene of the jasmonic signaling pathway known to be involved in the plant resistance to S. littoralis. Therefore, we added two additional treatments to the ones exposed above. As control of PIN-II induction, plants were sprayed with a 0.5 mM MeJA (Sigma-Aldrich, Germany) in 0.8% ethanol and 0.1% tween-20® (Merck, Germany) aqueous solution until the point of run-off and a mock treatment with 0.8% ethanol (etOH) and 0.1% tween-20® (Merck, Germany) aqueous solution used as control. Three composed samples (third and fourth leaf of each plant was taken) of each genotype and treatment were harvested and frozen in liquid nitrogen and stored at −80 °C until further processing for RT-qPCR. RNA extraction and RT-qPCR were performed according to the procedure described in Ontiveros et al. Citation2022. Briefly, total RNA was isolated with Trizol reagent (Ambion) and then treated with DNase-Free RNase (Roche). The cDNAs were generated from 500 ng of total RNAs using BioRAD iScript™ cDNA Synthesis Kit in a reaction volume of 20 µl. Quantitative PCR (qPCR) were done using as template 1 µl of cDNA using TAKARA SYBR Green PCR kit on a CFX96 Real-time PCR detection system (Bio-Rad, USA). The following cycling conditions were used: 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative quantification of specific RNAs were normalized to the elongation factor 1-α (SlEF1α) gene (López-Ráez et al. Citation2010). Primers for tomato PR-P6 and PIN-II amplification were reported by Sarmento et al. (Citation2011) and Uppalapati et al. (Citation2005). Relative quantifications were measured by using the comparative Ct method, as described by Livak and Schmittgen (Citation2001).
Assays with hormone impaired genotypes
The multiplication of M. euphorbiae was compared on tomato genotypes that differ in their ability to operate hormonal pathways and hence, to induce plant defenses. In a first assay, we compared aphid multiplication in the mutant spr2, impaired for the biosynthesis of JA and its wild type CM. Then in a second assay, we compared aphid multiplication on NahG, a transgenic line that prevents the accumulation of SA, and MM. In both assays, for each genotype, ten 3-week-old plants were manually infested with three aphids and every five days the number of aphids was noted down. The two assays were conducted in a greenhouse with cooling capacities and with a temperature ranging from 25 ± 2 °C during the day and 20 ± 2 °C at night, and with a relative humidity of 60–70% and a 16/8 h light/dark photoperiod. All plants from all treatments were caged individually with tul-mesh (i.e. confined within a cage of W30 × D30 × H70 cm). The experiments were kept for 20 days after plants received the aphids.
Statistical analysis
All statistical analyses were carried out with the R software version 4.1.0 (R Core Team Citation2021) using the ‘lme4,’ ‘emmeans,’ ‘lsmeans,’ ‘lmperm,’ ‘permuco’ and ‘performance’ packages. Plots were designed using ‘ggplot2.’
Model assumptions (i.e. homogeneity of variance, collinearity, normality of random effects and residuals) were checked for all models.
For the priming assay, differences on aphid multiplication due to the priming stimulus were evaluated by means of a GLMM (adjusting for a Poisson error structure using log link function). Again, the number of aphids was set as the response variable, genotype and treatment (i.e. different priming stimuli) were included as fixed factors. Greenhouse bench and date were included as random factor.
For the analysis of expression levels and given that the dependent variable did not fulfill the assumptions for a GLMM (i.e. homogeneity of variance and normality of residuals), a two-way permutational analysis of variance (aovp) was conducted using the R package ‘lmperm.’ Aovp is an analysis of variance that makes use of permutation tests instead of normal theory tests to calculate statistics. In this case, Log_fold value (as proxy for PIN-II expression) was included as dependent variable and treatment and genotype as fixed factors. For the estimation of model parameters 5000 permutations were run.
Aphid multiplication on the different mutants (i.e. for CM and spr2, in the first assay, and for MM and NahG in the second assay) was compared by means of two generalized linear mixed models (GLMMs) where the number of aphids was the dependent variable with a Poisson error structure (with a log link function) and tomato genotype was used as fixed factor. As experimental pots were placed in different benches in the greenhouse and aphids on plants were measured on four different occasions, bench and date (day of measurement) were included in both models as a random factor.
Results
Priming experiment
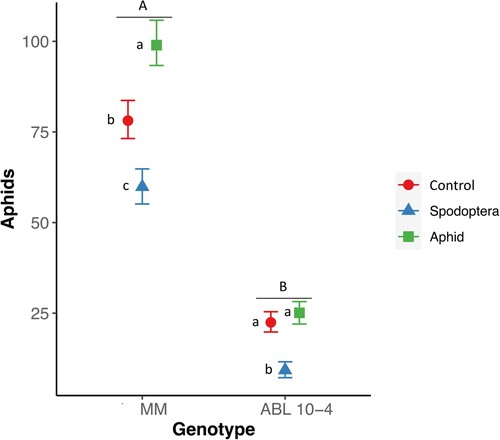
The mean number of aphids was significantly lower in ABL 10-4, the genotype with type-IV glandular trichomes and enhanced production of acylsucroses, than in MM (Z-ratioABL10-4 vs. MM = −26.04, df = 1, p ≤ 0.0001, ). While in ABL-10-4 the average number of aphids after 20 days was 14.4 ± 3.4 (mean ± standard error), in MM aphid number reached 61.5 ± 14.3. The assay revealed that for both genotypes, a previous herbivore attack had a strong effect on aphid multiplication: prior caterpillar feeding resulted in the lowest aphid multiplication with an average of 7.95 ± 2.1 aphids on ABL-10-4 and 47.68 ± 11.3 on MM. In contrast, previous aphid feeding resulted for both genotypes in the highest aphid multiplications; with an average of 20.6 ± 5 in ABL10-4 and 76.2 ± 18 in MM. Control plants, those that did not have any pre-treatment, showed intermediate values in both cases, i.e. with an average of 18.4 ± 4.5 aphids per plant in ABL10-4 and 64 ± 15.1 in MM. The interaction term showed that the effect of treatment differed between the two compared genotypes; while there were no significant differences between the pretreatment with aphids and control plants for ABL10-4 (Z-ratio aphids vs. control = −1.19, df = 1, p = 0.38), for MM there were clear differences (Z-ratio aphids vs. control = −3.55, df = 1, p ≤ 0.0008, ).
Figure. 1. Aphids (mean ± standard error) after 20 days of infestation in plants of Solanum lycopersicum cv. Moneymaker (MM) and ABL 10-4, previously primed with caterpillars of Spodoptera littoralis (Spodoptera) or M. euphorbiae (Aphid) or with no previous infestation (Control). Capital letters indicate significant differences between the two genotypes; letters in lowercase indicate differences among treatments within a genotype, Generalized Linear Mixed Model p ≤ 0.001, n = 10.
The analysis of expression of the proteinase inhibitor II gene (PIN-II) indicated that although there were significant differences between the compared genotypes (Parametric F-value = 12.37, df = 1, p = 0.0054) with average expression values slightly higher for MM of 1.91 ± 0.06 than for ABL10-4; with and average values of 1.58 ± 0.07. However, and more importantly, the treatment response was consistent for both genotypes, as the interaction term was not significant (Parametric F-value = 2.44, df = 3, p = 0.11). In other words, the proteinase inhibitor was only expressed after 48h when plants were either treated with MeJA or with caterpillars, showing no statistical differences between these treatments and with a similar response in both genotypes (Parametric F-value = 566.88, df = 3, p = 0.0002, Figure S1). In contrast, there was no expression of PIN-II when plants were treated with aphids or in the control plants, i.e. those that had received a mock treatment (Figure S1).
Assays with hormone impaired genotypes
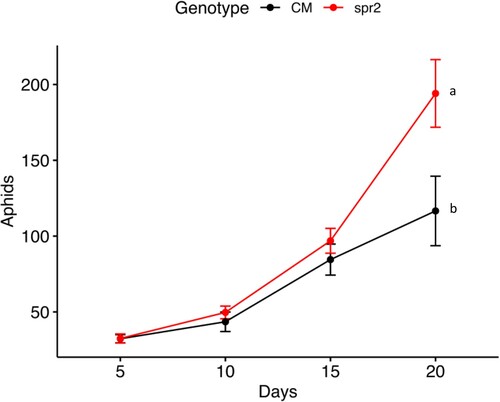
The number of aphids differed significantly between the two compared genotypes (spr-2 and CM) (). The spr2 genotypes impaired in the JA hormonal pathway showed an enhanced susceptibility to the aphid in comparison to the wild type (CM) (). After 20 days, the maximum multiplication of aphids was significantly higher on spr2 compared to CM; while on spr2 we found 194.1 ± 22 (mean ± standard error) aphids per plant; on CM, values averaged 116 ± 22.9 per plant (Z-ratioCM vs. spr-2 = −11.78, df = 1, p ≤ 0.0001, ).
Figure. 2. Population built-up (mean ± standard error) of M. euphorbiae on Solanum lycopersicum cv. CastleMart (CM, black line) and spr2. Aphid multiplication was monitored for 20 days. Different letters indicate significant differences in aphid multiplication between the compared genotypes according to a Generalized Mixed Linear Model with a Poisson error structure, p ≤ 0.001.
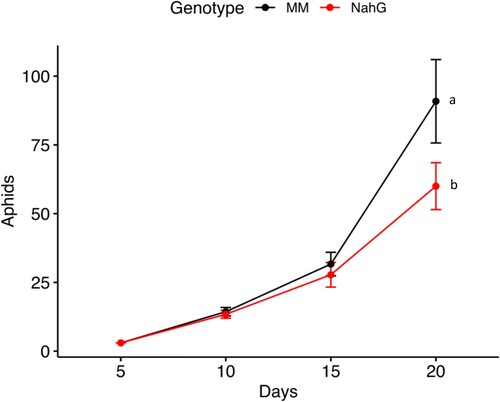
The comparison of aphid multiplication on MM and NahG revealed that aphid performance was significantly different between the compared genotypes (Z-ratioMM vs. NahG = −3.76, df = 1, p ≤ 0.0002, ). The number of aphids on NahG plants was considerably lower than in wild-type MM. The number of aphids after 20 days of infestation in MM plants was 90.8 ± 15.15 while in NahG plants was 62 ± 8.5 ().
Figure. 3. Population built-up (mean ± standard error) of M. euphorbiae on Solanum lycopersicum cv. Monemymaker (MM) and NahG. Aphid multiplication was monitored for 20 days. Different letters indicate significant differences in aphid multiplication according to a Generalized Mixed Linear Model with a Poisson error structure, p ≤ 0.001, n = 10.
Discussion
It is well known that prior herbivory can modify plant responses to subsequent herbivores, especially when exposed to different feeding guilds (Kroes et al. Citation2016; Johnson et al. Citation2020). However, little is known about how earlier herbivory can modify plant responses mediated by glandular trichomes to later arriving herbivores (Islam et al. Citation2022). Previous research has shown that the application of JA to tomato plants induces the production of acylsucroses by type IV glandular trichomes (Alba et al. Citation2015; Escobar-Bravo et al. Citation2016). Here, we investigated how sequential induction of plant defenses following attacks of M. euphorbiae or the caterpillar S. littoralis interfere on the dynamic of M. euphorbiae that subsequently infested two isogenic tomato lines that differ in the presence of type IV glandular trichomes and production of acylsucroses i.e. the cultivar MM that lacks glandular trichomes type-IV and its nearly-isogenic ABL 10–4 with high density of trichomes type-IV and production of acylsucroses. Using the two isogenic lines ABL 10–4 and MM, allowed us to distinguish the effects of a known resistance trait (i.e. the type IV trichomes) from the responses mediated by hormonal pathways and at the same time, indirectly evaluate the effect of different pathways on M. euphorbiae. Numerous studies have investigated how prior herbivory affects the performance of subsequent herbivores, particularly using contrasting feeding guilds with different outcomes in interaction (e.g. positive, neutral, or negative for the second herbivorous insect) (Rodriguez-Saona et al. Citation2010; Soler et al. Citation2012; Schweiger et al. Citation2014; Kroes et al. Citation2016; Leckie et al. Citation2016; Eisenring et al. Citation2018; Li et al. Citation2018; Johnson et al. Citation2020). Our results showed that ABL 10–4 plants, the ones with type-IV glandular trichomes, were overall more resistant to M. euphorbiae than MM, as previously reported for this aphid (Blanco-Sánchez et al. Citation2021; Planelló et al. Citation2022) and in the same line with previous studies using other herbivorous insects (Escobar-Bravo et al. Citation2016; Rodríguez-López et al. Citation2020). However, resistance differed depending on the type of herbivory previously faced. In this sense, plants previously infested with S. littoralis were more resistant than control plants, indicating that a first attack by the chewing herbivore, triggering the JA pathway resulted in an enhanced resistance against M. euphorbiae. Regardless of the presence of type IV glandular trichomes and acylsucrose production. Leaf-chewing herbivores inducing JA on the plant have been shown to reduce the performance of aphids (El-Wakeil et al. Citation2010; Ali and Agrawal Citation2014; Aslam et al. Citation2022). Our results also point that those plants previously infested with M. euphorbiae were more susceptible to a second attack of the aphid. Similar studies in other plant families and using other aphid clones have shown a lot of variability in the outcome of the interaction (Züst and Agrawal Citation2016). However, works focused on aphid choices at small scales indicated a preference and enhanced feeding on pre-infested leaves and a positive effect on nymph survival (Dugravot et al. Citation2007). In this sense, it has been hypothesized that specialized aphids seem to have gained the ability to manipulate plant defences to their benefit using the plant’s own hormonal crosstalk, a line of thought in accordance with our observations (Ali and Agrawal Citation2014; Züst and Agrawal Citation2016; Moreira et al. Citation2018).
Many inducible defenses are expressed rapidly (i.e. within hours) in leaves of herbivore-challenged plants. In tomato plants, wounding causes a systemic reprogramming of leaf cells that results in the synthesis of different defense-related proteins (Ryan Citation2000; Erb and Reymond Citation2019). The JA-responsive proteinase inhibitor II (PIN-II) gene, is a well-characterized marker of induced resistance to insects (Li et al. Citation2003) that inhibits the activity of digestive enzymes in the gut of the herbivore. Transcriptional activation of proteinase inhibitor genes in response to wounding and systemin depends on the action of JA (Ryan Citation2000). For this reason, in our experiment we evaluated after 48h the expression profile of PIN-II, when its maximum expression level is reached (Sanmartín et al. Citation2020), in order to verify that the priming stimulus worked. Our real-time qPCR analysis showed a considerable overexpression of PIN-II not only in plants sprayed with JA, as expected, but also in those primed with the caterpillar S. littoralis; indicating that this herbivore effectively triggered the JA signaling pathway. Previous studies have revealed that the application of MeJA in the ABL 10–4 plants increase the density of the glandular trichomes type-IV and their associated production of metabolites (Escobar-Bravo et al. Citation2016). Here we can notice a similar increase of the PIN-II levels in both genotypes, ABL 10–4 and MM, treated with a JA precursor solution and previously induced with the caterpillar. We attribute the lack of significant differences on the expression of PIN-II after infestation with M. euphorbiae to the fact that pierce sucking herbivores and specialized aphids in particular, activate the antagonist SA pathway (Züst and Agrawal Citation2016).
Considering the complexity of plant-aphid interactions and the equivocal role of hormonal pathways in plant defense to aphid herbivory, we further addressed the role of JA in the resistance to M. euphorbiae in tomato plants under experimental conditions. Moreover, and in order to avoid additional effects from the glandular trichomes that could mask the underlying defence pathway, we evaluated aphid’s performance on mutants impaired in the JA pathway. Our results showed that the number of aphids on spr2 plants was significantly higher than on the wild-type tomato CM; spr2 has no ability to synthesize Jas (Li et al. Citation2003) and therefore, these plants are unable to interact with SA or analogous pathways. Our results contrast with those reported by Avila and colleagues (Citation2012) where population growth of M. euphorbiae was significantly lower on spr2 than on CM or in the JA insensitive (jai1-1) mutant. These authors attribute the failure in population growth to the mutation on the JA pathway, either on the synthesis or perception. The results put forward in our experiment proof that this is not always the case and agree with other experimental evidence where a mutation in the JA pathway improves host suitability to herbivores (Li et al. Citation2004). In addition, Avila and colleagues proposed, like other previous studies, that SA can contribute to plant defenses against aphids in tomato genotypes (Li et al. Citation2006; Avila et al. Citation2012). Again, our experimental data are not in accordance with these observations, showing a reduction in the population of M. euphorbiae in the transgenic plants NahG. With these results, the hypothesis of an antagonism between the JA and SA pathways, where these phloem-feeding insects limit the induction of JA-dependent defenses by inducing SA (Zhu-Salzman et al. Citation2004; De Vos et al. Citation2005) gets underlined. Although it is difficult to pinpoint with the information available the exact reasons for the discrepancies among the studies compared, differences may be ascribed to the aphid densities used in the experimental set-ups, genotype variation or the used aphid clones, as it has been shown that variation in bacterial symbionts may strongly determine the outcome of plant aphid interactions. In any case, our results together with previous observations, indicate that the role of hormonal pathways may be strongly idiosyncratic.
Taken all the results of this study together the role of JA in the regulation of M. eurphorbiae and tomato should be reconsidered. At the same time, from an applied point of view, our results further support the breeding of tomato varieties with higher densities of glandular trichomes and higher levels of acylsugars as these genotypes show consistently very strong responses against aphids regardless of the induced defensive pathways.
Acknowledgement
We thank Prof. Alberto Fereres for kindly providing the clone of Macrosiphum euphorbiae.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors





Lidia Blanco-Sánchez
Lidia Blanco-Sánchez, PhD candidate, IHSM-UMA-CSIC, Málaga, Spain. She is currently finalizing her PhD dissertation addressing the role of glandular trichomes in the interaction of Macrosiphum euphorbiae and tomato.
Dr. Rosario Planelló, Associate professor, UNED, Madrid, Spain. Currently, her research is focused on: 1) describing the toxic effect of emerging pollutants and EDCs through molecular biomarkers on invertebrates; 2) characterizing, the physiological response of pest and non-pest insects to the natural defenses of plants.
Dr. Victoria Ferrero, Associate professor, Universidad de León, León, Spain. She is an evolutionary botanist with strong interests on breeding system in plants and animal-plant interactions. Her research goal is to understand the causal role of species interactions in driving the evolution of adaptive phenotypes, and the consequences of that evolutionary change in nature.
Dr. Rafael Fernández-Muñoz, tenured scientist (IHSM-UMA-CSIC), Malaga, Spain. Tomato genetics and breeding specialist interested in germplasm natural genetic variation and development of mapping populations (RIL, IL) from interspecific crosses, focussed on genetics of traits such as fruit quality, biochemical composition of the fruit cuticle, resistance to pests (spider mites and whiteflies) based on glandular trichomes, the induced plant resistance pathways, and searching for and inheritance studies on genetic resistance to both viruses transmitted by whiteflies and to the insect vector.
Dr. Eduardo de la Peña is a tenured scientist at the Spanish National Research Council (CSIC), affiliated with the Institute for Subtropical and Mediterranean Horticulture (IHSM-UMA-CSIC) in Malaga, Spain. He serves as the corresponding author. His current research focuses on plant-insect interactions and the early detection and management of emerging pests in tropical and subtropical crops. Dr. de la Peña's work also involves the implementation of crop management practices that emphasize biodiversity preservation and promote ecosystem services, including natural pest control.
Dr. Juan Antonio Díaz-Pendón, tenured scientist (IHSM-UMA-CSIC), Malaga, Spain. My research program focuses on understanding the complex interactions between plant viruses (in the context of single and mixed infections), host plants and insect vectors, and how these interactions result in virus transmission and diseases. Currently, we use as model Tomato yellow leaf curl virus (TYLCV), Tomato chlorosis virus (ToCV), the whitefly Bemisia tabaci and tomato (Solanum lycopersicum L.).
References
- Agrawal AA. 2014. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol. 28(6):1404–1412. doi:10.1111/1365-2435.12271.
- Alba JM, Montserrat M, Fernández-Muñoz R. 2009. Resistance to the two-spotted spider mite (Tetranychus urticae) by acylsucroses of wild tomato (Solanum pimpinellifolium) trichomes studied in a recombinant inbred line population. Experimental and Applied Acarology. 47. doi:10.1007/s10493-008-9192-4.
- Alba JM, Schimmel BCJ, Glas JJ, Ataide LM, Pappas ML, Villarroel CA, … Kant MR. 2015. Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 205(2):828–840. doi:10.1111/nph.13075.
- Ali JG, Agrawal AA. 2014. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol. 28(6):1404–1412. doi:10.1111/1365-2435.12271.
- Aslam H, Mushtaq S, Maalik S, Bano N, Eed EM, Bibi A, Tahir A, Ijaz I, Tanwir S, Khalifa AS. 2022. Exploring the effect of jasmonic acid for aphids control for improving the yield ofTriticum aestivum varieties. Peer J. doi:10.7717/peerj.14018.
- Avila CA, Arévalo-Soliz LM, Jia L, Navarre DA, Chen Z, Howe GA, … Goggin FL. 2012. Loss of function of FATTY ACID DESATURASE7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 158(4):2028–2041. doi:10.1104/pp.111.191262.
- Bhattarai KK, Xie QG, Pourshalimi D, Younglove T, Kaloshian I. 2007. Coi1-dependent signaling pathway Is Not required forMi-1—mediated potato aphid resistance. Molecular Plant-Microbe Interactions®. 20:276–282. doi:10.1094/MPMI-20-3-0276.
- Blanco-Sánchez L, Planelló R, Llorente L, Díaz-Pendón JA, Ferrero V, Fernández-Muñoz R, … de la Peña E. 2021. Characterization of the detrimental effects of type IV glandular trichomes on the aphid Macrosiphum euphorbiaein tomato. Pest Management Science. 77. doi:10.1002/ps.6437.
- Brading PA, Hammond-Kosack KE, Parr A, Jones JDG. 2000. Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J. 23:305–318. doi:10.1046/j.1365-313x.2000.00778.x.
- Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR. 2015. Priming for enhanced defense. Annu Rev Phytopathol. 53. doi:10.1146/annurev-phyto-080614-120132.
- Cooper WR, Goggin FL. 2005. Effects of jasmonate-induced defenses in tomato on the potato aphid Macrosiphum euphorbiae. Entomol Exp Appl. 115(1). doi:10.1111/j.1570-7458.2005.00289.x.
- da Silva AA, Andrade MC, Carvalho R, de C, Neiva IP, Santos DC, Maluf WR. 2016. Resistência à Helicoverpa armigera em genótipos de tomateiro obtidos do cruzamento de Solanum lycopersicum com Solanum galapagense. Pesquisa Agropecuária Brasileira. 51. doi:10.1590/S0100-204X2016000700002.
- De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, … Pieterse CM. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant-Microbe Interactions®. 18(9):923–937. doi:10.1094/MPMI-18-0923.
- Dugravot S, Brunissen L, Létocart E, Tjallingii WF, Vincent C, Giordanengo P, Cherqui A. 2007. Local and systemic responses induced by aphids in Solanum tuberosum plants. Entomol Exp Appl. 123(3):271–277. doi:10.1111/j.1570-7458.2007.00542.x.
- Eisenring M, Glauser G, Meissle M, Romeis J. 2018. Differential impact of herbivores from three feeding guilds on systemic secondary metabolite induction, phytohormone levels and plant-mediated herbivore interactions. J Chem Ecol. 44:1178–1189. doi:10.1007/s10886-018-1015-4.
- Ellis C, Karafyllidis I, Turner JG. 2002. Constitutive activation of jasmonate signaling in an Arabidopsis Mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Molecular Plant-Microbe Interactions®. 15. doi:10.1094/MPMI.2002.15.10.1025.
- El-Wakeil NE, Volkmar C, Sallam AA. 2010. Jasmonic acid induces resistance to economically important insect pests in winter wheat. Pest Management Science: Formerly Pesticide Science. 66(5):549–554.
- Erb M, Reymond P. 2019. Molecular interactions between plants and insect herbivores. Annu Rev Plant Biol. 70. doi:10.1146/annurev-Faarplant-050718-095910.
- Escobar-Bravo R, Alba JM, Pons C, Granell A, Kant MR, Moriones E, Fernández-Muñoz R. 2016. A jasmonate-inducible defense trait transferred from wild into cultivated tomato establishes increased whitefly resistance and reduced viral disease incidence. Front Plant Sci. 7:1732.
- Feng JL, Zhang J, Yang J, Zou LP, Fang TT, Xu HL, Cai QN. 2021. Exogenous salicylic acid improves resistance of aphid-susceptible wheat to the grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae). Bull Entomol Res. 111(5):544–552. doi:10.1017/S0007485321000237.
- Ferrero V, Baeten L, Blanco-Sánchez L, Planelló R, Díaz-Pendón JA, Rodríguez-Echeverría S, Haegeman A, de la Peña E. 2020. Complex patterns in tolerance and resistance to pests and diseases underpin the domestication of tomato. New Phytologist. 226(1):254–266. doi:10.1111/nph.16353.
- Fürstenberg-Hägg J, Zagrobelny M, Bak S. 2013. Plant defense against insect herbivores. Int J Mol Sci. 14(5). doi:10.3390/ijms140510242.
- Gao J, Tao T, Arthurs SP, Ye F, An X, Hussain M, Mao R. 2023. Plant jasmonic acid mediated contrasting effects of two citrus aphid species on Diaphorina citri Kuwayama. Pest Management Science. 79(2):811–820. doi:10.1002/ps.7249.
- Glas JJ, Schimmel BCJ, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR. 2012. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci. 13(12). doi:10.3390/ijms131217077.
- Goffreda JC, Steffens JC, Mutschler MA. 1990. Association of epicuticular sugars with aphid resistance in hybrids with wild tomato. J Am Soc Hortic Sci. 115(1):161–165. doi:10.21273/JASHS.115.1.161.
- Islam T, Moore BD, Johnson SN. 2022. Plant silicon defences reduce the performance of a chewing insect herbivore which benefits a contemporaneous sap-feeding insect. Ecol Entomol. 47(6):951–958. doi:10.1111/een.13183.
- Javed K, Wang Y, Javed H, Wang C, Liu C, Huang Y. 2023. Tomato aphid (Aphis gossypii) secreted saliva can enhance aphid resistance by upregulating signaling molecules in tomato (Solanum lycopersicum). Int J Mol Sci. 24(16):12768. doi:10.3390/ijms241612768.
- Johnson SN, Rowe RC, Hall CR. 2020. Aphid feeding induces phytohormonal cross-talk without affecting silicon defense against subsequent chewing herbivores. Plants. 9(8):1009. doi:10.3390/plants9081009.
- Juvik JA, Shapiro JA, Young TE, Mutschler MA. 1994. Acylglucoses from wild tomatoes alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J Econ Entomol. 87(2). doi:10.1093/jee/87.2.482.
- Kortbeek RWJ, Galland MD, Muras A, van der Kloet FM, André B, Heilijgers M, van Hijum SAFT, Haring MA, Schuurink RC, Bleeker PM. 2021. Natural variation in wild tomato trichomes; selecting metabolites that contribute to insect resistance using a random forest approach. BMC Plant Biol. 21(1). doi:10.1186/s12870-021-03070-x.
- Kroes A, Stam JM, David A, Boland W, van Loon JJA, Dicke M, Poelman EH. 2016. Plant-mediated interactions between two herbivores differentially affect a subsequently arriving third herbivore in populations of wild cabbage. Plant Biology. 18:981–12991. doi:10.1111/plb.12490.
- Kuśnierczyk A, Tran DHT, Winge P, Jørstad TS, Reese JC, Troczyńska J, Bones AM. 2011. Testing the importance of jasmonate signalling in induction of plant defences upon cabbage aphid (brevicoryne brassicae) attack. BMC Genomics. 12. doi:10.1186/1471-2164-12-423.
- Leckie BM, D’Ambrosio DA, Chappell TM, Halitschke R, de Jong DM, Kessler A, Kennedy GG, Mutschler MA. 2016. Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS ONE. 11(4). doi:10.1371/journal.pone.0153345.
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. 2003. The tomato suppressor of prosystemin-mediated responses 2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell. 15:1646–1661. doi:10.1105/tpc.012237.
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, … Howe GA. 2004. The tomato homolog of CORONATINE-INSENSITIVE1 Is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development[W]. Plant Cell. 16(1):126–143. doi:10.1105/tpc.017954.
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I. 2006. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Molecular Plant-Microbe Interactions®. 19(6):655–664. doi:10.1094/MPMI-19-0655.
- Li Y, Cai L, Ding T, Tian E, Yan X, Wang X, Zhang J, Yu K, Chen Z. 2023. Comparative transcriptome analysis reveals the molecular basis of brassica napus in response to aphid stress. Plants. 12(15):2855. doi:10.3390/plants12152855.
- Li YH, Meijer D, Dicke M, Gols R. 2018. Oviposition preference of three lepidopteran species is not affected by previous aphid infestation in wild cabbage. Entomol Exp Appl. 166:402–411. doi:10.1111/eea.12663.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25(4):402–408. doi:10.1006/meth.2001.1262.
- López-Ráez JA, Verhage A, Fernández I, García JM, Azcón-Aguilar C, Flors V, Pozo MJ. 2010. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot. 61:2589–2601. doi:10.1093/jxb/erq089.
- McDowell ET, Kapteyn J, Schmidt A, Li C, Kang JH, Descour A, … Gang DR. 2011. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 155(1):524–539. doi:10.1104/pp.110.167114.
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. 2005. Major signaling pathways modulate arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138(2). doi:10.1104/pp.104.053389.
- Moran PJ, Thompson GA. 2001. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 125(2). doi:10.1104/pp.125.2.1074.
- Moreira X, Abdala-Roberts L, Castagneyrol B. 2018. Interactions between plant defence signalling pathways: evidence from bioassays with insect herbivores and plant pathogens. J Ecol. 106(6). doi:10.1111/1365-2745.12987.
- Morkunas I, Mai VC, Gabryś B. 2011. Phytohormonal signaling in plant responses to aphid feeding. Acta Physiol Plant. 33(6). doi:10.1007/s11738-011-0751-7.
- Nguyen D, Rieu I, Mariani C, van Dam NM. 2016. How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol. 91:727–740. doi:10.1007/s11103-016-0481-8.
- Oldroyd GED, Staskawicz BJ. 1998. Genetically engineered broad-spectrum disease resistance in tomato. Proc Natl Acad Sci USA. 95:10300–10305. PMID: 9707642. doi:10.1073/pnas.95.17.10300.
- Ontiveros I, López-Moya JJ, Díaz-Pendón JA. 2022. Coinfection of tomato plants with Tomato yellow leaf curl virus and Tomato chlorosis virus affects the interaction with host and whiteflies. Phytopathology®. 112:944–952. doi:10.1094/PHYTO-08-21-0341-R.
- Pieterse CMJ, van der Does D, Zamioudis C, Leon-Reyes A, van Wees SCM. 2012. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 28. doi:10.1146/annurev-cellbio-092910-154055.
- Planelló R, Llorente L, Herrero Ó, Novo M, Blanco-Sánchez L, Díaz-Pendón JA, … de la Peña E. 2022. Transcriptome analysis of aphids exposed to glandular trichomes in tomato reveals stress and starvation related responses. Sci Rep. 12(1):20154. doi:10.1038/s41598-022-24490-1.
- R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
- Rodríguez-López MJ, Garzo E, Bonani JP, Fereres A, Fernández-Muñoz R, Moriones E. 2011. Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology. 101(10). doi:10.1094/PHYTO-01-11-0028.
- Rodríguez-López MJ, Moriones E, Fernández-Muñoz R. 2020. An acylsucrose-producing tomato line derived from the wild species Solanum pimpinellifolium decreases fitness of the whitefly trialeurodes vaporariorum. Insects. 11(9):616. doi:10.3390/insects11090616.
- Rodriguez-Saona CR, Musser RO, Vogel H, Hum-Musser SM, Thaler JS. 2010. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J Chem Ecol. 36:1043–21057. doi:10.1007/s10886-010-9854-7.
- Ryan CA. 2000. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1477(1-2):112–121. doi:10.1016/S0167-4838(99)00269-1.
- Sanmartín N, Sánchez-Bel P, Pastor V, Pastor-Fernández J, Mateu D, Pozo MJ, … Flors V. 2020. Root-to-shoot signalling in mycorrhizal tomato plants upon Botrytis cinerea infection. Plant Sci. 298:110595. doi:10.1016/j.plantsci.2020.110595.
- Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MGA, … Janssen A. 2011. A herbivore that manipulates plant defence. Ecol Lett. 14(3):229–110236. doi:10.1111/j.1461-0248.2010.01575.x.
- Schuurink R, Tissier A. 2020. Glandular trichomes: micro-organs with model status? New Phytol. 225(6):2251–2266. doi:10.1111/nph.16283.
- Schwartzberg EG, Tumlinson JH. 2014. Aphid honeydew alters plant defence responses. Funct Ecol. 28(2). DOI: 10.1111/1365-2435.12182
- Schweiger R, Heise AM, Persicke M, Müller C. 2014. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 37(7):1574–1585. doi:10.1111/pce.12257.
- Simmons AT, Gurr GM. 2005. Trichomes of lycopersicon species and their hybrids: effects on pests and natural enemies. Agric For Entomol. 7(4). doi:10.1111/j.1461-9555.2005.00271.x.
- Soler R, Badenes-Pérez FR, Broekgaarden C, Zheng SJ, David A, Boland W, Dicke M. 2012. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: from insect performance to gene transcription. Funct Ecol. 26:156–166. doi:10.1111/j.1365-2435.2011.01902.x.
- Stam JM, Kroes A, Li Y, Gols R, van Loon JJA, Poelman EH, Dicke M. 2014. Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol. 65. doi:10.1146/annurev-arplant-050213-035937.
- Steinbrenner AD, Muñoz-Amatriaín M, Chaparro AF, Aguilar-Venegas JM, Lo S, Okuda S, … Crubaugh D. 2020. A receptor-like protein mediates plant immune responses to herbivore-associated molecular patterns. Proc Natl Acad Sci USA. 117(49):31510–31518. doi:10.1073/pnas.2018415117.
- Tian D, Tooker J, Peiffer M, Chung SH, Felton GW. 2012. Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta. 236(4). doi:10.1007/s00425-012-1651-9.
- Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, Jones W, Bender CL. 2005. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 42(2):201–217. doi:10.1111/j.1365-313X.2005.02366.x.
- van Emden HF, Harrington R. 2007. Aphids as crop pests. Aphids as Crop Pests. doi:10.2135/cropsci2008.02.0001br.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling and Behavior. 7(10). doi:10.4161/psb.21663.
- Zhang Y, Li X. 2019. Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol. 50:29–36. doi:10.1016/j.pbi.2019.02.004.
- Zhu-Salzman K, Salzman RA, Ahn JE, Koiwa H. 2004. Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol. 134(1). doi:10.1104/pp.103.028324.
- Züst T, Agrawal AA. 2016. Mechanisms and evolution of plant resistance to aphids. Nat Plants. 2. doi:10.1038/nplants.2015.206.
